When Would Immunologists Consider a Nanomaterial to be Safe? Recommendations for Planning Studies on Nanosafety
Abstract
The immune system is professional in recognizing and responding to non-self, including nanomaterials. Immune responses by professional and nonprofessional immune cells are thus nearly inevitable upon exposure of cells and organisms to such materials. The state of research into taking the immune system into account in nanosafety studies is reviewed and three aspects in which further improvements are desirable are identified: 1) Due to technical limitations, more stringent testing for endotoxin contamination should be made. 2) Since under overdose conditions immunity shows unphysiological responses, all doses used should be justified by being equivalent to tissue-delivered doses. 3) When markers of acute inflammation or cell stress are observed, functional assays are necessary to distinguish between homeostatic fluctuation and genuine defensive or tolerogenic responses. Since immune activation can also indicate that the immune system considers a stimulus to be harmless and induces tolerance, activation markers by themselves do not necessarily imply a danger to the body. Guidelines such as these are necessary to approach the point where specific nanomaterials are classified as safe based on reliable testing strategies.
1 Introduction
The literature on nanotoxicity is by now exceeding 30 000 publications. It has been appreciated that methodical limitations and technical shortcomings are widespread in this body of literature.[1, 2] In many cases, studies have concluded that the particles tested elicit detrimental biological effects under the conditions chosen for the experiment. What can be done to step from there to the point where we can state with reasonable confidence that a specific nanomaterial (NM) is harmless to humans under realistic conditions? This step is urgently necessary, not only because we don't want to waste time and resources on extensively investigating the safety of NMs that are rather harmless in real life, but also because time and resources may then be lacking to analyze nano and other advanced materials that are more problematic. The risk and safety community should work primarily on materials that deserve the highest concern.
The definition on when a NM is considered to be harmless depends on the background of experts and certainly needs input from different fields. Genotoxicity specialists will define different data as necessary than experts on reproductive biology, but overall the amount of required information is finite. We recommend here the minimal requirements of immunologists for considering a NM as not dangerous.
Immunology plays a special role in the field, because the ensemble of immune cells and molecules is professional at recognizing non-self and will thus nearly always interact with nanoparticles (NPs).[3] Immune reactions, especially inflammation, are often reported as biological outcomes [4, 5] and a recent survey of nanosafety experts has identified reactive oxygen species (ROS) and immune system effects as the most frequently studied endpoints.[6] It is also worth noting that the innate branch of the immune system is shared between invertebrates and vertebrates, so information on inflammation is relevant for humans as well as for environmental species.[7]
The immune system may also provide a metaphor: Recognition of non-self by the immune system usually results in immune tolerance, an active response that becomes part of the immune memory. Tolerance is the default response of the immune system, since it needs to tolerate self. Several mechanisms exist to ensure also tolerance against harmless non-self, for example immunosuppressive regulatory T cells.[8] Immune tolerance is broken when agents are sensed that are genuinely dangerous, which is potentiated by signs of damage to the body. The clear distinction between harmless and harmful agents is the state that we need to achieve in nanosafety.
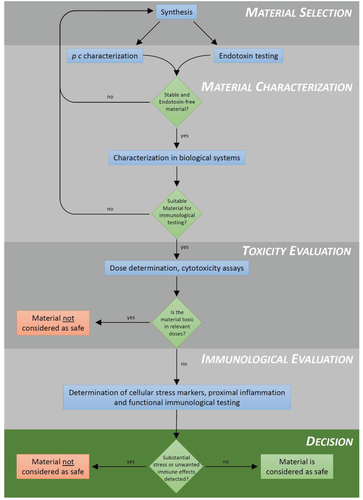
Figure 1 shows a simplified scheme for testing safety of NMs. We discuss here three types of data that are necessary to come to a conclusion about immunosafety. The critical questions are for immunologists: Is there a danger of false positives (or negatives) due to biological bystander substances? Do the doses used reflect the dose range that is experienced by immune cells in the body? Are observed changes in immune parameters signs of a defensive reaction, of a tolerogenic reaction, or a normal homeostatic fluctuation to adapt to a change in the environment? These questions can be addressed by immunological testing, provided that a sufficient amount of material is available for the required tests and no acute toxicity is manifest, which would stimulate unspecific inflammatory responses. The latter has to be verified by evaluating toxic effects for the duration of the intended experiments, which means that a dose-response curve for acute toxicity should be included on all toxicological studies. A further, critical requirement is that there is no unintentional contamination with proinflammatory agents like endotoxin (see below).
2 Endotoxin Contamination on NMs
2.1 Characteristics of Endotoxin
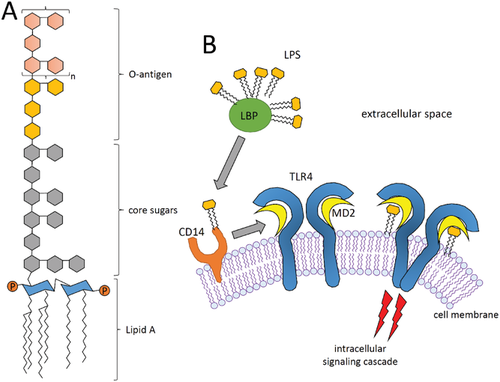
Endotoxin or lipopolysaccharide (LPS) is a molecule found in the outer membrane of Gram-negative bacteria.[9] It is nearly ubiquitous, due also to the bacterial flora that every human carries. LPS can easily persist in the absence of these bacteria and due to its high thermostability, it is resistant towards most standard sterilization methods applied in biological laboratories.[9-11] Chemically, bacterial endotoxin is a large molecule with a molecular weight of up to 1000 kDa, which consists of a polysaccharide region and a lipid region termed lipid A.[12] In addition, it carries phosphate groups—-thus, endotoxin has lipophilic as well as hydrophilic properties which enables it to bind to many kinds of surfaces.[10]
LPS is known to induce inflammatory reactions or even toxic effects. This is mediated by a signaling pathway involving the Toll-like receptor 4 (TLR4) which is expressed by many immune cell types such as dendritic cells (DCs)or macrophages.[13, 14] Binding of LPS to TLR4 is facilitated by the co-receptors CD14 and MD2.[15] Subsequent dimerization of TLR4 receptors initiates an intracellular signaling cascade involving several adaptor proteins including myeloid differentiation primary response gene 88 (MyD88), TIR domain-containing adaptor protein-inducing IFN-β (TRIF), and TRIF-related adaptor molecule (TRAM). Via two different pathways—-the MyD88 dependent and the MyD88 independent pathway—-LPS/TLR4 signaling continues leading to the activation of the transcription factors NF-κB and IRF, which in turn promote the secretion of proinflammatory cytokines and type I interferons.[16] This response can lead to fever, respiratory symptoms, inflammation and asthma.[12, 17, 18] A schematic structure of LPS and its mechanism for TLR4-mediated cell activation is given in Figure 2. LPS can even cause acute toxic effects: when entering the blood stream in higher concentrations, it causes systemic responses such as fever, hypotensive shock, impaired organ function or even multiple organ failure leading to death.[12, 19] Taken together, LPS is a crucial immunogenic factor that should be avoided when investigating biological responses of humans and animal cells towards different stressors, such as NMs, unless the presence of immune-stimulating bystander materials is desired.
2.2 Endotoxin Contamination on (Nano) Materials
As already mentioned, LPS contains hydrophobic and hydrophilic sections and thus is able to bind on very different surfaces/materials. This includes NPs, but also laboratory equipment in general, such as glass ware, pipettes, culture dishes, etc. Gorbet and Sefton determined the levels of endotoxin in different reagents and found levels of 20 endotoxin unit (EU) mL−1 in double-distilled water (ddH2O) or 76 EU mL−1 in phosphate-buffered saline (PBS) prepared from powder that was dissolved in endotoxin-free (!) H2O.[10] 1 EU refers to 100–200 pg LPS detected by various assays, see below. The severe health effects of endotoxins are especially important for biomaterials that are intended to be used as medical implants, e.g., for clinical treatment of orthopedic tissue injuries as reviewed earlier by Lieder et al.[20] For medical devices the FDA limits a maximum of 0.5 EU mL−1 for extracts of products that directly or indirectly contact the cardiovascular system and lymphatic system and 0.06 EU mL−1 for extracts of products in contact with cerebrospinal fluid (https://www.fda.gov/media/83477/download, visited 19th December, 2019).
Due to their small size and large surface to volume ratio, NMs are prone to bind large amounts of endotoxins onto their surface. Endotoxin binding to NMs likely involves Coulomb and van der Waals interactions and depends on the surrounding medium.[21] Furthermore, hydrophobic interactions between the lipid A part and the NM surface can play a role in adsorption of endotoxin to iron oxide NPs.[22] In addition, the polysaccharide chains have been shown to be involved in binding of endotoxins to the surface of Al2O3 NPs leading to LPS coating layers with a thickness of tens of nanometers.[23] Oostingh et al. investigated the LPS content in different preparations of Au NPs and found a high variability in endotoxin contents (between < 0.1 and 151.6 EU mL−1) among the different samples, concluding that handling conditions during synthesis or the use of contaminated glassware/reagents are major issues that play a role in the presence of endotoxin in the end product.[24] Additional NPs (gold, silver, iron oxide, polystyrene) were tested for endotoxin contamination by Li and colleagues with similar results (high variations between 0.5 and more than 1000 EU mL−1).[9] Thus, it is clear that endotoxin frequently occurs in NPs, which should be carefully considered when discussing their immunological effects.
Numerous studies have been published that investigate nanosafety with respect to human health and the environment [1, 2] and many more will follow soon.[25] In addition, immune effects of NPs have been critically reviewed earlier.[7, 26, 27] Here, the role of LPS comes into play: Li and Boraschi presented in a recent review numerous in vitro and in vivo studies that describe the biological effects of endotoxin associated with NPs.[12] They show a large bandwidth of results, mainly concluding that the presence of LPS in NP samples leads to increased inflammatory reactions accompanied by upregulation of typical proinflammatory cytokines such as IL-1β, IL-6, or TNF-α. However, inhibitory results were also presented, for example for LPS-treated Au and Pt NPs, which decreased inflammatory reactions compared to the NPs alone in rat's uveitis [28] and murine macrophages.[29] Thus it is evident that contamination of NPs with LPS can lead to misinterpretation of results since it is not clear whether an observed (anti) inflammatory reaction is a result of the NPs itself or is due to endotoxin contaminants.[9] Therefore, determination of possible endotoxin contamination in NP samples, or—-at best—-complete avoidance of endotoxin in any of the samples is a crucial issue when testing NPs for their safety.
2.3 Measurement Methods of Endotoxin
Determination of endotoxin contents on NMs is an essential step in immunosafety assessment of NMs in order to discriminate between effects resulted from endotoxin and intrinsic effects of the NMs themselves. The most prominent methods for endotoxin detection are the rabbit pyrogen test (RPT[30]) and the limulus amoebocyte lysate (LAL) assay.[31] Besides these, other bioassays can be used such as the monocyte activation test (MAT), the human PBMC activation assay and other commercial available kits (EndoZyme, EndoLISA, or transfected HEK-Blue TLR cells). Table 1 lists the different available test systems and discusses their function, advantages and disadvantages.
| Assay | Principle | (+) Advantages/(−) disadvantages | Refs. |
|---|---|---|---|
| Rabbit pyrogen test (RPT) | Injection of test substance into rabbits and monitoring of the animals' body temperature | (+) FDA-approved (−) Expensive, use of animals, not specific for endotoxin, not quantitative |
[30, 32] |
| Limulus amoebocyte lysate (LAL) assay | Reaction of endotoxin with an enzyme from the horseshoe crab, leading to protein clottinga), turbidityb), or formation of p-nitroaniline (pNA)c) | (+) Easy and cheap to performa)-c), Quantitative and highly sensitiveb), c) (−) only semiquantitativea), interferences with NMs possiblea)-c) |
[31, 33–35] |
| Endozyme/EndoLISA | Modified LAL assay involving a recombinant Factor C protein instead of LAL and a fluorescent detection method | (+) Easy to perform, high sensitivity, quantitative (−) Interferences with NMs possible, e.g., fluorescence quenching, inhibition of protein function or immobilization (EndoLISA) |
[36-38] |
| Monocyte activation test (MAT) | Activation of monocytes from human blood samples by endotoxin. Measurement of cytokine secretion by ELISA | (+) High sensitivity and quantitative. High biological relevance through the use of human cells (−) Lack of specificity, since also test materials (NMs) can induce inflammation themselves |
[39-41] |
| HEK-Blue hTLR4 assay | Activation of TLR4 pathway in HEK293 cells co-transfected with the human TLR4, MD-2, and CD14 co-receptor genes, and an inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene which is activated under the control of a minimal promoter fused to NF-κB-binding sites. | (+) Sensitive and quantitative. Specific for TLR4 activating ligands (−) Expensive, interferences with NMs still possible through unspecific binding to TLR4 receptor |
[42, 43] |
- The LAL assay is available in three variants:
- a) The gel-clot assay
- b) The turbidimetric assay and
- c) The chromogenic assay.
The RPT was the first described method for endotoxin detection already more than 100 years ago[30] and it was approved by the FDA in 1943. The principle is simple: the test substance is injected into a rabbit in which the temperature rise of the animal is subsequently monitored. Occurrence of fever indicates the presence of endotoxin in the sample. It is obvious that this test has several disadvantages: Use of animals, high costs, long test duration, no quantitative results or lack of specificity, i.e., also NMs (or other substances) could cause fever without endotoxin being present. Several alternative methods have been developed over the past decades.
One of the most common endotoxin assays is the also FDA approved LAL assay, which is often considered as the standard method for endotoxin detection. The LAL assay is an in vitro method involving an enzyme from the horseshoe crab Limulus polyphemus, which can be activated by endotoxin, initiating a cascade that in the end leads to the activation of a clotting enzyme inducing formation and clotting of coagulin.[44] Three different variants of the LAL assay are currently used, which are the gel-clot LAL assay, the turbidimetric LAL assay and the chromogenic LAL assay.[35] In the gel-clot assay, the protein clotting is directly observed, which makes this method the easiest and cheapest alternative, however with the disadvantages that it is only semi-quantitative and not very sensitive. The turbidimetric variant of the LAL assay involves turbidity measurements of the formed coagulin which makes the assay more sensitive and also quantitative. However, turbidimetric measurements can be easily affected due to the presence of other substances, such as NMs. The chromogenic LAL assay uses a synthetic substrate which is converted by the clotting enzyme to the colored compound p-nitroaniline (pNA), whose absorbance can be measured photometrically at 405 nm. This is probably the most frequently used variant of the LAL assay in biological labs. It is quantitative, highly sensitive and only affected by NMs that absorb at similar wavelengths and/or interfere with the enzyme reaction.
In addition to the classical LAL assays, some modifications are commercially available, like Endozyme and EndoLISA, which avoid the use of animal resources. These assays are based on the activation of a recombinant form of Factor C, which is one of the first elements involved in the Limulus coagulation cascade and involve a fluorogenic detection method.[36, 37] Endozyme is a homogenous test for soluble samples while EndoLISA uses a recombinant bacteriophage protein to immobilize endotoxins and detect them in a principle similar to an enzyme-linked immunosorbent assay (ELISA).[38] Both methods are highly sensitive and quantitative, however, they also can be affected by NMs due to interferences with the fluorescent light detection (e.g., quenching), inhibition of the protein function, or protein immobilization (EndoLISA).
Besides the vertebrate-based RPT and the invertebrate-based LAL assays, there are other types of in vitro tests for endotoxin detection using cell cultures. The MAT is based on human whole blood and is accepted by the European pharmacopoeia and the US FDA.[39, 41] In principle, monocytes isolated from blood samples are incubated with LPS or unknown test substances and their cytokine expression (IL-1β, IL-6, TNF-α) is monitored using ELISA. The test is highly sensitive and quantitative by using LPS standards; however, NMs can induce cell activation by themselves or interfere with the ELISA methods that should be carefully considered. Another disadvantage of this method is the presence of various TLRs on monocytes. Therefore, possible effects cannot be clearly attributed to LPS-induced TLR4 signaling. This problem is bypassed in the HEK-Blue hTLR4 assay, another alternative in vitro test using transfected human embryonic kidney (HEK) cells. HEK293 cells, which do not express any endogenous TLRs, are co-transfected with the human TLR4, MD-2 and CD14 co-receptor genes, and an inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene, which is activated under the control of a minimal promoter driven by the LPS-inducible transcription factor NF-κB. Upon stimulation with a TLR4 ligand (such as endotoxin), activation of NF-κB leads to the production of SEAP,[42, 43] which can then be quantified spectrophotometrically using the QUANTI-blue dye. Since it uses transfected cells, the HEK-Blue hTLR4 assay is a rather expensive and sophisticated method for endotoxin detection. It is sensitive and quantitative; assay interference with NMs is less likely, but assay interference still should be considered carefully as already described for the MAT.
2.4 Assay Interferences and “Masked” LPS
As already mentioned in the previous section, assay interferences of NMs (or other compounds) are a crucial issue which should be carefully considered when testing NMs for their endotoxin contents. The different endotoxin test methods as well as the importance of studying endotoxin contaminations on NMs in order to obtain appropriate data on their immunosafety assessment of NMs have been reviewed earlier.[9, 12, 45] Li et al. compared the different commercially available LAL assays regarding their interferences for detection of LPS contents on gold, silver and iron oxide NMs. They found that with the modified chromogenic LAL assay, the best results were obtained concerning the sensitivity and the assay interference with the NPs.[46] Smulders et al. obtained similar results with TiO2, Ag, CaCO3, and SiO2 NPs: in their study, the chromogenic LAL assay as well as the HEK-Blue hTLR4 showed no interferences with NPs.[45] In a recent study by Neun and Dobrovolskaia, the authors gave some recommendations on how to choose the proper LAL-based assay dependent on the respective NM of interest.[47] In general, factors to consider are the NM appearance, turbidity and absorption spectra (especially at wavelengths 405 and 540 nm, which are used in commercially available LAL assays). Using spiked standards as additional controls should be considered in order to validate the correct functioning of the respective assay.
Per definition low endotoxin recovery (LER) is a “masking effect” describing reduced detectability of endotoxin activity in biopharmaceutical product formulations containing chelating agents, surfactants and detergents. Because formulations used to stabilize NMs might contain similar components, LER should also be considered as potential problem for LPS detection in NM samples. LER is a temperature- and time-dependent process especially occurring in Factor C-based assays despite the fact that positive product controls (PPCs) show no evidence of test interference.[48-50] The precise molecular mechanisms behind LER are still not completely understood. It was suggested that chelators remove magnesium and calcium from LPS aggregates, changing the physical formation of LPS from an aggregated form into monomers, which are undetectable by Factor C-based assays.[51, 52] It was further demonstrated that LPS binding to factor C relies on the presence of multiple LPS binding sites and highly cooperative binding mechanisms, meaning that more than one LPS molecule is required for the activation of Factor C.[53] Because monomeric LPS may not activate Factor C sufficiently, there is a risk that Factor C-based LPS tests may display false negative results. In contrast, LPS detection assays involving TLR4 and its co-receptors CD14 and MD2 are particularly suitable for the detection of monomeric LPS, because the role of CD14 in vivo is to form a stable complex with monomeric LPS and deliver those complexes to the TLR4/MD-2 receptor. The dissociation of highly aggregated LPS to a monomeric form might even serve as the initial step in the activation of responding innate immune cells like monocytes.[54]
Direct comparison of different LPS detection assays revealed that Factor C based assays were unable to detect masked endotoxin whereas it was still detectable in a cell-based TLR4/CD14/MD2-NF-κB-luciferase reporter gene assay. Accordingly, primary human monocytes, which also express the TLR4/CD14/MD2 receptor complex responded strongly to masked LPS as shown by increased expression of proinflammatory cytokines and surface activation markers upon simulation with masked endotoxin.[49]
2.5 What Do We Need?
To answer the question, what data an immunologist needs to consider a NM as endotoxin-free, we give the following recommendations: 1) the material should be assayed for endotoxin contaminations by at least two different methods that follow different principles, e.g., a LAL-based assay (chromogenic LAL assay, or a modified recombinant Factor C assay (Endozyme, EndoLISA) and a cell-based assay (e.g., MAT or TLR4 reporter gene assay). 2) The used assays should be validated by including appropriate controls, i.e., spiked LPS standards, to verify the correct function of the assays. These data should be included to the LPS data obtained. 3) The results obtained should be reproduced at least two times with independently prepared samples. Measured endotoxin levels should be lower than 0.02 ng mL−1, i.e., <0.1 EU mL−1, in respective doses of NMs that will be applied (see Section 3), since such small amounts already are able to stimulate immune cells [55] and thereby can lead to misinterpretation of results.
3 Dose Justification in NM Safety Assessments
Non-self-recognition by professional immune cells is essential to generate immune responses against potential threats, including NMs. Immune cells can be found as circulating cells in blood and lymph, in defined structures of lymphoid organs, but they are also present as scattered cells in all tissues. Nevertheless, exposure and physical contact to NM starts in a healthy individual virtually always at a protective barrier, primarily the epithelia of either the skin, the lung or the gastrointestinal tract. At and behind these barriers, professional immune cells, upon non-self-recognition, depend on cellular decision making—-danger or not—-in order to initiate either an orchestrated vigorous defensive response or to do just the opposite, to calm down and arrange tolerance against NM. The dose of a non-self-agent determines not only the extent but also the type of the response, so it is extremely critical for immunity. While the margin of error here has to be kept low and the final cellular sum up for such a decision by professional immune cells may be very complex, the decision will largely rely on how much and at which rate non-self-NM is recognized beside the physical and chemical properties. Hence, the tissue delivered dose of NM is of exceptional importance and more strongly related to specific immune effects than the preceding exposure dose for the body. Adsorption, distribution and elimination over time are the factors that need to be considered to predict a tissue delivered dose in vivo. Dynamic NM modifications, such as protein corona formation, agglomeration or dissolution will further contribute to variations of the tissue delivered dose in the context of NM exposure. These effects are not limited to an in vivo context. They are equally determining the delivered dose in vitro, which represents the fraction of the administered NM dose reaching adherent or suspended cells in submerged cell culture systems. In this type of assays, the NM mass transfer is determined by particokinetics, in particular gravitational settlement and Brownian diffusion. Hence it is critical to link the exposure dose to a tissue-delivered dose in a first step and then to relate it in a second step to an equivalent cell surface-delivered dose and corresponding administered dose in vitro. Without such a multistep dose justification it is impossible to link in vitro nanotoxicity findings to real world exposure conditions, because observed effects may well be related to nonphysiological cellular responses due to unjustified dosing.
3.1 The Main Routes for NM Entry and Immunogenic Non-Self Recognition
Skin, lung, and gastrointestinal epithelia are the most likely ones exposed to NM. There is little evidence that NM, upon environmental or occupational exposure at intact skin, easily translocates to the deeper layers of living cells of the epidermis or beyond. For some consumer products (sunscreens, makeup), an accumulation of NM in hair follicles[56-58] could promote skin penetration as confirmed in human volunteers.[59] However, new ex vivo experiments with human skin and TiO2 NPs could not detect NM outside the stratum corneum of the epidermis both in intact or damaged skin after 24 h.[60] At gastrointestinal epithelial barriers, NM exposure is linked to food and fluid uptake contaminated with traces of NM, food additives or inhaled NPs subsequently cleared by the mucociliary escalator [61] into the esophagus.[62] The processing of non-self-material is the key task at the gastrointestinal epithelia and includes digestive and absorptive steps. These processes in general are associated with size reduction of food particles down to nanosized particular intermediates and finally molecular entities. In this process, particular matter, such as bacteria or micro and nanosized particles, are strictly separated from the intestinal epithelia and associated professional immune cells by a gel-forming mucus layer.[63, 64] The renewal and growth rate of this layer towards the lumen is up to 4 µm min−1 in humans,[65] therefore actively pushing away particular matter at a rate which is, for most nanosized particles, beyond the achievable distance of directed translocation by diffusion. Thus, the mucus layer provides an effective barrier, largely preventing transmucosal effects of particular matter while still allowing mass transfer of degraded nutrients to the epithelial cells by diffusion.[63] NM is therefore rapidly expelled with very little absorption. Additionally, the epithelial layer and the mucosa, the inner layer of the gut, are very rich of professional immune cells and well-structured local lymph node-like structures (Peyer's patches).[66] Its proximity to the commensal microbiota promotes a high degree of intestinal immune tolerance,[67] which would also cover non-self-NM of any origin.
There is some evidence in animal models that NP translocation through skin and gastrointestinal epithelia is possible and NPs were also found in lymphoid tissue, but these results are associated with excessive NP dosing when compared to human environmental or occupational exposure scenarios.[68] Therefore, exposure via the skin or ingestion is not the most pressing concern due to effective barriers, effective elimination and—- if any—-an expected very low tissue delivered dose. This is not true for the main route of NP exposure by inhalation and subsequent deposition at the epithelia of the respiratory tract. In a survey of nanosafety experts, the highest concern regarding uptake routes was expressed concerning the inhalation pathway.[6]
3.2 The Pressing Need for Dose Justification
Indeed, inhalation exposure to NM has been identified as a potential risk factor for human health.[69] It may be easily accepted that NM safety studies should include dose justification,[62] but the majority at least of submitted manuscripts is deficient in this respect. Accompanying dose finding studies are even rarer. Gangwal et al. described one of the first model-based approaches building the bridge from real world exposure to the effective delivered dose in vitro for inhalable NM in 2011.[70] Without convincing dose bridging,[71, 72] in vitro results lack practical relevance since interpretation of results could be wrong in any direction with false positive or false negative outcomes. Although strongly recommended in several publications and protocols,[73-76] this topic is still insufficiently addressed leaving room for mis-dosed studies.
NP overload in vitro can lead to nonphysiological immune responses. It is important to note that an overload dose always refers to the delivered dose effective at the cellular level and not to the administered dose, because both are frequently linked in a nonlinear manner [62, 77, 78] and administered dose is a poor surrogate for delivered dose. Overload effects can have their root cause either in an overall unjustified high NP delivered dose, but they could also result from cryptic overloading by excessive mass transfer rates at the start of an experiment and fading thereafter.
3.3 The Dose Finding Process for NP Assays
In contrast to classical dosimetry, where the effective dose of a molecular entity to be tested is mainly defined by a function of the administered mass concentration and the exposure duration, NP dosimetry is more complex and this is true in vivo as well as in vitro. In both cases, particokinetics determines to a large degree how much of the administered particle dose will be in close proximity to (immune) cells over time. Only this fraction can take part in the cellular bio–nano interactions driving the biological endpoint of a study.
Particokinetics in vivo is dominated by the principles of Brownian diffusion and impaction [79] and these determine the tissue delivered dose to the epithelia of the respiratory tract. In vitro, diffusion and gravitational settling are the main mechanisms for particle deposition at submerged adherent cells.[80] In both cases, however, consideration of particokinetics depends on the availability of particle characteristics data in situ, mainly information on size, shape, density, size distribution and in case of agglomeration, the size, size distribution and the effective density of agglomerates.[80, 81] Data on stability over time—-the rate of dissolution or ongoing agglomeration—-help to refine the outcomes of dose finding studies. All these data at hand, in silico modeling, based on validated tools, is the least time consuming and cost effective approach to start with dose finding studies. Established tools are computational fluid dynamics (CFD), distorted grid (DG), in vitro sedimentation diffusion dosimetry (ISDD) and its follow up version ISD3 for in vitro modeling [77, 78, 82], and multiple path particle dosimetry (MPPD) [83, 84] for in vivo simulation. Each in silico tool has its specific limitations whereof dealing with NP polydispersity is a shared problem.[77, 85] A comprehensive overview on the essential parameters for in silico modeling is provided in Tables 2 and 3.
| Parameter | I/Oa) | Req/Optb) | Description | |
|---|---|---|---|---|
| Exposure conditions | Aerosol concentration Exposure time Number of days Exposure and activity |
I I I I |
Req Req Opt Req |
NP concentration in the inhaled air Exposure time per day Number of days for repeated exposure Variable or constant scenario; description of body orientation, breathing frequency, tidal volume, inspiratory fraction, pause fraction, breathing mode (oral, nasal,…) |
| Lung/airway morphometry | Species Model FRC URT volume Pulmonary structure |
I I I I I |
Opt Req Req Req Req |
Specification of species morphometry Airway morphometry model, such as Yeh/Schum 5-Lobe, age specific symmetric, Functional residual capacity Upper respiratory tract volume Morphometric data for each pulmonary structure (bronchiole, distal alveolar region, …) such as length, volume, surface area, number of alveoli, number of macrophages (for overload calculations, clearance) |
| Clearance | Clearance settings Total mass retained Time to 50% clearance Clearance |
I O O O |
Opt Req Opt Opt Req |
Clearance rate, post exposure time Total NP mass retained in the different pulmonary tracts Half-life time of NP deposited Cleared mass in the different pulmonary tracts over time |
| Particle characteristics | Diameter Shape Density Dissolution rate Polydispersity |
I I I I I |
Req Opt Req Opt Opt |
Diameter of the particle Shape of the particle (spherical, rod, …) Density of the particle for dissolving particles only Size distribution |
| Agglomerate characteristics | Diameter Effective density Packing factor Polydispersity |
I I I I |
Opt | Agglomerate diameter Agglomerate density: is reduced due to intraagglomerate air fraction Solid fraction within an agglomerate Size distribution |
| Tissue delivered dose | Per pulmonary segment: Fraction deposited Mass deposited Surface area deposited Number deposited |
O O O O |
Req Req Req Req |
Fraction of exposure dose deposited in the pulmonary section Particle mass deposited; for overload evaluation: optional mass per macrophage Particle surface area deposited; for overload evaluation: optional surface per macrophage Particle number deposited; for overload evaluation: optional number per macrophage |
- a) I: Input; O: Output
- b) Req: required; opt: optional.
| Parameter | I/Oa) | Req/Optb) | Description | |
|---|---|---|---|---|
| Exposure conditions | Concentration Incubation time Boundary conditions |
I I I |
Req Req Opt |
Administered dose Incubation time with particles applied Stickiness (particle sticks at the cell surface with first contact): yes/no; 0 – 1 |
| Particle characteristics | Diameter Shape Density Dissolution rate Polydispersity |
I I I I I |
Req Opt Req Opt Opt |
Diameter of the particle Shape of the particle (spherical, rod, …) Density of the particle for dissolving particles only Size distribution |
| Medium/well conditions | Dish depth Well volume Temperature Viscosity Density |
I I I I I |
Req Req Req Req Req |
Medium height level Medium volume applied per well Medium temperature Medium viscosity Medium density |
| Agglomerate characteristics | Diameter Effective density Packing factor Polydispersity |
I I I I |
Opt | Agglomerate diameter Agglomerate density: is reduced due to Intraagglomerate medium Solid fraction within an agglomerate Size distribution |
| Delivered to cell dose | Fraction deposited Mass deposited Surface area deposited Number deposited |
O O O O |
Req Req Req Req |
Fraction of administered dose deposited Particle mass deposited Particle surface area deposited Particle number deposited |
- a) I: Input; O: Output
- b) Req: required; opt: optional.
Figure 3 illustrates a general in silico strategy for in vivo to in vitro dose bridging studies.
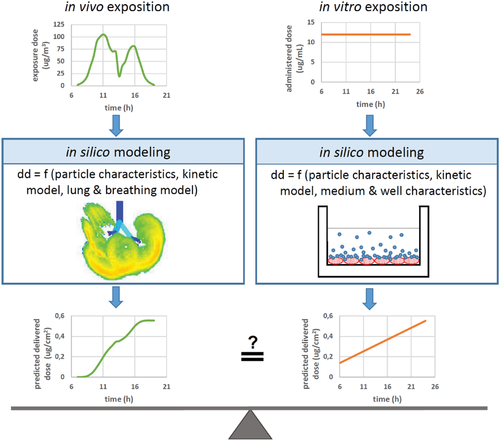
In vivo the particle size is the main selection criterion for particle deposition within different structures of the respiratory system. A larger size of particles selects for early deposition by impaction in the upper respiratory tract and airway structures of the lower tract, while small sized NPs effectively deposit by diffusion in the intrapulmonary regions, most notably in the alveoli.[62, 79, 86] Many deposition models are still based on the human respiratory tract model (HRTM) of the international commission of radiological protection (ICRP).[87] More stringent investigation of alveolar NP deposition proposed nonhomogeneity associated with hot spots of NP retention.[88] As such, the proximal alveolar region (PAR) was identified [89, 90] and animal lung histopathology confirmed an increase in the number of macrophages in this region, both 18 and 36 h post exposure.[91]
Under submerged in vitro conditions, NP mass transfer for particles in the upper nanosize range or for particles with high effective particle density is dominated by gravitational settling, while small nanosized particles deposit by diffusion and therefore independent of density. Consideration of media characteristics and well characteristics adds more complexity. Particle surface charge, the presence of ions and a possible protein content of the cell culture medium may promote protein corona formation or yield instable colloids with agglomerated primary particles, which dramatically changes the particokinetic profile. Agglomeration goes along with increased size and thereby promotes gravitational settlement, but it also means a loss of effective density of the agglomerates due to intraagglomerate fluid accumulation, which counteracts the aforementioned effect.[75] A thorough characterization of the particokinetic determinants is necessary, because at this point it is evident that the delivered dose may not reflect the administered dose in a linear manner. Additional nonlinearity may originate from the selected media and well characteristics. As aforementioned, denser and bigger particles come along with efficient deposition by gravitational settling, which means a high initial deposition rate (mass deposited per minute) and cryptic overloading. When undetected and not tested for, cryptic overloading may disqualify any data on biological endpoints of an in vitro assay. On the other hand, a high initial deposition rate in vitro could be part of an experimental setup mimicking a very high short time exposure in vivo, which is primarily relevant for medical use.
All these aspects have to be considered when determining a reasonable dose scheme for the experimental setting, which is indispensable to obtain reliable, comparable and relevant data.[73, 92] A high quality dose finding study should have two findings regarding doses: First, it should demonstrate that the delivered dose in vitro is reasonably linked to a human exposure dose in vivo.[72] Second, it should underpin that the in vitro deposition rate is also reasonably reflecting the in vivo exposure scenario and that it is free from cryptic overload effects. The immunologist would ask, which dose is encountered by immune cells in the body and is this mimicked in the experiment?
3.4 The Main Pitfalls in Dose Finding
Unjustified dose ranges, cryptic overdosing or colloid instability due to agglomeration are among the main sources of errors in assays with immunological endpoints. While particle dissolution and the release of ions is a matter for consideration in virtually all cellular assays, for professional immune cells, the recognition of non-self, the subsequent induction of immune tolerance or the initiation of a defensive response, depend on cellular decision making open for different outcomes.[93] The time elapsed for such a decision making together with the associated NP deposition rate links the accumulated delivered dose to the immune response at this point. Changes in gene regulation measured as specific alterations of mRNA, occur very fast and often peak within the first hours.[94] Small differences in the early stages of non-self-recognition may promote big differences in the response patterns with contrasting outcomes. Hence, special attention is needed i) to avoid supraphysiological responses due to overload, ii) to implement conclusive testing strategies for acute versus chronic exposure, and iii) to identify the most appropriate dose metric.
3.4.1 Overload
For the in vivo scenario and based on studies in rats, Morrow proposed already in 1988 the overload hypothesis.[95, 96] It postulates an impaired particle clearance due to the loss of macrophage mobility resulting in an accumulation of NPs in the lung, as well as related changes in the immunological responses and other biological effects, when associated with continued exposure. The uptake and disposal of NPs by macrophages is the crucial clearance pathway in the alveolar region.[86] As argued by Oberdörster et al.[62] and based on Morrow's findings, also human macrophages are expected to have a limited volumetric capacity for particle uptake beyond which macrophage overloading starts. Once a delivered dose reaches such a threshold, the response options on the side of professional immune cells between in vivo and in vitro are expected to be quite different: In vivo, this could imply the starting point for adaption by increasing the clearance capacity by recruitment of additional phagocytotic immune cells[97] without further adverse consequences. In vitro, compensatory options are not equally existent and adverse outcomes due to particle toxicity, noticeable by increased cellular stress and apoptosis[98] may occur earlier. In silico tools, such as MPPD, readily provide dose prediction data per alveolar macrophage and support overload screening. However, the high phenotypic plasticity of macrophages[99, 100] and the lack of established thresholds for volumetric overloading limit the value of such data.
3.4.2 Acute versus Chronic Exposure
Immune responses of professional and nonprofessional immune cells progress over time, but the decision about the appropriate type of immune response, tolerance versus defense, may come very early.[101, 102] This means that the rate of non-self-recognition in the very beginning of an exposure determines the outcomes more than the accumulated final delivered dose for specific biological endpoints. Hence, an experimental design ending up in the same final delivered dose but with different deposition rates over time, starting with an acute, initial high-dose exposure may result in a completely different immune response compared to a long-time, but low-dose exposure. Thus, a good accordance of the delivered dose and the rate of dose delivery over time goes along with a higher in vivo–in vitro coherence of experimental results mimicking an acute exposure. The immune response to an acute high dose could be transient in nature and without long lasting adverse effects. The immune response in vivo to a low-dose chronic exposure may result in long lasting inflammation associated with fibrosis.[103-105] However it may also induce tolerance against this permanent subclinical low dose or may result in an immunologic adaption process such as enhanced NP clearance via phagocytic immune cells or a hormetic-like biphasic dose response, which is characterized “by a low-dose stimulation and a high-dose inhibition.”[106] It is a general observation that the dose of any agent recognized by immunity does not only influence the extent, but also the type of the response. A familiar example is that allergies can be therapeutically treated by overdoses of the responsible allergen.
Typically, in vitro testing is simulating an acute exposure: One single administration with the full exposure dose and incubation for a time range of hours or days, a period that is still considered as acute exposure. This is due to the inherent limitations associated with routine cell cultures. Refined cell culture models, mostly based on advanced cell culture techniques and cell lines suitable for long-term culturing do exist.[69] However, the in vitro–in vivo coherence of achieved results remains elusive and these more elaborate cell cultures have their own set of limitations and specific root causes for result misinterpretation. Table 4 gives a short overview of the most important options when dealing with chronic to acute in vivo to in vitro mapping.
| Scenario | In vivo exposure | in vitro exposure | In vivo/in vitro coherence | Approach | Shortcomings |
|---|---|---|---|---|---|
| Acute | High dose, short time | High dose, short time | Good | Standard cell culture | Risk of cryptic overload |
| Chronic | Low dose, long time | High dose, short timea) | Very poor | Pretreatment of the cells with low doses | Risk of overload/cryptic overload Risk of unphysiological immune response No simulation of clearance mechanisms |
| Low dose, long timeb) | Poor | Use of primary cells surviving for prolonged timec) | Availability Limited availability of cells per isolation Donor-specific variations |
||
| Specific cell lines for long-term experiments up to six monthsd) | Unsuitable for co-culture Limited survival Necessity for subculturing Risk of (de-) differentiation maybe not appropriate for subchronic doses Difficulty to simulate postexposure time |
- Different approaches for simulation of chronic scenario in vitro:
- a) High dose, short time
- b) Low dose, long time; Different approaches for long-term in vitro experiments
- c) Primary cells or
- d) Cell lines.
3.4.3 Dose Metric
Consciously decision making on an appropriate dose metric for specific biological endpoints in cytotoxicity testing is often omitted. Traditionally, NP mass is used to describe doses for in vivo and in vitro toxicity studies. Alternative dose metrics are the particle number and NP surface area (SA). With porous particles, NP mass is decoupled from NP volume, and the latter could be used as dose metric in specific cases. For professional and nonprofessional immune cells, the majority of immunologically relevant endpoints for toxicity testing depend on 1) receptor mediated signaling associated with non-self or danger recognition, 2) cellular responses to signals with a root cause in NP surface reactivity, 3) instable NPs with release of toxic ions or 4) NPs induced damage of membrane enclosed cellular compartments, such as lysosomal damage subsequent to internalization and accumulation of crystalline NPs.[112] With decreasing particle size the SA to mass ratio increases. Hence, NPs in general are more biologically active when compared to bigger sized particles with same chemistry. Thus, description of dose–response data by NP mass or NP number is inappropriate[62, 113] or even misguiding for the majority of biological endpoints. Not surprising, 20 nm silver NPs compared to 113 nm silver NPs have been found to be ten times more toxic to macrophages when administered at the same NP mass concentration. This was demonstrated for a panel of different biological endpoints, such as markers of inflammation, of metabolic activity, of cellular production of reactive oxygen species and of cell membrane integrity.[114] A very detailed investigation of early gene expression changes in macrophages exposed to amorphous silica across a wide range of particle diameters (7–500 nm) unveiled that most observed changes correlated more with NP SA than with NP mass.[115] In vivo, demonstrated in animals (mouse, rat), the situation is not different.[116] This alleviates concerns about coherence of dose bridging studies from in vivo to in vitro.[90] A retrospective metaanalysis of particle induced pulmonary toxicity with very different types of NM and a wide range of primary particle diameters (9–535 nm) concluded, that the most relevant dose metric is indeed the SA, explaining about 80% of the observed variability in toxicity findings.[116] To sum it up, an unbiased step of prospective, systematic decision making about the most appropriate dose metric should precede toxicology testing. In the absence of specific reasons, dose description by NP SA should be used by default.
4 Functional Immunological Testing
Biological effects that NMs exert on the function of the immune system induce changes in different sets of immune parameters. These are usually determined via measurement of gene expression, upregulation of cell surface molecules, and production of secreted molecules (cytokines, chemokines, etc.) in different types of immune cells to monitor their activation state. This chapter will give an overview on the cellular and molecular entities that play a functional role in typical modes of activation such as acute inflammation and immune modulation for defensive and tolerogenic reactions. Inflammation precedes nearly all other immune responses, so detecting inflammation is particularly important for safety assessment.
4.1 Functional Entities of the Immune System
The immune system is composed of different cell types, which are distributed throughout the body to exert immunity, some of which also migrate to the lymphoid organs in order to initiate adaptive immune responses towards a novel antigen (Figure 4). A large array of soluble factors controls leukocyte migration and action (Tables 5 and 6).

| Term | Name | Functions |
|---|---|---|
| IL-1α | Interleukin 1α | Initiates self-perpetuating inflammatory responses; induces fever and vasoconstriction |
| IL-1β | Interleukin 1β | Initiates self-perpetuating inflammatory responses; induces fever and vasodilation |
| IL-6 | Interleukin 6 | Induces acute phase protein secretion, T and B cell growth and maturation |
| IL-8 | Interleukin 8 | Recruits neutrophils to site of infection |
| IL-12 | Interleukin 12 | Promotes differentiation of naive T cells into Th1; blocks angiogenesis; activates cytotoxic T cells |
| TNF-α | Tumor necrosis factor α | Promotes leukocyte extravasation; induces fever; promotes vasodilation |
| INF-γ | Interferon γ | Activates cytotoxic T cells and macrophages; upregulates MHC II expression in macrophages |
| MCP-1 | Monocyte chemoattractant protein-1 | Involved in monocyte trafficking; recruits monocytes, macrophages, NK cells |
| GM-CSF | Granulocyte macrophage-colony stimulating factor | Stimulates monocytic cells, promotes their survival and differentiation |
| Term | Name | Functions |
|---|---|---|
| IL-10 | Interleukin 10 | Downregulates antigen presentation and induces phagocytic activity of monocytes; promotes Th1→Th2 shift; inhibits TNF-α, IL-6 production in monocytes |
| IL-25 | Interleukin 25 | Promotes production of Th2 cytokines, attracts eosinophils, activates ILC2 |
| IL-33 | Interleukin 33 | Induces helper T cells, mast cells, eosinophils and basophils to produce type 2 cytokines, activates ILC2 |
| TGF-β | Transforming growth factor β | Induces peripheral tolerance, suppresses innate immune cells |
| TSLP | Thymic stromal lymphopoietin | Promotes T and B lymphocytes, induces release of T cell attracting chemokines from monocytes |
| FGF | Fibroblast growth factor | Involved in tissue repair, associated with cell proliferation, and angiogenesis |
| VEGF | Vascular endothelial growth factor | Promotes angiogenesis and vascular remodeling |
| EGF | Epidermal growth factor | Results in cellular proliferation, differentiation, and survival of epithelial cells |
Both cells and molecules can function in a quick but rather nonspecific way (innate immunity) or a slower but highly specific way (adaptive immunity).[117] Nonspecific entities have also to be considered, like mucosal tissue, which acts by physically entangling invaders and is located at most relevant body barriers, such as the airways or the intestinal tract.[118] Epithelium itself reacts to different invaders differently by secreting either molecules that initiate a proinflammatory or an antiinflammatory immune response.[119, 120] Mucosal tissue is patrolled by phagocytic cells, i.e., macrophages, which take up in a nonspecific way all sorts of substances appearing at these epithelial barriers, but also in a specific way upon labelling of non-self-antigens by factors of the complement system or antibodies.[121] Macrophages can also differentiate into different subgroups during the course of an immune response and act as immune modulators.[122-124] Underneath the epithelial barriers, antigen-presenting cells, i.e., DCs, wait for capturing foreign entities.[125] By use of their dendrites they can even sense the outside environment through the epithelial barrier, and such functions have previously been shown using advanced in vitro models with (nano) particles.[126] DCs use various receptors to monitor their local environment for signs of danger to the body. Based on these inputs, DCs may get activated and migrate to lymph nodes where they interact with T cells, forming thus a bridge between innate and adaptive immune responses. By use of antigen-specific signals, additional co-stimulatory cellular signals and release of cytokines, DCs orchestrate further immune responses. Different subsets of T cells can be differentiated in lymph nodes, such as naive, effector, central or effector memory, regulatory, Th1-, Th2-, Th9-, Th17-, or Th22-polarized T cells, which are defined by their surface markers and cytokine profiles they secrete.[127, 128] An important T cell subset are the immunosuppressive regulatory T cells (Treg), which mediate lasting tolerance. Soluble factors released by epithelial and other cells of the innate immune system contribute to immunity as depicted in Figure 4. This impact on immune activation and modulation further includes the invasion of effector cells, such as neutrophils and monocytes.
4.2 The Sequence of Events during Inflammation
The cellular and molecular entities outlined above act in a coordinated way to preserve tissue integrity against pathogens or reestablish it upon injury or penetration of noxious foreign (nano)material through the epithelial barrier. This process ensures homeostasis, yet homeostasis can be severely deregulated by inflammatory conditions. While inflammation is a crucial life-saving defense mechanism, protecting against infection and other environmental challenges, inflammation is established at the cost to homeostasis and thus needs to be tightly controlled. Inflammation as well as resolution of inflammation, which enables inflamed tissues to return to homeostasis is driven by the concerted action of cytokines, chemokines and growth factors in a timely well-controlled manner (Figure 5).
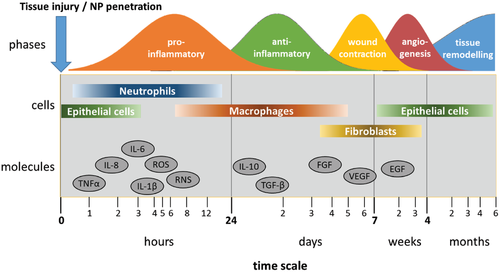
For safety assessment it is critical to distinguish which phase of the process is observed. Agents like TNF-α or IL-8 are quickly induced, but may disappear equally fast, reflecting nothing but a brief fluctuation. A persistent inflammatory program that advances via the regular factors and steps will, on the other hand, raise concern. A statement that proinflammatory factors were observed should include the information which step of inflammation they represent. In vivo, the acute phase of inflammation involves the release of proinflammatory cytokines and chemokines, such as TNF-α, IL-8, IL-6, by epithelial cells aiming to facilitate the penetration of neutrophils and monocytes into the site of injured or NM-penetrated tissue.[129-132] These two cell types are in charge of resolving a potential infection occurring at the affected site and exert their “destructive” role by means of releasing ROS, nitric oxide (NO), hydrolytic enzymes and more proinflammatory cytokines. Likewise, monocytes infiltrated on demand of MCP-1 and GM-CSF secrete proinflammatory cytokines, such as IL-1, themselves and, upon contact with LPS, TNF-α, and IFN-γ, start to differentiate into so-called classically activated macrophages (M1) facilitating increased and enduring ROS production required to effectively eradicating the invader.[133] Moreover, innate lymphoid cells contribute to M1 differentiation.[134] M1 can induce the polarization of T cells into the Th1 phenotype, thus, establishing a long-term inflammatory response including adaptive immunity. During the resolution phase, macrophages downregulate the production of inflammatory mediators. This switch is promoted by the antiinflammatory mediators TGF-β and IL-10, resulting in alternatively activated macrophages (M2), which display upregulated phagocytic capacity aiming at clearing the tissue from cellular debris left from the prior neutrophilic attack.[135] In the remodeling phase, macrophages contribute by maturation of the regenerated tissue through reorganization of the extracellular matrix and the vasculature, which has been initiated by VEGF. This final process can take up to years.[136]
4.3 Induction of Oxidative Stress, Autophagy, and Immunological Consequences
NMs taken up in cells exert effects including lysosome impairment, mitochondria dysfunction, endoplasmic reticulum stress, and inflammatory consequences of oxidative stress, mostly driven by activation of the NF-κB pathway.[3, 69, 137] These processes further include molecular mechanisms based on autophagy-related signaling pathways and mitophagy, i.e., the selective destruction of mitochondria by autophagy.[138-142] The tiered model of immune effects resulting from an oxidative imbalance has been coined as the hallmark of NP-induced toxicity and a number of adverse outcome pathways (AOPs) have been built on the underlying paradigms.[143] At present such quantitative structure–activity relationships (QSARs) have even enabled the establishment of predictive models.[144] The ongoing EU H2020 e-infrastructure project “NanoCommons” (www.nanocommons.eu) collects currently available, develops further NM toxicity prediction tools and facilitates their sustained open access. The future perspective of nanosafety assessment includes application of in silico methods based on established and verified QSAR models with grouping for read-across, a procedure being known as integrated approaches to toxicity assessment (IATA). However, such procedures strongly depend on verified QSARs and as such on functional immunological consequences exceeding transient fluctuations determined by predictive immunological parameters.
4.4 Measures of Functional Immune Responses Induced by NMs
As outlined above, changes in a number of cellular and molecular factors can be observed at several stages and thus during different time points during the course of an immune response. Fluctuations in inflammatory mediators are inherent to the daily contact of humans with foreign substances. It is, therefore, crucial to select relevant markers and time points to monitor whether NMs lead to a functional, defensive immune response. Detecting inflammation is comparatively simple, since useful data can be obtained by cell cultures with single cell types and through following established markers for which commercial tests are available. Studying adaptive immune responses usually involves more complex protocols, like co-cultures of different cells.
An important issue for in vitro hazard assessment is the selection of a realistic cellular model, which is often more demanding than single cell culture. Complex models have been developed; for example several systems for alveolar exposure to NMs have become available during the past years.[69, 145-147] Other advanced cellular models include macrophages and dendritic cells to monitor responses in a realistic interplay of these cells.[148] Including polarized macrophages (M1 vs M2) in such models enables a deeper look into immune modulation.[149] Ultimately, an involvement of T cell activation or polarization by antigen-presenting cells will give the strongest evidence for a functional impact on the human immune system. However, these assays require complex analyses of cyto/chemokine multiplex assays and sophisticated gating strategies applying flow cytometry workflows on peripheral blood mononuclear cells.[128] It may be reasonable to involve experts for these assays if it is necessary to proceed in that direction.
5 Conclusions, Outlook, Prioritization
We have considered here three aspects that are important to assess the immunosafety of NMs: Endotoxin, relevant dose and functional responses. Of these three problems, the first two can be well addressed by study design.
5.1 Endotoxin
It is simply not possible to determine ex post whether reported inflammatory effects were due to the NMs studied, if LPS effects cannot be excluded. This invalidates many older publications, where data on LPS are often missing altogether, which rules out to use them for hazard assessment, at least with respect to cell stress and inflammation. Due to the pleiotropic effects of LPS, other biological responses may have been affected as well. For today's studies the problem can be easily addressed with appropriate study design that involves LPS testing as discussed above.
5.2 Relevant Dose
Using NP doses that reflect actual exposures of human cells and tissues is important for all studies. With respect to immunity, dose may not only determine the extent but also the type of response. The models that have been worked out to estimate delivered doses for cells in culture and for cells in the body are useful tools that should be applied in study design.
5.3 Functional Response
This is the most difficult of the three aspects. The cellular assays required are not trivial and setting them up will be excessive for many more technology-oriented labs. On the other hand, if tests show that a material has in the relevant dose range no inflammatory potential, it is usually not necessary to proceed further in that direction. If there is doubt about whether or not a sustained immune response occurs, the question is already very specific and it should usually be possible to address it in a cooperation.
If all this is met, immunologists should be able to consider a NM either as problematic or as safe. We can then break away from the mantra that more testing is needed for everything and focus on those materials that are truly problematic.
Acknowledgements
The funding from the European Union's Horizon 2020 Research and Innovation Programme, under the Grant Agreements No. 814530 (NANORIGO), No. 671881 (PANDORA), and No. 731032 (NanoCommons) is acknowledged. The work was supported by the Allergy Cancer Bio-Nano Research Centre of the University of Salzburg.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Martin Himly studied chemistry and biotechnology with a focus on medicine at the University of Graz, Austria. Thereafter, he worked in the biomedical industry and was a research fellow at The Scripps Research Institute, La Jolla, CA, USA. He is presently Assoc. Prof. at the Dept. Biosciences, Paris Lodron University of Salzburg (PLUS) with Veniae docendi in biochemistry and immunology. He has a track record of research in allergy, immunology, and bio–nano interactions with a vivid interest in nanomedical applications. He has been developing education and training in nanotechnology and nanomedicine at different levels including collaborative research projects with schools.

Mark Geppert studied chemistry at the University of Bremen (Germany) and received his Ph.D. in 2012 in neurobiochemistry investigating nanoparticles on glia cells. He then worked for three years as a postdoc at Eawag, the Swiss Federal Institute of Aquatic Science & Technology as part of the EU Flagship project NanoValid. In 2015, he joined the University of Salzburg as a Senior Scientist. His key expertise is the characterization of nanomaterials and the investigation of their biological effects using in vitro cell culture models. He has deep experience with numerous analytical, physicochemical, and biological methods to address the aforementioned points.

Albert Duschl received his Ph.D. at the University of Giessen, Germany. He was a postdoc at the University of California in Irvine and at the Max-Planck Institute for Biochemistry at Munich. After 10 years as an assistant professor at the University of Würzburg, he became chair of Biochemistry at the University of Salzburg, Austria, in 2001, where he is now speaker of the University's excellence cluster Allergy-Cancer-BioNano. His research focuses on regulatory processes in the human immune system, methods for the assessment of nanosafety, and understanding bio–nano interactions.




