Integrating grafting and companion planting to improve crop performance in intensive high-tunnel tomato production
Abstract
Introduction
Tomatoes are among the most popular horticultural crops cultivated in high tunnels. Due to space limitations in these enclosed environments, some tomatoes are produced in succession (i.e., without rotation) across years, which could lead to yield reductions over time. However, the impact of tomato monocultures on productivity in high tunnels and strategies to buffer against yield declines need further testing.
Materials and Methods
We used a 4-year field experiment whereby heirloom tomatoes (cv. Cherokee Purple and Black Krim) were grown in the same high tunnel soil over time. We tested the impact of both tomato grafting and companion planting on aboveground vegetative biomass and fruit yield. For the grafting treatment, we used the commercially available Maxifort and wild tomato, Solanum pimpinellifolium, as rootstocks. For companion planting, we seeded open alleys with clover and spatially shifted crop beds each year such that tomatoes were transplanted into the previous year's clover planting.
Results
Our data indicate that the companion (clover) treatment had little to no impact on tomato production, while grafting to Maxifort rootstock had a significant positive effect on biomass and yield. However, beneficial effects of grafting were only observed over time, in years three (+71% inc. in biomass) and four (+77% inc. in biomass, +38% inc. in yield) of the experiment; not during the initial 2 years. Leaf (SPAD) and fruit (Brix) metrics were unaffected by any of the treatments.
Conclusion
Overall, our results suggest that grafting tomatoes with commercially available rootstock is an effective tool for maintaining production in high tunnel monocultures over time.
1 INTRODUCTION
As the demand for locally produced food increases, growers are challenged to adopt new strategies to keep up with the demands for year-round produce. Unfortunately, cold weather constrains crop production for much of the year in temperate climates, preventing many growers from meeting demand and thereby limiting farm profitability (Conner et al., 2009). A leading solution to the dilemma of seasonality is to employ high tunnels, a form of protected agriculture that aids in season extension by creating a more favourable microclimate using trapped solar heat. High tunnels allow for vegetable production outside of the traditional summer months into periods when outside temperatures are suboptimal (i.e., extending into the ‘shoulder seasons’ of spring and fall); for instance, by transplanting vulnerable seedlings before the frost-free date in spring. Because they allow farmers to generate a crop earlier in the spring and continue production later into the fall compared with open-field cultivation, their popularity among fruit and vegetable growers is on the rise worldwide (Lamont, 2009), particularly in the United States where adoption has been more recent (Carey et al., 2009). The season extension is especially beneficial in the US Midwest region, which falls into the agriculture hardiness zones 4–6 (USDA Hardiness zone map, 2012) where only 6 months of frost-free weather constrains year-round crop production. As a result, Midwestern producers have been quick to adopt high tunnels. For example, >170 new high tunnels were constructed across Indiana as part of a USDA high tunnel initiative (Bruce et al., 2017) and >1000 high tunnels have been added across other Midwestern states (Foust-Meyer & O'Rourke, 2015).
Several unique aspects of high tunnel production affect their management compared with traditional field agriculture. First, most farms own only one or a few high tunnels and, consequently, growing space is often a limiting factor. This constraint causes growers to prioritize high-value specialty crops, leading to a diversity bottleneck whereby a smaller subset of crops is cultivated in this space. Second, by extending the growing season, high tunnels are often filled with crops for the 9 months from early spring to late fall, and in some cases even through the winter. Therefore, cover crops or other forms of cropping system diversity are difficult or impossible to implement. Third, since most high tunnels are stationary, the soil within these areas is repeatedly used. Best practices for field-grown crops are to incorporate a rotation or fallow period, but this may be impractical in a high tunnel, leading to concerns about soil health (Knewtson, Janke, et al., 2010). Altogether, high tunnel production systems inherently select for a low diversity of crops, grown intensively throughout the year, and in the same soil.
Sequential planting of the same crop increases the likelihood of accumulation of soil-borne pathogens and insect pests, eventually leading to a decline in plant yield and performance through a process called negative plant–soil feedback (PSF). In general, PSF describes the indirect effect between two plants mediated by changes in soil properties (Van Der Putten et al., 2013). These changes can be more or less conducive to plant growth and performance. PSFs are usually negative, meaning plants grow or produce less and are less vigorous when planted in soil that was previously planted with the same species. In contrast, plants tend to perform better in soil that is sterilized or that was cultivated with a different, nonself species (Dias et al., 2014; Kulmatiski et al., 2008).
Historically, crop rotation has been the primary method for combating negative PSF (Huang et al., 2013). Because rotations are often impractical in high tunnels, alternative approaches are needed (DiGiacomo et al., 2023). For example, companion crops such as low-growing legumes (e.g., clover, vetch) could be integrated into open space between rows without competing with the focal crop as a means to break the cycle of monoculture. In addition to enhancing plant diversity, nitrogen-fixing leguminous companion crops improve the soil nutrient profile (Rudisill et al., 2015; Schipanski et al., 2014; Sharma et al., 2018). In alternating seasons, crop rows could then be spatially offset so that new transplants are always rotating into the previous year's companion planting. This ‘micro-rotation’ would allow high tunnel growers to reap the benefits of rotation without sacrificing crop production; however, this approach has not been scientifically validated or tested as a mechanism to maintain yields.
Grafting is another tool that could mitigate the effects of soil-borne pathogens and reduce negative PSF in high tunnel systems. While vegetable grafting is still growing in popularity in the United States, in other parts of the world, it is widely used (Kubota et al., 2008). Grafting is the physical combination of a desired plant cultivar aboveground (scion) with the root system of another plant (rootstock), selected for traits such as resistance or tolerance to stressors (Louws et al., 2010; Thies, 2021). With the phasing out of bio-fumigants such as methyl bromide, there has been a push to develop rootstocks conveying resistance to diseases. Although studies are beginning to emerge that test grafted vegetable performance in high tunnel environments (e.g., Djidonou et al., 2020; Gong et al., 2022; Jenkins et al., 2022; Lang and Nair, 2019; Lang et al., 2020; Masterson et al., 2016; Soltan et al., 2017), these tend to be shorter-term (mostly 2-year) experiments that may underestimate the benefits of grafting if negative PSFs accumulate over time, as expected.
Tomato is the most popular high-tunnel crop (Carey et al., 2009). Although tomatoes generally respond well to growing in high tunnel environments (Kandel et al., 2020; O'Connell et al., 2012), half of surveyed growers do not incorporate a rotation (Knewtson, Carey, et al., 2010). This outcome matches anecdotal observations of local farms where tomatoes were grown in consecutive years on the same high tunnel soil. By comparison, field-grown tomatoes are rarely replanted into the same field for at least 3 years between consecutive plantings to avoid build-up of pathogens. Studies experimentally growing tomato in succession generally show negative effects on vegetative growth (Carrillo et al., 2019) and yield (Kaplan et al., 2020). This effect could be especially strong in high tunnels where heirloom cultivars that lack disease resistance are commonly grown, making them particularly vulnerable (Rivard & Louws, 2008).
In this study, we combine the use of companion crops along with grafting, using a factorial design, inside intensive high tunnel tomato production in a 4-year field experiment. We empirically test the hypothesis that more diverse rotations and grafted plants produce higher tomato yield compared to the traditional ungrafted monoculture system.
2 MATERIALS AND METHODS
Research was conducted in six high tunnels over 4 years (2018–2021) at the Meigs Horticultural Farm, a part of the Throckmorton Purdue Agricultural Centre (Lafayette, Indiana). These six tunnels were constructed directly adjacent to one another along a north-south gradient with 5 m space between neighbouring tunnels. Before the start of the experiment (2017 and years prior), the whole field was planted in a corn–soybean rotation, but since then has been used to grow tomatoes. We used gothic-style high tunnels (15 × 9 m LW) covered with a double-layer of 6 mm polyethylene SunClim film with infrared diffusion properties (Nifty Hoops, Ann Arbor, Michigan). All tunnels were equipped with motorized roll-up sidewalls and louvres on endwalls for cooling. Automated ventilation was determined by a thermostat set to 27°C.
The focal crop in this experiment was tomato, specifically two heirloom varieties: Cherokee Purple (CP) and Black Krim (BK) (Johnny Selected Seeds, Winslow, Maine). These cultivars were selected based on horticultural characteristics and popularity among growers and consumers alike.
2.1 Experimental design
The companion plant treatment was employed as the main plot factor applied at the tunnel level (n = 6 total high tunnels, three each for with vs. without companion plants). The six tunnels were divided into three blocks across the north–south gradient. Within each of the blocks, one tunnel was randomly designated for tomato alone and the other for tomato cultivated with a companion planting. The control tunnels were planted with only tomato across the 4-year experiment to replicate a standard high-intensity production system. Tunnels assigned to companion planting were cultivated with tomato combined with white clover (Trifolium repens). Aside from the addition of clover, all other aspects of production were identical (e.g., irrigation, pruning, fertilization). For the initial 2 years of the experiment, kale was also implemented as an overwintering crop in the clover-added tunnels due to its cold tolerance and use by growers in winter production. However, due to poor and inconsistent establishment this practice was abandoned.
The grafting treatment was employed as a subplot factor within all high tunnels. All tunnels contained both heirloom tomato cultivars (CP, BK) as scions, which were grafted onto two alternative rootstocks—the commercially available Maxifort (MF) and Solanum pimpinellifolium (SP), a wild relative of tomato. MF was selected due to its high vigour and resistance to multiple soil-borne pathogens (Grieneisen et al., 2018; Rivard & Louws, 2008), as described by the seed vendor (Johnny Selected Seeds, Winslow, Maine). The wild tomato S. pimpinellifolium was selected because wild relatives of tomato are thought to have greater resistance and/or tolerance to pests and pathogens (Ferrero et al., 2020; Zhang et al., 2017). Also, wild tomatoes showed weaker negative PSFs than domesticated tomatoes (Carrillo et al., 2019), suggesting that wild species like S. pimpinellifolium have roots that are less prone to yield reductions when grown consecutively in the same soil. In addition to MF and SP rootstocks, ungrafted and self-grafted treatments were included to control for the effect of grafting alone, independent of rootstock. These combinations resulted in eight plant types: two scions × four graft types (BK, BK/BK, BK/MF, BK/SP and CP, CP/CP, CP/MF, CP/SP). Within each tunnel, three of the six rows were cultivated with BK (rows 1, 3, 5), while the other three were cultivated with CP (rows 2, 4, 6). Within each row, all possible grafting combinations were present in a randomized complete block design. Each row consisted of four replicated blocks with each block having one plant of the four grafting treatments (i.e., 16 plants per row). At the tunnel level, this resulted in 12 plant replicates of each of the eight grafting treatments, and 72 plant replicates per treatment combination across all six tunnels included in the experiment (for a visual depiction of the layout of these treatments, see Supporting Information: Figure S1).
2.2 Transplant production
Tomato seeds were sown in 72 cell trays filled with soilless potting mix (Metro mix; Sun Gro Horticulture) and propagated in a greenhouse on the Purdue University campus under supplemental high-pressure sodium lamps (1000 W; 06:00-22:00). Tomato seedlings designated for grafting were grown for 3–4 weeks or until the diameter of the stem was ~3 mm. Grafting was performed using the cleft technique where a ‘V’ shape was cut into the bottom end of the scion and inserted into a vertical cut made into the rootstock. A grafting clip was placed over the graft union and the grafted tomatoes were placed into plant trays covered with humidity domes. Grafted seedlings were kept in darkness at ~100% relative humidity for 1 week for the graft union to heal and show signs of callous formation. Thereafter, grafted plants were moved into low light conditions for another week before moving to standard light levels for plant growth.
2.3 High tunnel preparation and management
For initial preparation of the high tunnels in 2018, all weeds were mowed, and the ground was prepared using a rotary tiller. For subsequent years, the rotary tiller was only used for rows that were designated for tomato plants. Six beds were prepared in each tunnel with a hand-operated bed maker using black plastic mulch with 1.1 m spacing between adjacent rows and 0.75 m within row spacing between consecutive plants. From 2018 to 2021, tomatoes were transplanted on 28 May, 27 May, 1 June and 1 June, respectively. Each year, transplants that did not survive were replaced within 2 weeks, after which there were no further replacements throughout the growing season. In each tunnel, we installed an automated irrigation system (Rainbird, Model 1ZEHTMR) that connected to drip irrigation (12 mm) lines, which extended along the full length of each of the six beds beneath black plastic mulch used to control weeds.
In the companion plant treatment, the open soil between rows was sown with white clover at a rate of 10 lbs./acre. Clover was broadcast seeded during the first week of May and reseeded later in the growing season as needed to ensure establishment. However, in tunnels dedicated to the monoculture system, the interrow space was covered with black ground cloth to smother emerging weeds and ensure that tomato was the only plant growing in the soil. We also hand-removed weeds as needed throughout all tunnels in both treatments to maintain a consistent plant presence. Herbicides and other pesticides (e.g., insecticides, fungicides) were never applied. In control tunnels, tomatoes were always planted in the same location across years, whereas in the companion tunnels, tomato rows were shifted into the space cultivated with clover in the prior year.
For fertility, Jack's professional 20-20-20 fertilizer was applied via fertigation system at three times over the course of the growing season based on agronomic recommendations: one application at transplant, another application during the vegetative growth phase and one final application at fruit set to ensure the tomatoes were not deficient of macronutrients. Weekly throughout the growing season, all tomato plants were pruned and trellised continuously as they grew. For pruning, suckers and stems that were too close to the ground and/or diseased leaves were removed and discarded from the high tunnel to avoid contamination.
2.4 Crop performance
Once the fruits reached maturity (i.e., pink and ripe stage, ~75 days) they were picked and weighed from individual plants weekly. At the end of the growing season, total yield was calculated per plant. Yield data from the initial year of the experiment (2018) were collected based on a per tunnel average for each graft treatment so that we have sums for all plants within a grafting treatment. For this reason, we only present yield data from 2019 to 2021. Above-ground plant vegetative biomass was measured at the end of the growing season after the final harvest. To measure biomass, each tomato plant was detached from the root system at the soil surface, then individual plant fresh weights were recorded.
We used a Minolta SPAD 502 Plus Chlorophyll Metre to measure the chlorophyll content of the leaves. Three mature leaves were selected per plant, and leaf position was standardized across all treatments. Three measurements were taken per leaf for a total of nine measurements per plant and averaged to give one SPAD reading per plant. SPAD measurements were taken on three separate occasions (28 July, 27 August and 25 September) from all experimental plants during the final (2021) growing season.
To determine potential effects of treatments of fruit quality, tomatoes were collected from the different graft treatments and analyzed using a Brix refractometer (Atago, Japan). From each high tunnel, five representative tomatoes of each graft treatment were collected across all rows for a total of 180 tomatoes sampled and measured in 2021. Using a mortar and pestle, individual fruits were ground to a pulp and approximately 3 mL of liquid was transferred into the refractometer and Brix measurement was recorded. The instrument was cleaned thoroughly before the next measurement was taken.
2.5 Statistical analysis
All statistical analyses were performed using JMP Pro 16 using linear mixed models. Fixed effects included year, companion plant, tomato cultivar and grafting type with all possible two-way interactions tested. Tunnel and row nested within tunnel were included as random effects. Biomass data were square-root transformed to improve normality, whereas raw data were used for all other response variables (yield, SPAD, Brix). Year was not included as a factor for SPAD or Brix since these were only measured in 2021. For SPAD data, measurements over time were averaged across dates to create a single measurement per plant, which was used for analysis. Post hoc pairwise comparisons were performed using Tukey's HSD.
3 RESULTS
3.1 Biomass
There was no overall statistical difference in tomato biomass between management systems (monoculture = 1.44 ± 1.22, companion = 1.09 ± 0.84; kg/plant, mean ± SE); however, there was a significant interaction effect of Year*Companion (Table 1). Plant biomass increased across both tunnel treatments from 2018 to 2021, but the rate of increase was proportionally greater in monoculture (219%) than with clover added (163%). The only year where there was a significant difference between tunnel treatments was in 2020 when monoculture plants had 38% higher biomass than their counterparts with clover (Figure 1). There was no statistical interaction between companion planting and the other two variables in the model (Cultivar & Grafting).
| Source | DFNum | DFDen | F | p Value |
|---|---|---|---|---|
| Year | 3 | 1386.8 | 92.53 | <0.0001* |
| Grafting | 3 | 1373.9 | 9.41 | <0.0001* |
| Cultivar | 1 | 114.4 | 2.86 | 0.0934 |
| Companion | 1 | 4.6 | 1.21 | 0.3258 |
| Year*Companion | 3 | 1386.3 | 6.93 | 0.0001* |
| Year*Cultivar | 3 | 1392.1 | 1.96 | 0.1181 |
| Companion*Cultivar | 1 | 25.6 | 2.33 | 0.1388 |
| Year*Grafting | 9 | 1378.3 | 13.43 | <0.0001* |
| Companion*Grafting | 3 | 1378.1 | 2.59 | 0.0517 |
| Cultivar*Grafting | 3 | 1386.6 | 4.35 | 0.0046* |
- Note: Significant effects are designated by an asterisk and bold text based on a level of p < 0.05. The variable ‘companion’ refers to clover addition as a companion plant for tomato. DFNum and DFDen show the numerator and denominator degrees of freedom, respectively.
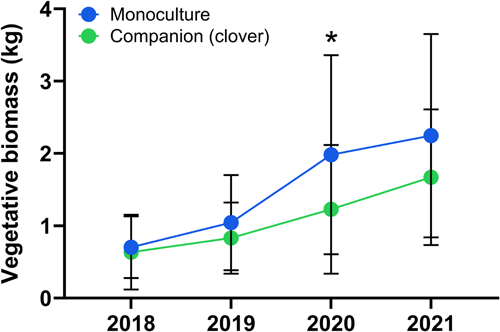
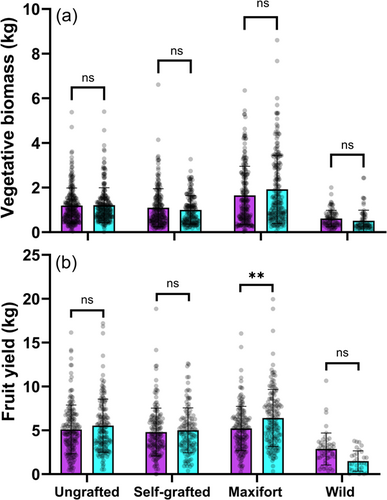
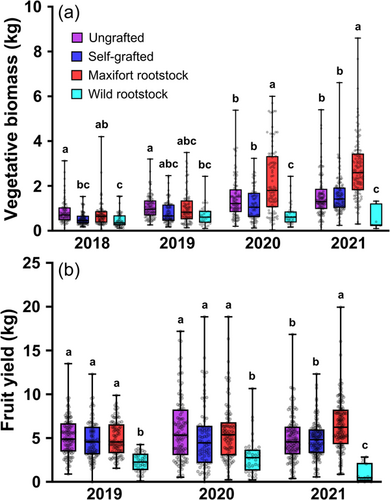
Similarly, there was no overall statistical difference in tomato biomass between cultivars (BK = 1.29 ± 1.1, CP = 1.21 ± 0.98; kg/plant, mean ± SE); however, there was a significant interaction effect of Cultivar*Grafting (Table 1). This interaction was seemingly caused by cultivar-based differences in compatibility with the wild rootstock, where the median of biomass accumulation was 78% higher in CP than BK (Figure 2a). The grafting treatment also significantly interacted with year (Table 1). The Graft*Year interaction was primarily driven by changes in the performance of MF-grafted plants over time, which were not different from the ungrafted or self-grafted plants in years one and two but were significantly higher in years three (+71%) and four (+77%) (Figure 3a). The relative performance of the other three treatments remained comparatively stable over time with the ungrafted and self-grafted controls showing similar biomass accumulation and wild-grafted tomatoes growing nearly half as large (49% lower biomass).
3.2 Yield
Grafting had a significant main effect on fruit yield, but graft type also significantly interacted with year (Table 2). Similar to biomass, this interaction effect was primarily caused by yield increases in MF-grafted plants over time compared to the other three treatments; namely, a 38% higher yield than the ungrafted and self-grafted controls in the final year of the experiment (Figure 3b). Over all years combined, tomatoes grafted to wild rootstock had 81% lower yields.
| Source | DFNum | DFDen | F | p Value |
|---|---|---|---|---|
| Year | 2 | 984.6 | 3.78 | 0.0231* |
| Grafting | 3 | 974.8 | 27.45 | <0.0001* |
| Cultivar | 1 | 43.3 | 0.06 | 0.8033 |
| Companion | 1 | 56.4 | 0.85 | 0.3591 |
| Year*Companion | 2 | 984.6 | 0.43 | 0.6504 |
| Year*Cultivar | 2 | 989.7 | 0.09 | 0.9052 |
| Companion*Cultivar | 1 | 30.2 | 1.76 | 0.1943 |
| Year*Grafting | 6 | 971.8 | 2.38 | 0.0270* |
| Companion*Grafting | 3 | 974.4 | 0.46 | 0.7033 |
| Cultivar*Grafting | 3 | 973.5 | 3.23 | 0.0216* |
- Note: Significant effects are designated by asterisk and bold text based on a level of p < 0.05. The variable ‘companion’ refers to clover addition as a companion plant for tomato. DFNum and DFDen show the numerator and denominator degrees of freedom, respectively.
There was also a significant interaction effect of Cultivar*Grafting (Table 2). MF rootstock performed better when grafted to BK compared to CP scions (Figure 2b). While a nonsignificant effect, there was a trend for higher yields when CP was grafted to wild rootstock compared to BK; a pattern that was also apparent in the biomass data.
Companion planting had no effect on tomato yields, either alone or interacting with other variables in the model. While time was a significant factor, tomato yields were far less variable from year to year compared with biomass.
3.3 Crop quality
None of the experimental factors significantly affected SPAD values, which mostly ranged from 46 to 48 (see means according to treatment in Table 3). Brix values were affected by the interaction between Companion*Cultivar (F = 7.56, p = 0.0066) and Cultivar*Grafting (F = 3.25, p = 0.0410). However, the biological magnitude of treatment differences was minimal, with the majority of fruits scoring between 4.8 and 5.3 (see Table 3 for values).
| Treatment | SPAD | Brix (%) |
|---|---|---|
| Cultivar: Black Krim | ||
| Monoculture | ||
| Ungrafted | 48.04 ± 0.62 (28) | 4.95 ± 0.05 (15) |
| Self-grafted | 46.96 ± 0.47 (21) | 5.04 ± 0.04 (15) |
| Maxifort rootstock | 47.12 ± 0.50 (34) | 5.05 ± 0.10 (15) |
| Companion | ||
| Ungrafted | 48.09 ± 0.45 (34) | 4.86 ± 0.05 (15) |
| Self-grafted | 47.45 ± 0.51 (28) | 5.04 ± 0.08 (15) |
| Maxifort rootstock | 46.94 ± 0.60 (34) | 4.94 ± 0.08 (15) |
| Cultivar: Cherokee Purple | ||
| Monoculture | ||
| Ungrafted | 47.40 ± 0.59 (29) | 5.36 ± 0.04 (15) |
| Self-grafted | 48.08 ± 0.46 (34) | 5.11 ± 0.05 (15) |
| Maxifort rootstock | 46.91 ± 0.45 (31) | 5.23 ± 0.04 (15) |
| Companion | ||
| Ungrafted | 46.94 ± 0.60 (33) | 4.96 ± 0.08 (15) |
| Self-grafted | 47.79 ± 0.55 (35) | 4.99 ± 0.10 (15) |
| Maxifort rootstock | 46.79 ± 0.50 (30) | 4.84 ± 0.11 (15) |
- Note: Both variables were measured in 2021. SPAD values are the average of three separate measurements taken on the same plant across the growing season. Data from the wild rootstock treatment are excluded due to low sample size.
4 DISCUSSION
Sustainable agricultural practices, including enhancing biodiversity through crop rotations and cover crops, are gaining popularity as alternatives to monoculture. These practices improve crop productivity, soil health, pest management and economic sustainability. However, these approaches are more challenging to implement in high tunnels compared with open-field horticultural crop production. Encouraging sustainable practices, including the use of grafted vegetables, among high tunnel growers is crucial for long-term success.
4.1 Changes to tomato productivity in high tunnel soil over time
We tracked tomato biomass and fruit yield as proxies for plant productivity, expecting that both variables would decrease over time in the monoculture system, while maintaining production in the companion plant treatment. Our data did not support either of these predictions. Neither vegetative aboveground biomass nor yield declined over 4 years of growing in the same high tunnel soil. In fact, biomass increased over time in both treatments, even though this pattern was not reflected in fruit yield. The differential response in vegetative biomass and fruit production is not surprising given that different factors affect these two variables. For example, heat accumulation may constrain tomato fruit production due to limitations in pollination or pollen viability (Pressman et al., 2002; Sato et al., 2000). Prolonged daytime temperatures >32°C negatively affect fruit set; a threshold that is routinely exceeded during summer production months in our region, even in well-ventilated tunnels (Ingwell & Kaplan, 2019).
The lack of observed yield penalties in serial tomato production could have been caused by a few factors. Our field trial was conducted on land that had never been used to cultivate tomato. Although we did not systematically sample soil or roots for pathogen presence, plants exhibiting foliar symptoms of disease were sent for diagnosis. This process only revealed a single case of confirmed soil-borne disease out of the >1000 tomato plants. This outcome suggests that 4 years was insufficient to develop disease pressure in a tomato-naïve soil. Notably, Fu et al. (2017) found that continuous tomato monoculture on naïve soil improved soil quality and plant yields in the short term while only observing negative effects after >10 crop cycles. Thus, the timeframe of our experiment, while twice the duration of many published studies, still might be insufficient for observing negative effects. Future studies would benefit from implementing on-farm trials using commercial high tunnels that have soil with a much longer history (i.e., a decade or more) of tomato cultivation.
Because the monoculture system did not develop disease pressure and/or reduce yields, it is not surprising that the clover treatment had little to no effect. Field trials of cover crops on tomatoes, in general, show variable effects on crop performance with positive, negative or neutral effects changing across years and sites (Chahal & Van Eerd, 2021; DiGiacomo et al., 2023; Summers et al., 2014). Other authors have demonstrated that positive effects of companion plants depend on the presence of soil pathogens such as Pythium or plant–parasitic nematodes (Hannula et al., 2020; Ma et al., 2018). Even in the absence of disease pressure, leguminous covers like clover could have beneficial effects on soil fertility, but this mechanism is more likely to operate in low-input or organic cropping systems (Abdul-Baki et al., 1996; Lenzi et al., 2009). Since we applied synthetic fertilizer, these applications could have masked any potential nutritional benefits of clover-derived nitrogen.
4.2 Impact of grafting on tomato productivity
Our study clearly shows that grafting affects biomass and yield of tomato plants, though, selecting an adequate grafting combination is key to its success. Our data suggest that grafting scions of both cultivars of heirloom tomatoes on the commercially available MF rootstock is beneficial. MF-grafted tomatoes showed increased biomass and yield compared to ungrafted and self-grafted plants. Interestingly, this effect was only apparent in years 3 and 4 of the experiment, demonstrating that grafting benefits may accrue over time rather than appearing immediately. Given that disease pressure did not appear to increase over time, the mechanism responsible for this pattern remains unclear. MF-induced benefits to tomato can be highly variable for unknown reasons (Buller et al., 2013; Rivard & Louws, 2008) and thus 2020 and 2021 may have simply presented environmental conditions conducive to positive effects, unrelated to time since establishment. Earlier work by Masterson et al. (2016) on MF-grafted tomatoes in high tunnels showed fairly dramatic (+53%) and immediate yield increases. However, a meta-analysis by Grieneisen et al. (2018) on tomato grafting emphasizes the variable outcomes, which are more typical in these studies. For example, grafted tomatoes produced significantly higher yields in only about one-third of trials. Despite this variation, tomato grafting, overall, caused a 37% yield increase for all studies combined; an effect size that is nearly identical to what we observed (+38%) in the final year of the study. Recent studies also emphasize that grafted plants can strengthen beneficial microbial associations in the rhizosphere (Ge et al., 2022; Ruan et al., 2020), including in MF-grafted tomato (Poudel et al., 2019). This opens the possibility that grafting benefited tomato growth and yield in our experiment via the microbiome.
In contrast to MF, our wild grafted tomatoes performed poorly in terms of establishment and production, resulting in >50% reductions in growth and yield. Tomato has long been known to show compatibility with interspecific grafts, including closely related Solanum sp. and other genera in the Solanaceae family (Dawson, 1942). Moreover, recent research demonstrates successful implementation of tomato grafting with its closest wild ancestor, S. pimpinellifolium (Mauro et al., 2020; Naik et al., 2021; Zhou et al., 2022). These experiments, however, were performed using hybrid varieties as scions. Heirloom tomatoes may be less compatible with wild rootstock due to morphological or developmental differences.
5 CONCLUSIONS
As a whole, our data indicate that tomatoes are resilient to negative feedbacks in naïve high tunnel soils. This could mean that growers producing in soils without a long history of tomato cultivation or disease incidence can engage in a high-intensity monoculture in the short to medium term. Over longer periods, however, this approach is likely to fail without more targeted soil management. Our data further indicate that MF rootstock provides a boost to tomato growth and yield, but it remains unclear whether these sporadic increases offset the additional costs (e.g., seeds or seedlings, labour, equipment) associated with the production of grafted plants (Rysin & Louws, 2015; Rysin et al., 2015).
Finally, despite the lack of effect documented for companion planting in our study, this tactic has been understudied relative to grafting for high tunnel management, even though both approaches can be used to generate similar effects with potential synergies among the two. As with grafting, profitability associated with diversifying rotations or integrating nontomato plants in the limited space occupied by high tunnels is a challenge that needs to be overcome before grower adoption occurs (DiGiacomo et al., 2023). It would be helpful to test these approaches using multiple diversification strategies on soils with varying histories of disease and cultivation intensity.
AUTHOR CONTRIBUTIONS
Wadih Ghanem collected the data. Both authors (Wadih Ghanem and Ian Kaplan) contributed to formulating the research questions, designing the study, analyzing the data, interpreting the findings and writing the article.
ACKNOWLEDGEMENTS
We would like to thank the following individuals who offered advice on the design of this experiment and/or assisted in data collection—Gabriella Altmire, Jennifer Apland, Laramy Enders, Robert Grosdidier, Lori Hoagland, M. Ross Hunter, Laura Ingwell, Maisha Lucas, Tristand Tucker and Tyler Vandermark. This work was supported by the U.S. Department of Agriculture, National Institute of Food & Agriculture (USDA NIFA), award # 2017-67013-26255. The USDA had no role in the design, analysis or writing of this article.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that they followed the ethical policies of the journal.
Open Research
DATA AVAILABILITY STATEMENT
All data from this work are publicly available on the Purdue University Research Repository (purr.purdue.edu).




