Desmopressin for bleeding in non-severe hemophilia A: Suboptimal use in a real-world setting
All members of the DYNAMO Study Group are listed in Appendix S1.
Handling Editor: Dr Neil Zakai
Abstract
Background
Desmopressin is an important treatment option in nonsevere hemophilia A because it has several benefits compared with factor (F) concentrates, including no inhibitor risk and much lower costs. Despite these advantages, data are limited on the real-world use of desmopressin in the treatment of bleeds.
Objective
To describe the clinical use of desmopressin in relation to other therapeutic modalities in the treatment of bleeding episodes in patients with nonsevere hemophilia A.
Methods
Patients with nonsevere hemophilia A aged 12–55 years were included from the DYNAMO cohort study. Data on the desmopressin test response and treated bleeding events in the period January 2009 to July 2020 were retrospectively collected from medical files. An adequate desmopressin test response was defined based on a peak FVIII level of ≥30 IU/dl.
Results
A total of 248 patients with a median age of 38 years (interquartile range 25–49) were included. An adequate desmopressin test response was documented in 25% and 73% of patients with moderate and mild hemophilia, respectively. In adequate responders, 51% of bleeds were exclusively treated with FVIII concentrates, 24% exclusively with desmopressin, 21% with a combination of both and 4% with other treatments. In 54% of bleeds treated with a single dose of factor concentrates, the expected FVIII level after desmopressin exceeded the level targeted.
Conclusion
Most bleeds in patients with an adequate response to desmopressin are treated with factor concentrates. These findings may indicate a suboptimal use of desmopressin and that barriers to the use of desmopressin should be explored.
Essentials
- Desmopressin (DDAVP) is an important therapeutic agent in non-severe hemophilia A.
- The real-world use of DDAVP to treat bleeds was assessed in an international cohort.
- DDAVP was only used exclusively in 24% of bleeds in patients with an adequate DDAVP response.
- In 54% of 1-dose treated bleeds, the DDAVP response exceeded the level targeted with concentrate.
1 INTRODUCTION
Hemophilia A is an X-linked inherited coagulation disorder that is caused by a deficiency in clotting factor VIII (FVIII). The residual amount of FVIII corresponds with the bleeding phenotype, in which patients with moderate (FVIII 1–5 IU/dl) or mild hemophilia A (FVIII >5–40 IU/dl) mainly suffer from bleeds elicited by trauma or surgery. Important therapeutic options for patients with nonsevere hemophilia A include clotting factor concentrates and desmopressin (1-deamino-8-D-arginin vasopressin [DDAVP]). Desmopressin is a synthetic analogue of vasopressin that induces the release of von Willebrand factor (VWF) from endothelial Weibel-Palade bodies and leads to a simultaneous 3- to 5-fold increase in circulating FVIII.1-3 Although the underlying mechanism that drives the release of endogenous FVIII is still not fully understood, desmopressin has been used in the treatment of bleeding disorders for more than 40 years.2, 4
The reason behind the long-standing clinical use is not surprising, as desmopressin has several benefits when compared with clotting factor concentrates. These advantages include the lack of inhibitor risk, lower costs, no potential transmission of bloodborne infections and the possibility of subcutaneous or nasal administration and home treatment. Desmopressin is considered safe and is overall well tolerated, with mild transient side effects including flushing, tachycardia, and headache.5, 6 According to the World Federation of Hemophilia guideline, it can be used up to 3 consecutive days as after repeated exposure tachyphylaxis occurs because of depletion of stored FVIII, reducing its therapeutic effects.7, 8 When used twice in a single day, it is recommended to limit the subsequent doses to once per day.7 Therefore, desmopressin may be especially useful to treat minor bleeds that require a relatively short treatment period. Obviously, desmopressin treatment should only be initiated when no contraindications exist, such as age <2 years, an increased risk to develop hyponatremia, and patients at high risk of cardiovascular disease or thrombosis.7, 9
Because of a large inter-individual variation in the response to desmopressin, it is not an effective treatment option for all patients. Determinants that are reported to influence the biologic responsiveness include age, baseline FVIII level, VWF level, mode of administration, and the F8 mutation type.3, 10, 11 In mild hemophilia A, most patients may benefit from desmopressin because of previous studies reported an adequate response with a peak FVIII ≥30 IU/dl in 66%–76% of patients.3, 12, 13 It is therefore recommended as the treatment of choice for this patient group with an adequate response.14 Although patients with moderate hemophilia A are less likely to respond to desmopressin, around 21%–40% has been reported to achieve peak levels ≥30 IU/dl.15 As a consequence, a desmopressin test to assess the individual response and rise in FVIII is recommended before its therapeutic use. If desmopressin administration results in sufficient FVIII levels, it is an important modality to prevent and treat bleeds in this population.
Despite the advantages, little research has been done on the actual use of desmopressin to treat bleeds in patients with nonsevere hemophilia A. One previous study evaluated treatment strategies among a large cohort of 377 patients with nonsevere hemophilia A receiving treatment at one of the London hemophilia facilities.16 This work demonstrated that most patients received factor concentrates for hemostatic management, although no information was provided on desmopressin responsiveness and type of bleeds. A single-center Italian cohort study reported on the clinical efficacy of desmopressin in 27 patients with an adequate response.12 Importantly, desmopressin was effective in 92% of treated bleeds. Because of the study mainly focused on desmopressin treatment, details on type of bleeds treated with other modalities were limited.
These studies do not provide detailed information that may put these findings in further context, such as data on desmopressin response or other treatment agents used. Real-world data from a large international multicenter cohort may provide more comprehensive insight into the current hemostatic management and opportunities to improve care. Therefore, the aim of this study is to describe the real-world clinical use of desmopressin in relation to response and other therapeutic modalities in the treatment of bleeding episodes in patients with nonsevere hemophilia A.
2 METHODS
2.1 Setting and patients
The DYNAMO study was a cohort study including patients with non-severe hemophilia at 15 hemophilia treatment centers located in the Netherlands, United Kingdom, Italy, Austria, and Canada. The participating centers are listed in Appendix S1. Recruitment took place between January 2018 and May 2021. The DYNAMO study included patients with moderate (FVIII/FIX 2–5 IU/dl) or mild (FVIII/FIX >5–35 IU/dl) hemophilia A or B aged 12–55 years, in which the upper age cutoff was set based on the historical availability of factor concentrates. For the present work, only the patients with hemophilia A were selected. Patients were excluded in case of a history of or current presence of an inhibitor against FVIII, if participating in a trial with use of an investigational product or when using anticoagulant or antiplatelet agents. Approval was obtained from the institutional review boards and all participants provided written informed consent. The study was registered in advance at ClinicalTrials.gov (Identifier: NCT0362395).
2.2 Study outcome
The primary outcome is the type of treatment used for bleeding episodes, which included factor VIII concentrates, desmopressin, desmopressin in combination with factor concentrates and other treatment such as antifibrinolytics, blood transfusion, and surgical hemostasis.
2.3 Data collection
Data were retrospectively collected from medical files and included demographics, lifetime lowest measured FVIII activity irrespective of assay type, lifetime lowest measured VWF activity and antigen, F8 genotype according to the Human Genome Variation Society numbering, body mass index calculated from the highest measured weight and height in the last 10 years, current treatment regimen, bleeding history, and details on desmopressin test response. For the bleeding history, information was collected on all bleeding episodes requiring any form of treatment that occurred in the period from January 2009 to July 2020. The latter cutoff was chosen as at that moment desmopressin nasal sprays were withdrawn worldwide because of manufacturing problems and therefore were (temporarily) unavailable. Data were retrieved from the complete hospital file including (home) treatment logs if used. Data collected on each bleeding episode included location and treatment and if available, the number of treatment days, type of product for factor concentrates, and total dose given over the treatment course. For desmopressin test response, details of the most recent desmopressin test were collected and included age, weight, pretest FVIII level and highest FVIII level measured during the test as obtained with the local testing protocol, which was usually at 1 h. Definitions can be found in Appendix S1.
2.4 Classifications
2.4.1 Treatment
The type of treatment was classified based on the main therapeutic agent provided and included factor concentrates, desmopressin, desmopressin in combination with factor concentrates, or other treatment. The latter option was used when bleeds were exclusively treated with antifibrinolytics, blood transfusion, or surgical hemostasis. Desmopressin treatment entailed all administration routes.
2.4.2 Desmopressin test response
We classified response based on the peak FVIII level achieved and defined this as no response (<30 IU/dl), partial response (30 to <50 IU/dl) and complete response (≥50 IU/dl).3, 10, 17-19 An adequate response entailed both complete or partial response (≥30 IU/dl). In some studies, a 2-fold increase in FVIII levels after desmopressin has been used to qualify as a partial responder.3, 17 For the present work, we solely used absolute peak level in this definition, as bleeds are generally treated with the aim to obtain certain (peak) FVIII levels.
2.4.3 F8 mutations with high inhibitor risk
The following mutations were classified as conferring a high inhibitor risk based on previous literature of frequently occurring mutations: Arg612Cys, Arg2169His, Arg2178Cys, and Trp2248Cys.20
2.5 Study analysis
Descriptive data were presented as medians and interquartile range (IQR) for continuous variables or as frequencies and percentages for categorical variables. The treatment provided was described for the total cohort and for subgroups: (A) according to desmopressin test response and (B) FVIII level categories based on lifetime lowest FVIII activity. For bleeds treated for a single day with factor concentrates in adequate responders, the FVIII level targeted was estimated using the formula of the Dutch hemophilia guideline (IU given = target FVIII level – baseline FVIII level/2 × bodyweight in kilograms).9 In this calculation, the highest measured weight of the patient in the last 10 years was used. We assumed that these bleeds were all treated with a single dose of factor concentrates given the short treatment course of a single day. The FVIII level targeted was compared with the peak FVIII level obtained after desmopressin. The analyses were conducted with SPSS 25 (IBM SPSS Statistics).
3 RESULTS
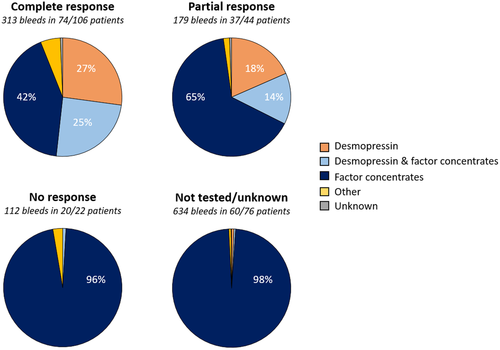
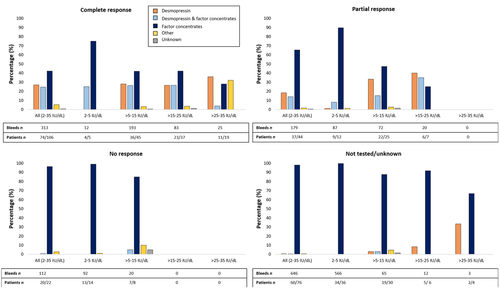
In total, 248 patients (67 moderate and 181 mild hemophilia) with a median age of 38 years (IQR 25–49) were included. The median observation period was 11 years (IQR 10–12). The desmopressin test response was known in the majority of the population (69%). In the total cohort 150 of 248 patients (60%) had an adequate response, which was 87% when considering the 172 tested patients only. In patients with mild hemophilia, 73% had an adequate response, 4% had no response and 22% were not tested. In comparison, fewer patients with moderate hemophilia had an adequate response (25%) and a larger proportion had no response (21%) or was not tested (54%). When restricted to tested patients only, respectively, 55% and 94% of moderate and mild hemophilia patients achieved an adequate response. More patients with baseline FVIII levels ≥3–5 IU/dl achieved an adequate response (15 of 25) in comparison to those with a baseline FVIII level <3 IU/dl (2 of 6) after testing for a response. However, 43% and 74% of patients with baseline FVIII levels ≥3–5 IU/dl and <3 IU/dl were not tested, respectively (Appendix S1). Table 1 summarizes characteristics of the total cohort and different desmopressin response groups. As for desmopressin, it was more frequently used in patients with an adequate desmopressin test response and in patients with higher baseline FVIII levels (Figures 1 and 2). Desmopressin was administered exclusively in 27%, 18%, 0%, and 1% of bleeds in patients with a complete, partial, no or unknown response, respectively. For patients with a complete response there was a shift toward less exclusive use of factor concentrates (42%) in comparison to those with a partial response (65%). F8 mutations were available in 189 (76%) patients. Mutations present in at least three patients are presented in relation to desmopressin response in Appendix S1.
| Total cohort n = 248 | Complete response n = 106 | Partial response n = 44 | No response n = 22 | Not tested/unknown n = 76 | |
|---|---|---|---|---|---|
| Age in years, median (IQR) | 38 (25–49) | 39 (25–50) | 28 (21–40) | 39 (25–49) | 42 (28–50) |
| Lifetime lowest FVIII levels in IU/dl, median (IQR) | 11 (5–16) | 16 (12–22) | 9 (5–13) | 5 (3–6) | 6 (3–11) |
| VWF activity levels in IU/dl, median (IQR) | |||||
| Lifetime lowest activity | 87 (66–111) | 81 (64–107) | 85 (68–111) | 77 (63–117) | 101 (65–121) |
| Unknown | 106 (43) | 38 (36) | 17 (39) | 8 (36) | 43 (57) |
| VWF antigen levels in IU/dl, median (IQR) | |||||
| Lifetime lowest antigen | 91 (71–111) | 81 (64–105) | 94 (69–112) | 106 (71–150) | 93 (81–131) |
| Unknown | 111 (45) | 43 (41) | 14 (32) | 8 (36) | 46 (61) |
| Severity classification, n (%) | |||||
| Moderate hemophilia | 67 (27) | 5 (5) | 12 (27) | 14 (64) | 36 (47) |
| Mild hemophilia | 181 (73) | 101 (95) | 32 (73) | 8 (36) | 40 (53) |
| Treatment regimen, n (%) | |||||
| Prophylaxis | 9 (4) | 0 (0) | 0 (0) | 0 (0) | 9 (12) |
| Intermittent prophylaxis | 4 (2) | 0 (0) | 1 (2) | 0 (0) | 3 (4) |
| On demand | 235 (95) | 106 (100) | 43 (98) | 22 (100) | 64 (84) |
| BMI in kg/m2, n (%) | |||||
| BMI | 24 (22–28) | 24 (22–28) | 24 (21–27) | 27 (22–32) | 26 (22–29) |
| Unknown | 43 (17) | 16 (15) | 3 (7) | 3 (14) | 21 (28) |
| Blood group, n (%) | |||||
| O | 80 (32) | 25 (24) | 18 (41) | 6 (27) | 31 (41) |
| Non-O | 87 (35) | 38 (36) | 11 (25) | 10 (45) | 28 (37) |
| Unknown | 81 (33) | 43 (41) | 15 (34) | 6 (27) | 17 (22) |
| Ethnicity, n (%) | |||||
| African American | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Asian | 4 (2) | 0 (0) | 1 (2) | 1 (5) | 2 (3) |
| Caucasian | 149 (60) | 72 (68) | 26 (59) | 13 (59) | 38 (50) |
| Middle Eastern | 7 (3) | 4 (4) | 2 (5) | 1 (5) | 0 (0) |
| Mixed | 5 (2) | 3 (3) | 2 (5) | 0 (0) | 0 (0) |
| Unknown | 82 (33) | 26 (25) | 13 (30) | 7 (32) | 36 (76) |
| At DDAVP test | |||||
| Age in years, median (IQR) | |||||
| Age | 22 (13–36) | 25 (15–37) | 14 (10–26) | 29 (16–38) | NA |
| Unknown | 19 (11) | 13 (12) | 2 (5) | 4 (18) | NA |
| Pre-DDAVP FVIII activity in IU/dl, median (IQR) | |||||
| Pre-DDAVP level | 16 (10–23) | 20 (15–26) | 12 (7–15) | 5 (4–8) | NA |
| Unknown | 6 (3) | 4 (4) | 1 (2) | 1 (5) | NA |
| Peak FVIII activity in IU/dl, median (IQR) | 58 (40–82) | 76 (60–104) | 40 (33–46) | 21 (15–26) | NA |
| Time at measured peak levels in min, median (IQR) | |||||
| Time | 60 (60–60) | 60 (60–60) | 60 (60–78) | 60 (60–60) | NA |
| Unknown | 58 (34) | 38 (36) | 14 (32) | 6 (27) | NA |
- Note: Values are given in medians and interquartile ranges (IQR) or n (%). Ethnicity was self-reported by participants.
- Abbreviations: BMI, body mass index; DDAVP, 1-deamino-8-D-arginin vasopressin; min, minutes; n, number; NA, not available.
3.1 Adequate responders
3.1.1 Treatment characteristics
Among 111 of 150 patients with an adequate response (peak FVIII ≥30 IU/dl) a total of 492 bleeds occurred that required treatment. Half of these bleeds (51%) were treated exclusively with FVIII concentrates, 24% exclusively with desmopressin, 21% with a combination of both, and the remaining 4% with other treatments. As a result, 84 treated patients (76%) were exposed at least once to factor concentrates and 20 treated patients (18%) received desmopressin exclusively for the treatment of bleeds during the observation period. The treatment duration was available for 90% of bleeds, with a median duration of 2 days (IQR 1–4) for factor concentrates, 1 day (IQR 1–2) for desmopressin, and 2 days (IQR 1–2) for both products when combined. In the latter case, factor concentrates and desmopressin were mostly provided on a consecutive basis (60%) instead of on the same day (16%). In 86% of bleeds treated with factor concentrates the product type was available which were all standard half-life products.
3.1.2 Type of bleeds
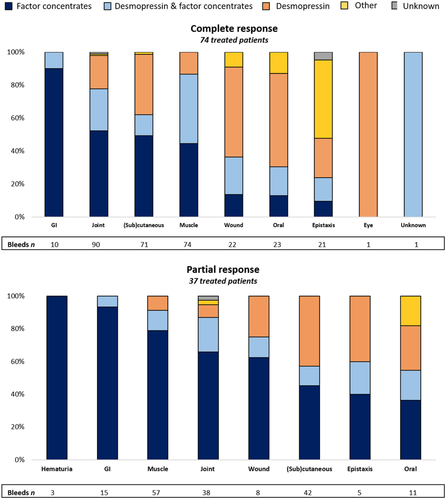
The treatment provided was largely determined by the type of bleed (Figure 3). Desmopressin was most frequently used to treat minor wounds, oral cavity bleeds, and soft-tissue/(sub)cutaneous bleeds and was used as the exclusive treatment in 47%, 47%, and 39% of these bleeds, respectively.
3.2 Comparison of peak factor levels for single-dose treated bleeds
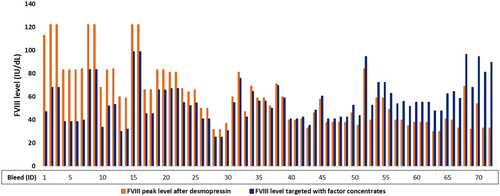
A subset of 85 bleeds in adequate responders were treated with a single dose of factor concentrates. Information on dose and weight was known for 72 of these bleeds. Hematuria was excluded from this analysis because it requires a high fluid intake that is contraindicated for desmopressin. Most bleeds were classified as soft-tissue/(sub)cutaneous bleeds (38%), muscle bleeds (26%), or joint bleeds (21%). In more than half (54%) of the bleeds, the estimated peak FVIII level targeted with factor VIII concentrates was lower compared with the expected FVIII level after desmopressin, based on results of the desmopressin test (median levels: 53 IU/dL [IQR 40–67] versus 69 IU/dl [IQR 59–83], respectively). Furthermore, in 14% of bleeds, the estimated FVIII level targeted with factor concentrates resulted in levels that were only up to 5 IU/dL higher compared with the FVIII level after desmopressin (median levels: 42 IU/dL [IQR 41–44] versus 39 IU/dl [IQR 38–42], respectively) (Figure 4).
3.3 High inhibitor risk
Of the 58 patients with a high inhibitor risk F8 mutation, 81% had an adequate response to desmopressin, 2% had no response, and 17% had an unknown response. In adequate responders with a high-risk mutation, treatment was exclusively with desmopressin in 23% of bleeds whereas factor concentrates (exclusive or in combination with desmopressin) were given in 72% of bleeds. In comparison to adequate responders with unknown mutations or mutations unidentified as high-risk mutation, similar rates of bleeds treated with desmopressin and factor concentrates were observed.
4 DISCUSSION
In this international cohort study among 248 patients with non-severe hemophilia A, we assessed the use of desmopressin in the treatment of bleeding events over a median observation period of 11 years. The majority of included patients had an adequate desmopressin response as 60% achieved a peak FVIII level ≥ 30 IU/dl. When restricted to tested patients only, 87% obtained sufficient peak levels in the total cohort. This was 55% and 94% for tested patients with moderate and mild hemophilia, respectively. Nonetheless, we found that most bleeds were treated with FVIII concentrates and desmopressin was exclusively used in merely 27% and 18% of bleeds in patients with a complete or partial response, respectively. As a result, 76% of treated adequate responders were exposed to factor concentrates during the observation period when treated for a bleed. Although most bleeds in this population were classified as joint or muscle bleeds, the median treatment duration of 2 days suggests that some were relatively minor or early-phase events. Strikingly, we estimated that in more than half of the bleeds treated with a single dose of factor concentrates, the expected peak FVIII level after desmopressin would have at least equaled the FVIII level targeted with the factor concentrates. These findings suggest that desmopressin may be considered more often as treatment option for bleeds in this population.
4.1 Desmopressin response
In our study cohort, 73% of all patients with mild hemophilia achieved a peak FVIII level ≥ 30 IU/dl after desmopressin, which was 94% including tested patients only. This is in line with previous literature reporting similar response rates in 66%–76%.3, 12, 13 In moderate hemophilia, we observed that 25% of patients showed an increase in FVIII to ≥30 IU/dl at desmopressin testing. This is lower than reported by the largest study to date in moderate hemophilia that showed that 40% had an adequate response.15 This could be explained by their approach in which only patients tested or treated with desmopressin were included, which may have led to the exclusion of patients with an expected low response. Also in our study, we observed a much higher response rate in moderate patients who underwent desmopressin testing (55%) and most of these patients had factor levels ≥3–5 IU/dl. We demonstrated that desmopressin test results were lacking in half of our moderate hemophilia study population and in 22% of patients with mild hemophilia. This calls for more desmopressin testing to identify a potential response and to facilitate optimal use of desmopressin, especially in patients with factor levels ≥3 IU/dl. We observed a younger median age in patients with a partial response compared to those with a complete response (14 years vs. 25 years). This raises the question whether young children respond less well to desmopressin, which also has been suggested by data from pediatric cohort studies.21, 22 These findings may support the recommendation to repeat desmopressin testing at a later age in children who initially failed to response. Previous research also identified other factors associated with a better desmopressin response, including lower VWF antigen levels and certain mutations (Arg612Cys, Asn637Ser, Arg1960Gln, Arg2178Cys).3, 10, 15 In our study, all patients carrying any of these mutations that were tested had an adequate response. In addition, median VWF antigen levels indeed seemed to be lower in those with an adequate response in comparison to those without a response, although these levels were not collected during the desmopressin test. The influence of determinants of response was beyond the scope of the current work and warrants future studies focused on this research question using more advanced statistical techniques.
4.2 Treatment of bleeds
We demonstrated that 76% of adequate responders treated for a bleed were exposed to factor concentrates, whereas 18% was exclusively treated with desmopressin. These findings are comparable to a previous study among 377 patients with nonsevere hemophilia A, demonstrating that factor concentrates were used in 78% of patients, whereas desmopressin was used exclusively in 20%.16 Another study reported that 32% of bleeds in adequate responders over a 12-year period were treated with desmopressin without the need for additional factor concentrates, which were mainly mucosal and subcutaneous bleeds.12 In patients with von Willebrand disease (VWD), desmopressin is considered as an important treatment option as well.23 Also in this population, discrepancies in the response to desmopressin and its actual clinical use have been noticed.24-26 A study demonstrated that 30% of patients with type 1 VWD received factor concentrates over the last year, in spite of 69% of these patients having an adequate response.24 This was confirmed by a survey among physicians involved in the management of VWD in Brazil that reported an unexpected lower use of desmopressin compared with factor concentrates in the treatment of VWD.26 It is unclear what the underlying reasons are for these observations and whether perceived side effects, estimated to occur in around 30% of patients,5 pose a substantial barrier for desmopressin use. Another possibility for suboptimal use of desmopressin may be related to local differences in costs of desmopressin or the hospital infrastructure, in which the slow infusion rate and in young children monitoring of sodium levels7 may be considered time-consuming. Additionally, clinicians may resort to factor concentrates when patients present with an early joint or muscle bleed to ensure they are not undertreating a potentially serious bleed. This is supported by our finding that most bleeds treated with a single dose of factor concentrates were classified as suspected joint or muscle bleeds, although it is unlikely that these were true fulminant bleeds considering the short treatment length. Furthermore, desmopressin was used to a lesser extent in patients with a partial response (≥30 to < 50 IU/dl) in comparison to patients with a complete response (≥50 IU/dl). Factor levels ≥30 IU/dl may be regarded as sufficient to treat minor bleeds and as preoperative treatment for minor surgery, especially in lower dose practice patterns.7, 27 Our data may indicate that a factor level ≥30 IU/dl is perceived as too low for adequate treatment in the countries where we performed our study with higher dose practice patterns. Despite this, a previous study reported that desmopressin was efficacious in 92% of all bleeds and in 78% of muscle and joint bleeds, with resolution of the bleed without need for additional factor concentrates.12 Partial responders more frequently required factor concentrates after initial desmopressin treatment than complete responders (5% vs. 22%).12 Because this was based on small patient numbers, further studies into the efficacy of desmopressin are needed.
4.3 Strengths and limitations
The DYNAMO study collected detailed information on bleeding episodes in a relatively large cohort of patients with nonsevere hemophilia, reflecting care for this patient group in an international setting. The meticulous data collection including data from the complete clinical file provided a unique opportunity to assess real-world use of treatment modalities in this population. Data collection was restricted to bleeds requiring any form of treatment, leading to a potential overrepresentation of severe bleeds and an underestimation of minor bleeds that were not reported in the clinical files. As a result, we may have missed small bleeds that resolved after treatment with desmopressin nasal spray or subcutaneous desmopressin in the home setting and for which no (telephone) contact was sought with the treatment center. However, the majority of the participating investigators confirmed that bleeds treated with desmopressin at home are generally discussed at routine clinic visits and reported in the clinical files at their center, reducing the risk that such bleeds are lacking. Another limitation was that our study did not address the considerations driving the choice for a specific treatment modality. The reasons to prefer factor concentrates over desmopressin in complete and partial responders may be influenced by the clinical scenario (type of bleed, symptoms, timing of presentation, first or rebleed, cause of bleed), patient preferences, previous side effects or contraindications, variables that we were unable to retrieve and need to be investigated in future research. Additionally, some patients with an initial adequate response may have an increased FVIII clearance, resulting in lack of a sustained response.19 In our study, data were only collected on the highest peak FVIII level measured during the desmopressin test, which was usually at 1 h. Information on the route of administration, brand, and dose used for desmopressin testing were not part of the data collection, which could have affected the response rates reported. Consequently, the presented data need to be interpreted with these issues in mind.
4.4 Clinical implications
Desmopressin is considered an important alternative to factor concentrates because it is less expensive and carries no risk for the development of inhibitory antibodies. Because it is estimated that 60%–70% of all hemophilia patients worldwide still lack access to factor concentrates,28 desmopressin has the potential to be an effective product for a relatively large patient group in resource-constrained countries. It is therefore also included in the World Health Organization list of essential medicines.29 Considering that 60% of patients with non-severe hemophilia A achieved adequate factor levels, desmopressin is indeed an important treatment option for this population. Despite this, for only 24% and 21% of bleeds in adequate responders desmopressin was used exclusively or in combination with factor concentrates, respectively. Moreover, we found that desmopressin test results were lacking for one-third of patients. This calls for a more systematic assessment of desmopressin response. Strikingly, the use of desmopressin would have resulted in similar peak FVIII levels in more than half of cases treated with a single dose of factor concentrates. Suboptimal use of desmopressin should especially be addressed in patients with a high risk of inhibitor development. Combined treatment with factor concentrates and desmopressin could reduce the need for high peak FVIII doses, thereby reducing the risk of inhibitor development.30, 31 Therefore, future research is needed to explore reasons for potential underuse of desmopressin from both a patient and physician perspective. If side effects prevent patients from using desmopressin, further studies should explore potential opportunities to improve motivation for desmopressin.
5 CONCLUSION
Desmopressin was used as exclusive treatment in 24% of bleeds in patients with an adequate desmopressin test response, which may reflect suboptimal use of this treatment option.
AUTHOR CONTRIBUTIONS
A. Zwagemaker analyzed the data and wrote the manuscript. A. Zwagemaker and F.R. Kloosterman collected, cleaned, and interpreted the data. K. Fijnvandraat designed the study. M. Coppens, S.C. Gouw, S. Boyce, C.N. Bagot, E.A.M. Beckers, P. Brons, G. Castaman, J. Eikenboom, S. Jackson, M.J.H.A. Kruip, F.W.G. Leebeek, K. Meijer, L. Nieuwenhuizen, I. Pabinger, and K. Fijnvandraat collected data or supervised data collection and reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by a grant from Novo Nordisk. The funding sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. The authors thank all local research staff for their help in setting up and conducting the study.
RELATIONSHIP DISCLOSURE
This work was supported by a grant from Novo Nordisk. M.C. has received financial support for research from Bayer, Roche, UniQure, and Novo Nordisk; honoraria for lecturing from Bayer, CSL Behring, Pfizer, and Alexion; fees for consulting from Sobi, Viatris, CSL Behring, Novo Nordisk, and Daiichi Sankyo; travel support for attending meetings: Alexion, Sobi. S.C.G. has received an unrestricted research grant from Sobi, a Heimburger grant from CSL Behring, and a EAHAD research grant. The institution of P.B. received consulting fees from Oxford PharmaGenesis. S.B. received research support from Sangamo Therapeutics. G.C. is a speaker at Company Symposia during Scientific meetings for Bayer, Grifols, LFB, Roche, Sobi, Novo Nordisk, Werfen, and Kedrion; research funding directly to his institution from CSL Behring, Pfizer, and SOBI; during the last 2 years, participating in Advisory Boards of Bayer, Takeda, CSL Behring, Novo Nordisk, Pfizer, Roche, Sanofi, SOBI, and Uniqure. J.E. received research support from CSL Behring and he has been a teacher on educational activities of Roche. He is DSMB member of the Alphabet Study. S.J. has received financial support for research from Sanofi Genzyme and honoraria from Bayer, Takeda, Sanofi, Pfizer, Thrombosis Canada, Roche, Octapharma, and Hemalytic. The institution of M.J.H.A.K. has received unrestricted research grants from Sobi; her institution received speakers fees from Sobi, Roche, and BMS. F.W.G.L. received unrestricted research grants from CSL Behring, Shire/Takeda, Sobi, and uniQure. He is a consultant for CSL Behring, Shire/Takeda, Biomarin, and uniQure, of which the fees go to the University. He was DSMB member of a study sponsored by Roche. K.M. reports speaker fees from Alexion, Bayer, and CSL Behring, participation in trial steering committee for Bayer, consulting fees from Uniqure, participation in data monitoring and endpoint adjudication committee for Octapharma; all fees paid to her institution. I.P. received honoraria for Lectures and or Advisory Boards from Bayer, Biotest, CSL Behring, Sobi, Roche, Takeda, Pfizer, NovoNordisk, and received Research Grants to the Institution from CSL Behring, NovoNordisk, Sobi, Takeda. The institution of K.F. has received unrestricted research grants from Novo Nordisk, Sobi, and CSL Behring and her institution received consultancy fees from Roche, Takeda, and Sobi. A.Z., F.R.K., C.N.B., E.A.M.B., and L.N. have no potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
For original data, please contact [email protected].




