Post-discharge thrombosis and bleeding in medical patients: A novel risk score derived from ubiquitous biomarkers
There was no funding provided to complete this study.
Handling Editor: Pantep Angchaisuksiri.
Abstract
Background
Some hospitalized medical patients experience venous thromboembolism (VTE) following discharge. Prophylaxis extended beyond hospital discharge (extended duration thromboprophylaxis [EDT]) may reduce this risk. However, EDT is costly and can cause bleeding, so selecting appropriate patients is essential. We formerly reported the performance of a mortality risk prediction score (Intermountain Risk Score [IMRS]) that was minimally predictive of 90-day hospital-associated venous thromboembolism (HA-VTE) and major bleeding (HA-MB). We used the components of the IMRS to calculate de novo risk scores to predict 90-day HA-VTE (HA-VTE IMRS) and major bleeding (HA-MB IMRS).
Methods
From 45 669 medical patients we randomly assigned 30 445 to derive the HA-VTE IMRS and the HA-MB IMRS. Backward stepwise regression and bootstrapping identified predictor covariates from the blood count and basic chemistry. These candidate variables were split into quintiles, and the referent quintile was that with the lowest event rate for HA-VTE and HA-MB; respectively. A clinically relevant rate of HA-VTE and HA-MB was used to inform outcome rates. Performance was assessed in the derivation set of 15 224 patients.
Results
The HA-VTE IMRS and HA-MB IMRS area under the receiver operating curve (AUC) in the derivation set were 0.646, and 0.691, respectively. In the validation set, the HA-VTE IMRS and HA-MB IMRS AUCs were 0.60 and 0.643.
Conclusions
Risk scores derived from components of routine labs ubiquitous in clinical care identify patients that are at risk for 90-day postdischarge HA-VTE and major bleeding. This may identify a subset of patients with high HA-VTE risk and low HA-MB risk who may benefit from EDT.
Essentials
- Some medical patients after hospital discharge will experience thrombosis.
- Complete blood count– and basic metabolic profile–derived risk scores find those at risk for postdischarge VTE and major bleeding.
- Automated calculation of risk can reside in the electronic medical record and be presented for virtually every patient upon discharge.
- Individual patient risk can inform decision-making to prevent VTE at hospital discharge.
1 BACKGROUND
Approximately 8 million patients are hospitalized in the United States for a nonsurgical indication each year.1 An estimated 75% of all hospital-associated venous thromboembolism (HA-VTE) and 70% to 80% of fatal pulmonary emboli occur among hospitalized medically ill patients.2, 3 The rate of symptomatic VTE more than doubles over the first 21 days after hospital discharge4 and greater than one-half and as many as three-quarters of all HA-VTE events are believed to occur after hospital discharge.5, 6 Postdischarge anticoagulant prophylaxis, referred to as extended duration thromboprophylaxis (EDT) may reduce VTE but is burdensome and carries a risk of bleeding. While it is estimated that nearly one in four discharged medical patients may benefit from EDT,7 prospective randomized control trials have demonstrated the challenges associated with identifying the best candidates for EDT.8-11 A robust effort to identify those risk factors most associated with thrombosis and bleeding among hospitalized medical patients was recently completed.12 Yet there exists a very narrow margin between the rate of postdischarge VTE reduction and the rate of major bleeding observed among patients enrolled in prospective randomized studies evaluating EDT.13-15 The results from these studies suggest imprecision in the ability to predict those patients at highest risk for postdischarge VTE and low risk for bleeding. Therefore, a risk score that is easily calculable from lab tests ubiquitous in routine clinical care and can discriminate patients that would experience a net clinical benefit from EDT represents an unmet need.
Risk assessment models (RAMs) that include clinical factors have been developed and variably validated that identify hospitalized medical patients at risk for VTE.16-25 However, these RAMs may not precisely identify patients who would experience net clinical benefit from EDT; and can be complex and difficult to implement.9, 10 Biomarkers have been proposed as predictive of HA-VTE to enrich and study populations.26, 27 However, the incorporation of biomarkers such as D-dimer (not obtained routinely as part of clinical care among hospitalized medical patients) employed to better identify patients at high risk for EDT has met with limited success.8, 11
We recently reported the performance of a risk score derived for the prediction of 1-year mortality (Intermountain Risk Score [IMRS]) and compared that score to two formerly described clinical RAMs in predicting 90-day HA-VTE among discharged medical patients.28 The IMRS was poorly predictive of HA-VTE, and when compared to the clinical RAMs, it did not meaningfully improve predictiveness of 90-day HA-VTE. Therefore, we elected to ascertain if performing a derivation and validation study to select different parameters for the components of the IMRS could generate a HA-VTE–specific IMRS (HA-VTE IMRS) that better identifies those discharged medical patients at greatest risk to experience postdischarge VTE. We hypothesized that if a pragmatic risk score that uses laboratory results obtained as part of routine care would identify patients at high risk for postdischarge VTE, then two things may occur. First, others would be able to easily replicate our observations in their data set. Second, this exercise could identify a candidate population that might benefit from EDT.
Likewise, while prediction models for bleeding risk among hospitalized medical patients have been described29 and preliminarily validated,30, 31 these risk models include clinical factors that are burdensome to ascertain. Therefore, analogous to our approach to derive and validate the VTE-IMRS, we derived and validated a hospital-associated major bleeding risk score (HA-MB IMRS) to predict 90-day hospital-associated major bleeding (HA-MB) among this patient cohort. Here we derive and validate a RAM that would be simple to deploy in any electronic medical record (EMR) to calculate the estimated risk of both 90-day HA-VTE and HA-MB. These risk estimates could inform decision making regarding the prescription of EDT.
2 METHODS
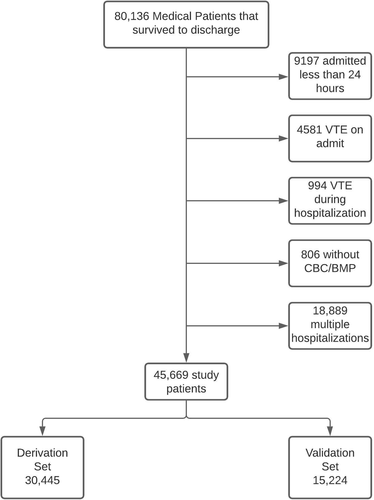
2.1 Study design and patient population
This retrospective derivation and validation cohort study included hospitalized medical patients discharged from an Intermountain Healthcare hospital between January 2010 and December 2014 including patients from the data sets that comprised the Intermountain Healthcare Venous Thromboembolism Reduction Initiatives 1 and 3 (VRI-132 and VRI-2).33 Among 80 136 eligible patients, the study cohort of 45 669 included those that were hospitalized on a first admission for longer than 24 hours, discharged alive, did not have VTE detected on admission or during inpatient stay, and had a complete blood count (CBC) and basic metabolic profile (BMP) obtained (Figure 1). None of the patients in our study received EDT as part of routine care. The clinical outcomes of 90-day postdischarge VTE and major bleeding were identified as we have formerly reported. VTE was identified upon EMR interrogation of radiology reports using a natural language processing (EMR interrogation software that identifies the presence of VTE outcomes from unstructured, radiologist-dictated text) to identify outcomes of thrombosis that we recently reported.34 We included only clinically overt VTE identified during clinical care defined as any pulmonary embolism (PE) or deep vein thrombosis (DVT) in the lower extremities (excluding unusual site thrombosis). We identified major bleeding events by EMR interrogation as we formerly reported32, 33 and considered bleeding as major if, as defined by Schulman,35 there existed an International Classification of Diseases, Ninth and Tenth Revisions (ICD-9/10) code representative of bleeding into a critical space including the spinal cord, brain, eye, retroperitoneum, or pericardium. Major bleeding was also considered present if clinically overt bleeding was documented on the basis of an ICD-9/10 code in conjunction with the laboratory requirement of a drop in hemoglobin by ≥2 grams per deciliter, or the concomitant transfusion of ≥2 units of packed red blood cells during the same clinical encounter (Appendix S1). Patients were randomly assigned to the derivation or validation cohort based on a two-thirds versus one-third split of the available Intermountain population. The derivation cohort was evaluated using Cox regression to fit the risk models and create risk scores. The derivation was conducted initially using separate Cox regressions entering age, CBC parameters, and BMP factors in 500 bootstrapped samples to evaluate the sensitivity of the Cox models to variations in the composition of the derivation population. The 500 Cox models used backward stepwise variable selection. Based on the results of those 500 analyses, a final consensus set of parameters was chosen for inclusion in the model that made it the most predictive and robust to be fit in Cox regression for the full derivation population. Then, the validation analysis was performed as the study hypothesis test when the risk scores were applied to the other 33% of patients who had been held aside at the beginning of data analysis. Patient demographics are summarized in Table 1.
| Overall (n = 45,669) | Derivation (n = 30,445) | Validation (n = 15,224) | |
|---|---|---|---|
| Patient characteristics | |||
| Age, y, mean (SD) | 61.4 (19.5) | 61.4 (19.5) | 61.5 (19.5) |
| Female, n (%) | 24 873 (55) | 16 592 (55) | 8281 (54) |
| Race, n (%) | |||
| White | 40 631 (89.0) | 27 047 (88.8) | 13 584 (89.2) |
| Asian | 397 (0.9) | 272 (0.9) | 125 (0.9) |
| Black | 555 (1.2) | 386 (1.3) | 169 (1.1) |
| Pacific Islander | 779 (1.7) | 512 (1.7) | 267 (1.8) |
| Native American | 397 (0.9) | 272 (0.9) | 125 (0.8) |
| Other/Unknown | 2844 (6.2) | 1915 (6.3) | 929 (6.1) |
| Ethnicity, n (%) | |||
| Hispanic/Latinx | 1230 (2.7) | 784 (2.6) | 446 (2.9) |
| Not Hispanic/Latinx | 44 439 (97.3) | 29 661 (97.4) | 14 778 (97.1) |
| Married | 21 834 (47.8) | 14 609 (48.0) | 7225 (47.5) |
| Insurance, n (%) | |||
| Medicare | 23 940 (52.4) | 15 949 (52.4) | 7991 (52.5) |
| Medicaid | 3671 (8.0) | 2417 (7.9) | 1254 (8.2) |
| Self-pay | 4750 (10.4) | 3201 (10.5) | 1549 (10.2) |
| Commercial insurance | 13 308 (29.1) | 8878 (29.2) | 4430 (29.1) |
| Comorbidities | |||
| Congestive heart failure, n (%) | 10 273 (22) | 6853 (23) | 3420 (22) |
| Diabetes, n (%) | 10 174 (22) | 6730 (22) | 3444 (23) |
| Current tobacco use, n (%) | 11 481 (25) | 7630 (25) | 3851 (25) |
| Infection, n (%) | 12 477 (27) | 8304 (27) | 4173 (27) |
| PICC line, n (%) | 3150 (7) | 2077 (7) | 1073 (7) |
| Sepsis n (%) | 9108 (20) | 6083 (20) | 3025 (20) |
| Central venous catheter n (%) | 3969 (9) | 2709 (9) | 1260 (8)* |
| Bleed n (%) | 623 (1.4) | 420 (1.4) | 203 (1.3) |
| Received VTE chemoprophylaxis n (%) | 32 853 (72) | 21 865 (72) | 10 988 (72) |
| Had contraindication for prophylaxis | 1207 (2.6) | 798 (2.6) | 409 (2.7) |
| APACHE II, mean (SD) | 11.8 (6.0) | 11.8 (6.1) | 11.7 (6.0) |
| Charlson Comorbidity Index, mean (SD) | 3.4 (3.0) | 3.4 (3.0) | 3.4 (3.0) |
| VTE risk factors, n (%) | |||
| Cancer | 4877 (11) | 3266 (11) | 1611 (11) |
| Prior VTE | 6231 (14) | 4178 (14) | 2053 (13) |
| Thrombophilia | 2052 (4.5) | 1367 (4.5) | 685 (4.5) |
| Surgerya | 4926 (11) | 3247 (11) | 1679 (11) |
Note
- Race, ethnicity, and social determinants of health are self-reported data and may be incomplete.
- Abbreviations: PICC, peripherally inserted central catheter; SD, standard deviation; VTE, venous thromboembolism.
- a Defined as surgery with anesthesia for a duration longer than 1 hour within 30 days.
- * P < .05 for validation vs derivation.
2.2 Laboratory testing and HA-VTE IMRS and HA-MB IMRS generation
Similar to our formerly reported methodology36 we used elements of CBC (hemoglobin, red blood cell count, hematocrit, white blood cell count, platelet count, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red cell distribution width, mean platelet volume) and BMP (sodium, potassium, chloride, bicarbonate, calcium, blood urea nitrogen, glucose, creatinine) along with age to generate the HA-VTE IMRS. This score was derived as a linear combination of the weighted regression β-coefficients from Cox regression that were estimated for each of the CBC and BMP parameters. To estimate regression weightings, each CBC and BMP component was divided into quintiles in the derivation population. CBC testing used an automated Coulter hematology device (Beckman Coulter Corp, Hialeah, FL, USA) and the BMP panel used automated chemistry analyzer (Ortho Clinical Diagnostics, Raritan, NJ, USA), both of which have low intra- and interrun assay variability. We used the CBC and BMP results most proximal to the time of hospital discharge for both derivation and validation analyses, as we hypothesized those would be most relevant to predicting postdischarge VTE events. Cox regression modeled the risk of VTE-free survival with multivariable adjustment for age. The quintiles of each laboratory factor were modeled as dummy variables, with the referent quintile assigned as the category with the lowest risk (the exception being age, where decade groupings were used and compared to 40-49 years—18-29, 30-39, 50-59, 60-69, 70-79, and ≥80 years—given the familiarity in considering age based on decade as opposed to quintile). Quintiles were used to create clinically representative cohorts to mitigate random variation in biomarker result variability from impacting model development. Analogous to our previously published work,36 we adopted quintiles to avoid “overfitting” random laboratory variability in data points. Risk weightings were assigned to Cox regression β-coefficients based on an algorithm that initially evaluated the data to identify a minimally meaningful β-coefficient. The minimal coefficients that were considered were selected as follows. First, they were between 0.10 and 0.25 and based on variables whose P values in the final regression model in the derivation set were close to P = .05. Also considered was the distribution of the variables’ coefficients in the bootstrapped samples, and the related impact that random variation between populations could have. Integer multiples of a proposed minimal β-coefficient were examined to determine if a broad distribution of categories would be created when the variables in the final model were assigned weightings based on equally spaced β-coefficient ranges. Authors (SCW, ELW, BDH) then discussed the merits of the possible weighting schemes and chose the one most likely to provide the best spread of patient results to empower distinction in prognostic predictions between the most patients. Integer weightings were 0 for coefficients below the minimally beneficial β-coefficient (including the lowest risk referent value), 1 for those between the threshold and two times the threshold, 2 for between two and three times the threshold, and so forth. A patient’s VTE-IMRS was calculated from his or her individual data as the sum of each component variable’s integer weighting values.
Demographics, comorbidities, and CBC and BMP variables are reported frequencies with percentages for dichotomous or categorical variables and mean ± standard deviation for continuous variables. Comparisons of baseline characteristics used Student’s t test or Pearson’s chi-square test, as appropriate. Two-tailed P values were used, with P ≤ .05 designated as significant.
In an analogous fashion used for the ascertainment of the HA-VTE IMRS, we derived and validated a HA-MB IMRS to predict 90-day postdischarge major bleeding risk. Analogous to our genesis for the HA-VTE IMRS, we used the CBC and BMP most proximal to the time of hospital discharge to derive and validate the HA-MB IMRS, as we hypothesized those would be most relevant to predicting postdischarge HA-MB.
2.3 Additional statistical considerations
For the HA-VTE IMRS and the HA-MB IMRS we computed the area under the receiver operating characteristic curve (AUC) to generate this common measure of predictive accuracy for binary outcomes. We compared the AUC for the outcome of 90-day postdischarge VTE for scores using the derivation population and controlled for multiple comparisons using the false discovery rate. Further, risk scores were categorized into low and high risk based on a threshold for HA-VTE IMRS where the risk of VTE was >2% (HA-VTE IMRS ≥7) and for HA-HB IMRS where risk of bleeding was >1% (HA-MB IMRS ≥8). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated for these risk thresholds. All analyses were performed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).37
2.4 Outcomes
The primary outcomes are the predictiveness of the HA-VTE IMRS and the HA-MB IMRS for 90-day HA-VTE and 90-day HA-MB, respectively, described using the derived c-statistic.
3 RESULTS
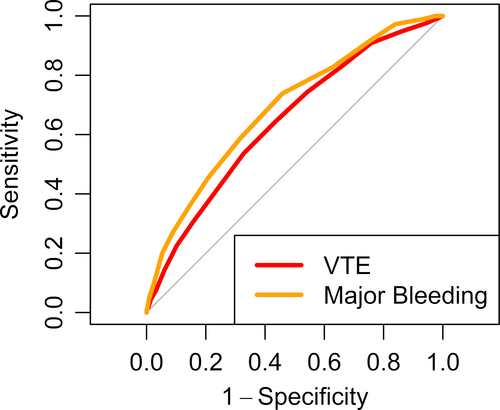
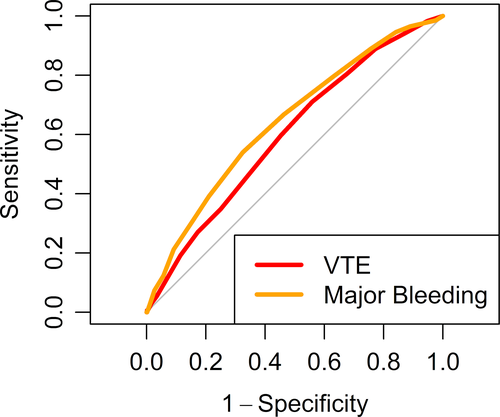
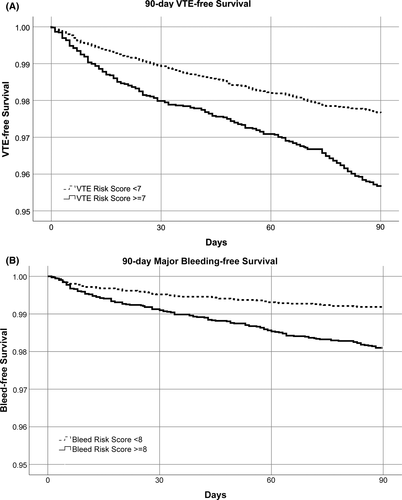
The overall demographics of the derivation set of 30 445 patients and the validation set (n = 15 224) were similar (55% female; mean age, 61.4 ± 19 years; Table 1) and are presented across categories of thrombosis and bleeding risk (Table 2). In addition to age, the HA-VTE IMRS calculated in the derivation set retained the covariates red blood cell distribution width (RDW), white blood cell count, platelet count, blood urea nitrogen, glucose, and sodium. Integer points assigned to each quintile of covariates selected may be found in Table 3. In the derivation set a HA-VTE IMRS ≥7 was elected to represent high risk for 90-day HA-VTE because this threshold was associated with a ≥2% rate of 90-day HA-VTE, which we considered clinically important and yielded an AUC of 0.65. In the validation set, this was 0.60 (Figure 2). The rate of HA-VTE in the high-risk group compared with that of the low-risk group defined by the threshold of a HA-VTE IMRS ≥7 was 2.6% versus 1.6% (hazard ratio, 1.69; 95% confidence interval [CI], 1.35-2.13; P = .008, Figure 4A). Numerically, for the HA-VTE IMRS at 90 days, 137 patients with a risk score ≥7 had VTE (2.6% of 5242) and 160 patients with a score <7 had VTE (1.6% of 9982). For the HA-VTE IMRS at 90 days, 500 patients (9.5%) and 369 patients (3.7%) who had a score ≥7 and <7, respectively, died. In addition to age, the HA-MB IMRS in the derivation set retained RDW, red blood cell count, and mean platelet volume; creatinine; and sodium. Integer points assigned to each quintile of covariates selected are found in Table 3. In the derivation set, a HA-MB IMRS ≥8 was elected to represent high risk for 90-day HA-MB because this threshold was associated with a ≥1% rate of 90-day major bleeding, which we considered clinically important and yielded an AUC of 0.691. In the validation cohort, this was 0.64 (Figure 3). The rate of HA-MB in the high-risk group compared with that of the low-risk group defined by the threshold of a HA-MB IMRS ≥8 was 1.9% versus 0.8% (relative risk, 2.35; 95% CI, 1.75-3.16; P <.001 (Figure 4B). Numerically, for the HA-MB IMRS, 135 patients with a score ≥8 had major bleeding (1.9% of 7101) and 67 patients with a score <8 had major bleeding (0.8% of 8123). For the HA-MB IMRS, 651 patients (9.2%) and 218 patients (2.7%) who had a score ≥8 and <8, respectively, died by 90 days.
|
HA-VTE IMRS <7 (n = 9982) |
HA-VTE IMRS ≥7 (n = 5242) | HA-MB IMRS <8 (n = 8123) | HA-MB IMRS ≥8 (n = 7101) | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, y, mean (SD) | 57.5 (21.5) | 69.1 (11.6)* | 54.1 (20.7) | 70.0 (13.7)* |
| Female, n (%) | 5583 (56) | 2698 (52)* | 4594 (57) | 3687 (52)* |
| Race, n (%) | ||||
| White | 8942 (89.6) | 4642 (88.6)* | 7194 (88.6) | 6390 (90.0)* |
| Asian | 96 (1.0) | 45 (0.9) | 81 (1.0) | 60 (0.8) |
| Black | 119 (1.2) | 50 (1.0) | 89 (1.1) | 80 (1.1) |
| Pacific Islander | 172 (1.7%) | 95 (1.8%) | 155 (1.9%) | 112 (1.6%) |
| Native American | 90 (0.9) | 35 (0.7%) | 75 (0.9%) | 50 (0.7%) |
| Other/Unknown | 563 (5.6) | 375 (7.2) | 529 (6.5) | 409 (5.8) |
| Ethnicity | ||||
| Hispanic/Latinx | 316 (3.2) | 130 (2.5)† | 268 (3.3) | 178 (2.5)† |
| Not Hispanic/Latinx | 9666 (96.8) | 5112 (97.5) | 7855 (96.7) | 6923 (97.5) |
| Married | 4506 (45.1) | 2719 (51.9)* | 3746 (46.1) | 3479 (49.0)* |
| Insurance, n (%) | ||||
| Medicare | 4328 (43.4) | 3663 (69.9)* | 3137 (38.6) | 4854 (68.4)* |
| Medicaid | 956 (9.6) | 298 (5.7) | 826 (10.2) | 428 (6.0) |
| Self-pay | 1312 (13.1) | 237 (4.5) | 1170 (14.4) | 379 (5.3) |
| Commercial insurance | 3386 (33.9) | 1044 (19.9) | 2990 (36.8) | 1440 (20.3) |
| Comorbidities | ||||
| Congestive heart failure, n (%) | 1603 (16.1) | 1817 (34.7)* | 1075 (13.2) | 2345 (33.0)* |
| Diabetes, n (%) | 1720 (17.2) | 1724 (32.9)* | 1445 (17.8) | 1999 (28.2)* |
| Current tobacco use, n (%) | 2731 (27.4) | 1120 (21.4)* | 2401 (29.6) | 1450 (20.4)* |
| Infection, n (%) | 2683 (26.9) | 1490 (28.4)† | 2119 (26.1) | 2054 (28.9)* |
| PICC line, n (%) | 590 (5.9) | 483 (9.2)* | 475 (5.8) | 598 (8.4)* |
| Sepsis, n (%) | 1977 (19.8) | 1048 (20.0) | 1625 (20.0) | 1400 (19.7) |
| Central venous catheter, n (%) | 576 (5.8) | 684 (13.0)* | 459 (5.7) | 801 (11.3)* |
| Bleed, n (%) | 445 (4.5) | 514 (9.8)* | 311 (3.8) | 648 (9.1)* |
| Received VTE chemoprophylaxis, n (%) | 7116 (71.3) | 3872 (73.9)* | 5863 (72.2) | 5125 (72.2) |
| Had contraindication for prophylaxis, n (%) | 196 (2.0) | 213 (4.1)* | 117 (1.4) | 292 (4.1)* |
| APACHE II, mean (SD) | 10.6 (5.4) | 13.9 (6.3)* | 10.1 (5.3) | 13.6 (6.1)* |
| Charlson Comorbidity Index, mean (SD) | 2.73 (2.74) | 4.59 (3.22) * | 2.47 (2.56) | 4.40 (3.21)* |
| VTE risk factors, n (%) | ||||
| Cancer | 813 (8.1) | 798 (15.2)* | 603 (7.4) | 1008 (14.2)* |
| Prior VTE | 1104 (11.1) | 949 (18.1)* | 827 (10.2) | 1226 (17.3)* |
| Thrombophilia | 377 (3.8) | 308 (5.9)* | 264 (3.3) | 421 (5.9)* |
| Surgerya | 829 (8.3) | 850 (16.2)* | 645 (7.9) | 1034 (14.6)* |
- Abbreviations: HA-MB IMRS, hospital-associated major bleeding–Intermountain Risk Score; HA-VTE IMRS, hospital-associated venous thromboembolism–Intermountain Risk Score; PICC, peripherally inserted central catheter; SD, standard deviation; VTE, venous thromboembolism.
- a Defined as a surgical procedure with anesthesia for a duration longer than 1 hour within 30 days.
- * p<0.001 for high vs. low risk score.
- † p<0.05 for high vs. low risk score.
| Score characteristic | HA-VTE IMRS | HA-MB IMRS |
|---|---|---|
| Red blood cell count (quintiles: <3.23, 3.23-3.64, 3.65-3.99, 4.00-4.40, >4.40 × 103/μL) | ||
| <3.23 × 103/μL | 1 | 2 |
| ≥3.23 × 103/μL | 0 | 0 |
| White blood cell count (quintiles: <5.6, 5.6-6.9, 7.0-8.4, 8.5-10.5, >10.5 × 103/μL) | ||
| <7.0 × 103/μL | 0 | N/A |
| 7.0–8.4 × 103/μL | 1 | N/A |
| 8.5–10.5 × 103/μL | 0 | N/A |
| >10.5 × 103/μL | 2 | N/A |
| Platelet count (quintiles: <147, 147-184, 185-222, 223-278, >278 × 103/μL) | ||
| <147 × 103/μL | 1 | N/A |
| ≥147 × 103/μL | 0 | N/A |
| Red cell distribution width (quintiles: <13.3%, 13.3%-13.8%, 13.9%-14.6%, 14.7%-16.1%, >16.1%) | ||
| <13.9% | 0 | 0 |
| 13.9%-14.6% | 1 | 0 |
| 14.7%-16.1% | 2 | 0 |
| >16.1% | 4 | 4 |
| Mean platelet volume (quintiles: <7.9, 7.9–8.8, 8.9–9.6, 9.7–10.4, >10.4 fL) | ||
| <8.9 fL | 0 | N/A |
| ≥8.9 fL | 1 | N/A |
| Sodium (quintiles: <135, 135-136, 137-138, 139-141, >141 mmol/L) | ||
| <135 mmol/L | 1 | 1 |
| ≥135 mmol/L | 0 | 0 |
| Glucose (quintiles: <86, 86-95, 96-106, 107-129, >129 mg/dL) | ||
| <96 mg/dL | 0 | N/A |
| 96–106 mg/dL | 1 | N/A |
| 107–129 mg/dL | 0 | N/A |
| >129 mg/dL | 2 | N/A |
| Creatinine (quintiles: <0.64, 0.64-0.76, 0.77-0.90, 0.91-1.16, >1.16 mg/dL) | ||
| <0.64 mg/dL | N/A | 1 |
| 0.64–0.90 mg/dL | N/A | 0 |
| >0.90 mg/dL | N/A | 2 |
| Blood urea nitrogen (quintiles: <9, 9-11, 12-16, 17–24, >24 mg/dL) | ||
| <17 mg/dL | 0 | N/A |
| ≥17 mg/dL | 2 | N/A |
| Age | ||
| <40 y | 0 | 0 |
| 40-49 y | 0 | 4 |
| 50-59 y | 0 | 5 |
| 60-69 y | 4 | 5 |
| 70-79 y | 3 | 5 |
| ≥80 y | 0 | 5 |
Note
- Quintiles that did not differ from the referent quintile for either score are not displayed. We did not observe a linear relationship between laboratory quintiles and integers of risk that were derived from the predictiveness of data as described in Methods. Given that these laboratory variables are being assessed for prognostic (not diagnostic) predictiveness, uncertainty exists that familiar ranges of “normal” might apply.
- Abbreviations: HA-MB IMRS, hospital-associated major bleeding–Intermountain Risk Score; HA-VTE IMRS, hospital-associated venous thromboembolism–Intermountain Risk Score.
As an exploratory analysis we repeated the calculations using the laboratory results most proximal to hospital admission. The mean HA-VTE IMRS calculated using the laboratory results closest to the time of discharge was significantly higher (P <.001) in patients with 90-day postdischarge VTE than those without postdischarge VTE (6.4 vs 5.3) and did not substantively differ from the results when the HA-VTE IMRS was calculated on the first labs (7.9 vs 7.0) following admission. We included all patients discharged and censored those patients that died at the time of death. The exploratory analysis excluding those patients who died did not meaningfully affect our reported results.
4 DISCUSSION
The HA-VTE IMRS and the HA-MB IMRS are risk scores that predicted HA-VTE and HA-MB, respectively. These scores are derived from patient age and laboratory markers ubiquitous in routine care and are easily calculated in an automated fashion. As such, VTE and major bleeding risk estimates may be automatically surfaced in physician workflow at the time of discharge. We observed that among eligible patients CBC and BMP were present 99% of the time. The benefits of a risk score derived from laboratory markers associated with a hospitalization include that the covariates are coded data that, when programmed in an EMR, may automatically present personalized risk estimates for 90-day HA-VTE and HA-MB. These coded data are temporally related to the patient’s illness and contemporary risk at the time of discharge, and therefore represent a timely assessment of the patient’s risk. Furthermore, this risk assessment is not reliant on physician manual data entry/chart review (eg, “history of thrombosis”). Also, reliance on ICD codes (with inherent limitations described38, 39) is unnecessary, and no supplementary EMR interrogation programming to ascertain former clinical events (eg, employing natural language processing to find prior DVT or PE), such as the EMR interrogation programs that we and others have created and described, is required.21, 32, 40
A meta-analysis of prospective randomized controlled trials that assessed outcomes of extended thromboprophylaxis suggested a number needed to treat to protect against the outcome of symptomatic VTE/VTE-related death of 250 in comparison with a number needed to harm of 333 for the outcome of major/fatal bleeding.13 However, important heterogeneity existed among patients included in the studies that led to these estimates. The only trial that expressly studied the benefit of postdischarge extended duration thromboprophylaxis (MARINER) did not meet the primary end point. The close margin of safety reported in MARINER has been identified as a potential barrier in the broader application of postdischarge EDT.14, 41 However, if a patient’s personalized postdischarge thrombosis and bleeding risk may be estimated at the time of discharge, then that information may inform decision making regarding the prescription of EDT, and the net clinical benefit may be improved. Exciting advances in understanding individual prognostic factors for VTE and bleeding in hospitalized medical patients were recently reported.12 Yet the authors reported comparatively less certainty surrounding the predictiveness of the factors indicative of bleeding risk, and they highlighted limitations of the prognostic factors’ adoption into clinical practice including barriers to reliably ascertain and present these risk factors in an actionable fashion.12
Two percent is a 90-day rate of HA-VTE that is formerly described as actionable for the application of EDT.23, 42-44 Placing value on the avoidance of a major bleeding complication, we elected a 1% rate of 90-day HA-MB as a threshold that would be permissible for thromboprophylaxis. Alshouimi estimated that EDT reduces risk for symptomatic VTE compared with a “hospital-only” prophylaxis regimen by 41% yielding a number needed to treat of 314 to prevent one VTE event, yet an associated relative risk of major bleeding of 1.95 (95% CI, 1.25-3.04) and a number needed to harm of 343 was described.41 At the threshold of 2% 90-day risk for HA-VTE, we observed that a HA-VTE IMRS ≥7 was associated with a 69% increase risk for thrombosis. Assuming a 1% 90-day risk for major bleeding, the HA-MB IMRS ≥8 was associated with a 235% increase risk for major bleeding. If we further estimate that EDT confers a 40% risk reduction for 90-day HA-VTE and that those patients with a 90-day HA-MB risk of 1% are excluded, then 105 patients would need to be treated to prevent 1 venous thromboembolic event with a number needed to harm of 770 to realize a major bleed.
Clinical risk factors for HA-MB have been explored and in a subset of patients from a large prospective randomized controlled trial assessing rivaroxaban and enoxaparin for the prevention of HA-VTE, five factors are predictive of major bleeding. Those five factors were active cancer, dual antiplatelet therapy, bronchiectasis/pulmonary cavitation, gastroduodenal ulcer, or bleeding within 3 months before randomization. Upon statistical exclusion of those patients, 35 days of treatment with rivaroxaban conferred overall benefit with a number needed to treat of 55 to 481 versus a number needed to harm from 455 to 1067.45 The ascertainment of these clinical factors does not lend itself to automation, making routine risk assessment at the time of discharge cumbersone and challenging. These observations highlight the inherent attractiveness of a risk score that is easily calculable from coded data residing in the EMR.
To our knowledge, ours represents the first 90-day HA-VTE and HA-MB risk score that is derived from laboratory tests that are ubiquitous in routine clinical care. Because EDT was not used in our study cohort, we were able to derive a risk score that identified patients at risk for 90-day HA-MB in the absence of post-discharge chemoprophylaxis. This is valuable because should these scores be validated, then those patients for which EDT would be considered too great a risk for bleeding may be identified. An especially attractive aspect of this work is that EMR programming of this risk assessment tool could likely occur regardless of an institution’s EMR. Most EMRs can integrate equations with threshold parameters such as those that the HA-VTE and HA-MB IMRS require.
Considerations for future research include the external validation of our proposed HA-VTE and HA-MB IMRS. The external validation in a retrospective cohort and also prospective validation of these scores is ideal before adoption into routine clinical care.46 To facilitate collaboration and external validation of our work, we publish the HA-VTE IMRS and HA-MB IMRS covariate quintile thresholds and the weighted scoring (Table 3). We formerly reported the comparative benefit of adding the IMRS to clinical VTE risk assessment models, the UTAH score and the Kucher score.28 Assessing whether combining the HA-VTE IMRS with the clinical risk assessment models to enhance predictiveness for 90-day HA-VTE may be performed.
Limitations of our work include that while derivation and validation encompassed many patients from five hospitals, all are part of an integrated 23 hospital health care network. Limitations inherent in using EMR interrogation to identify outcomes of thrombosis and bleeding events exist, although we have formerly published our ability to do so with a high degree of certainty21 using these same techniques.32, 33 We are unable to account for missing outcome data that might arise from patients seeking care outside of our EMR catchment; however, we believe that this occurs rarely given our integrated health care system. A criticism of our work may be that we ignored the influence of patient history (eg, prior VTE, cancer, paresis, thrombophilia) and that including this might enhance the performance of our proposed risk scores. However, we believe that the simplicity of automating calculation and presentation of a HA-VTE and HA-MB IMRS in the EMR could lead to standardized VTE risk assessment at discharge. Another criticism may be that the HA-VTE IMRS threshold that results in a HA-VTE rate ≥2% that we chose is not what others would have considered as actionable. For this reason, we provided additional threshold result estimates in Appendix S1. While our data were derived from a 23-hospital health care system, external validation beyond our integrated system is lacking and advised before clinical implementation might be entertained. However, if validated, these estimates could reliably provide risk assessment for virtually every discharging patient, and independent of robust EMR programming to interrogate for clinical history markers often only available at academic institutions.
In conclusion, we have derived and validated biomarker risk scores predictive for 90-day hospital-associated VTE and major bleeding. We hypothesize that should these scores be validated, then following programming into the EMR, a personalized risk for 90-day postdischarge HA-VTE and HA-MB may be ascertained and presented to the discharging physician at no additional cost. Taken together, medical patients for whom the net clinical benefit of EDT is favorable to reduce the burden of HA-VTE may be identified.
RELATIONSHIP DISCLOSURE
SCW, SMS, MF, JFL, ELW, GLS, JRB report nothing to disclose. BDH is an inventor of clinical decision tools licensed to CareCentra and Alluceo, is principal investigator of grants funded by Intermountain Healthcare’s Foundry innovation program, the Intermountain Research and Medical Foundation, CareCentra, Sysmex, GlaxoSmithKline plc, and AstraZeneca, and is a coinvestigator for a grant from the Patient-Centered Outcomes Research Institute.
ACKNOWLEDGMENTS
The authors would like to acknowledge the administrative support of Ms. Jana Johnson in the Intermountain Medical Center Department of Medicine.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept, design, drafting of manuscript: SCW, SMS, GLS, ELW, JRB, MF, and BDH. Manuscript critical review and refinement: SCW, GLS, SMS, JFL, ELW, JRB, MF, and BDH. Statistical analysis: ELW, SCW, BDH, and JFL.




