Phase III trial of 8% vaginal progesterone gel for luteal phase support in Japanese women undergoing in vitro fertilization and fresh embryo transfer cycles
Abstract
Aim
This study evaluated the efficacy and safety of vaginal progesterone gel that was administered daily for luteal phase support as part of in vitro fertilization/embryo transfer (IVF/ET) cycles in Japanese women.
Methods
This was a phase III, multicenter, open-label, single-arm trial in Japanese women undergoing IVF/ET, using the Japanese Society of Obstetrics and Gynecology 2009 registry as a historical control. The primary objective was to demonstrate the non-inferiority, with regard to the clinical pregnancy rate per ET, of vaginal progesterone gel that was administered once daily, compared with the historical standard value in IVF/ET cycles in Japan. The biochemical pregnancy (positive serum β-hCG pregnancy test but no clinical pregnancy) rate per ET also was investigated, as were the safety and tolerability of the vaginal progesterone gel.
Results
Of the 178 women who were enrolled, 123 underwent IVF/ET. The clinical pregnancy rate per ET was non-inferior in the prospective arm, compared with the historical population. The biochemical pregnancy rate per ET was 7.3%. The safety profile of the vaginal progesterone gel was as expected, with no new safety issue identified.
Conclusion
The vaginal progesterone gel was efficacious, with a safety profile as expected, in this study in Japanese women undergoing IVF/ET cycles.
1 Introduction
Exogenous luteal phase support is important for stimulated in vitro fertilization (IVF) cycles and a meta-analysis of luteal phase progesterone support has demonstrated that its use is associated with higher ongoing pregnancy and live birth rates, compared with placebo (odds ratio [OR] 1.77; 95% confidence interval [CI] 1.09-2.86).1 This meta-analysis also found that progesterone was well tolerated, with no increased risk of ovarian hyperstimulation syndrome (OHSS), compared with placebo.
Progesterone for luteal phase support is typically given from the day of oocyte retrieval up to weeks 7-10 of pregnancy and may be administered orally, vaginally (as a tablet, capsule, or gel), or intramuscularly. Each route of administration has different characteristics in terms of pharmacokinetics and potency, as well as adverse event (AE) profiles.2-4 As such, it is important that an effective and well-tolerated form is used to ensure both optimal outcomes and patient well-being. Oral progesterone requires the administration of high doses to reach appropriate serum concentrations, which can result in a number of side-effects, including sedation, drowsiness, and nausea.5, 6 Vaginal administration of progesterone delivers high local concentrations to the vagina and uterus with low peripheral serum concentrations, reducing the risk for systemic side-effects that can be observed with oral or intramuscular administration.3 There is, however, the inconvenience of vaginal discharge, and possibly irritation, following this route of administration. Intramuscular progesterone is typically prepared in oil, is rapidly absorbed following injection, and maintains a steady state for ≤72 hours.5-7 There is the possibility of injection site reactions, including pain, inflammation, and abscesses, with this route of administration, as well as rare complications, including a severe allergic reaction.5-7
Outside of Japan, vaginally administered progesterone is widely used as luteal phase support for IVF/embryo transfer (ET) cycles, whereas in Japan, until recently, there was no approved vaginal progesterone preparation for luteal phase support. Injected progesterone or in-house formulations of progesterone were therefore used. Since 2014, a vaginal tablet containing 100 mg of progesterone has been available in Japan for luteal phase support during assisted reproductive technology (ART) cycles.8 Previous studies, outside of Japan, that compared vaginal progesterone gel and vaginal progesterone tablets indicated that there were no substantial differences in outcomes with either formulation. Patient ease-of-use, satisfaction, and convenience were greater with the gel, compared with the tablets, resulting in a better overall impression of the gel formulation, compared with the tablets.9-13
Here, the results of a phase III trial that was conducted to evaluate the efficacy and safety of 8% vaginal progesterone gel that was administered daily for luteal phase support in IVF/ET cycles in Japanese women are presented. The data from this trial were compared with the historical standard values in Japan from the Japanese Society of Obstetrics and Gynecology (JSOG) 2009 registry.14
2 Materials and Methods
This was a phase III, multicenter, open-label, single-arm trial in Japanese women undergoing IVF/ET (ClinicalTrials.gov identifier: NCT01863680), using the JSOG 2009 registry14 as a historical control, that was designed in line with regulatory requirements. Women were enrolled at 10 centers in Japan and the trial was conducted between July 2013 and October 2014. The study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki, the International Conference on Harmonisation–Good Clinical Practice guidelines, and all applicable regulatory requirements, with all the participants providing written informed consent prior to entry into the trial.
2.1 Study participants
Healthy, premenopausal Japanese women (aged between 20 and 45 years, inclusive) with a history of infertility, in whom IVF/ET was indicated and who were to undergo controlled ovarian stimulation with a gonadotropin-releasing hormone (GnRH) analog (either an antagonist or an agonist) in combination with a follicle-stimulating hormone (FSH)-containing preparation, were enrolled in the trial if they met the following criteria: body mass index (BMI) between 17.0 and 25.0 kg/m2 (inclusive); a negative pregnancy test (urinary β-human chorionic gonadotropin [β-hCG]) prior to starting controlled ovarian stimulation; a normal cervical smear test result within 12 months prior to the date of informed consent; and no clinically significant abnormal findings in the screening hematology, biochemistry, and urinalysis parameters.
The main exclusion criteria included: a history of recurrent pregnancy loss, defined as three or more previous spontaneous abortions; a history of three or more consecutive canceled or failed (no clinical pregnancy) IVF/ET cycles; ovarian enlargement or a cyst of unknown etiology; a uterine myoma requiring treatment; a history of severe OHSS, classified according to the Japan Reproductive/Endocrine Working Group guidance;15 or a contraindication for pregnancy, controlled ovarian stimulation, or vaginal progesterone gel.
2.2 Study treatments and interventions
Vaginal progesterone gel (COL-1620 8%; Crinone® 8%, Merck KGaA, Darmstadt, Germany) was provided in a single-use, one-piece vaginal applicator that delivered 1.125 g of progesterone gel containing 90 mg of progesterone. The women were instructed to administer the vaginal progesterone gel once daily at approximately the same time each day, preferably in the morning.
The study was divided into four main periods (Fig. 1). Within 2 months of screening, conventional controlled ovarian stimulation was initiated, according to the procedures of the center, using a GnRH analog (agonist or antagonist) in combination with an FSH-containing preparation, followed by hCG administration prior to oocyte pick-up. The vaginal progesterone gel was initiated on the day of oocyte pick-up and the fresh ET was performed 2-6 days after this (defined as day 1). The women then were followed-up for a maximum of 10 weeks.
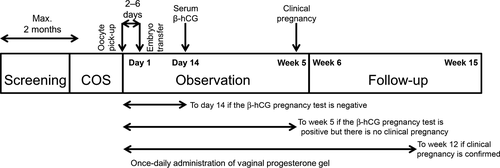
Administration of the vaginal progesterone gel was discontinued if the serum β-hCG pregnancy test 14 days after ET was negative or if a confirmed miscarriage occurred before a clinical pregnancy was confirmed at week 5. If the vaginal progesterone gel was discontinued for these reasons, a safety follow-up visit was conducted 3 weeks after the last dose. If a clinical pregnancy was confirmed, the vaginal progesterone gel was continued up to week 12 or until confirmation of a miscarriage or an extra-uterine pregnancy. A final safety visit was performed at week 15, or 3 weeks from the last use of the vaginal progesterone gel, whichever was earlier. The women were expected to be in the trial for 3-8 months, depending on the pregnancy outcome and the duration of the screening period.
2.3 Study objectives and endpoints
The primary objective of the trial was to demonstrate the non-inferiority of the clinical pregnancy rate per ET with the vaginal progesterone gel administered once daily, compared with the historical standard value in IVF/ET cycles in Japan (JSOG 2009 registry data).14 Clinical pregnancy was defined as the presence of a fetal sac on transvaginal ultrasound during the visit 5 weeks after the ET or the presence of an extra-uterine pregnancy (confirmed during surgery when no gestational sac was identified by transvaginal ultrasound following two positive serum β-hCG results, 1 week apart from week 5).
The other objectives of the trial were to assess the biochemical pregnancy rate per ET, as well as the safety and tolerability of the vaginal progesterone gel being administered once daily. Biochemical pregnancy was defined as any miscarriage without evidence of a fetal sac on transvaginal ultrasound during the visit 5 weeks after the ET, but with a positive serum β-hCG pregnancy test (serum β-hCG>10 mIU/mL) at the visit 14±3 days after the ET. For participants who had experienced a miscarriage between the visits 14±3 days and 5 weeks after the ET, biochemical pregnancy was defined as a positive serum β-hCG pregnancy test at the visit 14±3 days after the ET, with no data recorded at the visit 5 weeks after the ET on transvaginal ultrasound, no fetal hearts recorded on transvaginal ultrasound at the final safety visit, and with no occurrence of ectopic pregnancy recorded in an unscheduled transvaginal ultrasound.
Safety, including the incidence and severity of AEs and physical examination findings such as vital signs and laboratory tests, was also assessed. The AEs were classified by their severity and the causal relationship to the study treatment. AEs with an onset date occurring on or after the administration of the vaginal progesterone gel were defined as treatment-emergent AEs (TEAEs).
2.4 Statistical analysis
Based on the reference clinical pregnancy rate per ET of 24.3% (JSOG 2009 registry)14 and a 10% non-inferiority margin, overall, 117 participants were planned to be enrolled to demonstrate that the lower limit of the 95% two-sided CI (based on the Clopper–Pearson approach) of the observed rate was ≥14.3%, with a power of at least 80%. Allowing for a drop-out rate of 10%, an estimated total of 130 participants were planned to be enrolled (ie to start controlled ovarian stimulation following successful screening).
The primary efficacy endpoint of clinical pregnancy rate per ET was assessed in both the intention-to-treat (ITT) and per-protocol (PP) populations. The ITT population was defined as all the participants who underwent IVF/ET. The PP population included all participants who were treated according to the protocol, were compliant with all the entry criteria, did not have any major protocol violations that were likely to affect the efficacy of the treatment, and were adequately compliant with the vaginal progesterone gel treatment. The exact (Clopper–Pearson) two-sided 95% CI of the primary endpoint was calculated and if the lower bound of the 95% CI minus 24.3% (historical standard value) was above −10% in both analysis sets then non-inferiority was assumed.
The secondary efficacy analysis of the biochemical pregnancy rate per ET was calculated as a percentage, with 95% CI values calculated using an exact (Clopper–Pearson) method. Safety was assessed in the safety population, which included all the participants who received at least one dose of the vaginal progesterone gel.
3 Results
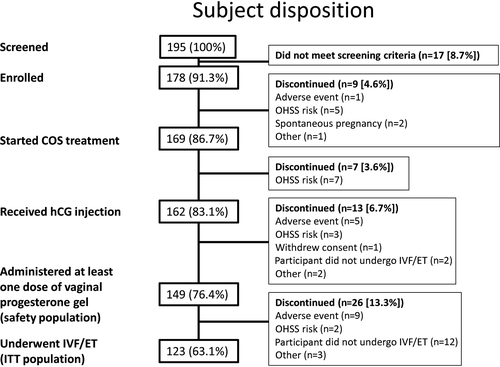
A total of 195 women were screened for inclusion in this trial and 178 were enrolled and entered the screening period (Fig. 2). Of these women, 169 started controlled ovarian stimulation and 162 received an hCG injection to trigger ovulation, with all the women who discontinued before the hCG injection doing so because of risk of OHSS. Following the hCG injection, 149 women received at least one dose of the vaginal progesterone gel, representing the safety population, and 123 women underwent IVF/ET, representing the ITT population. The PP population included 115 participants and, in total, 121 women completed the trial. Overall, the most frequent reasons for discontinuation were risk of OHSS (n=17), AEs (n=17), and the participant not undergoing IVF/ET (n=14).
3.1 Baseline characteristics and demographics
In the ITT population, the mean (standard deviation [SD]) age of the women who received the vaginal progesterone gel was 34.5 (3.8) years, the mean (SD) weight was 52.4 (5.1) kg, and the mean (SD) BMI was 20.6 (2.0) kg/m2 (Table 1). The majority of the women had primary infertility (69.1%), with 46.3% of the women having female-only infertility and 44.7% having unexplained infertility. The most common cause of female infertility was a tubal factor. The data were similar for the PP population (Table 1). The baseline demographics and patient characteristics were not available for the historical control data.
| Characteristic | ITT Population (n=123) | PP population (n=115) |
|---|---|---|
| Age, years (mean [SD]) | 34.5 (3.8) | 34.4 (3.8) |
| Weight, kg (mean [SD]) | 52.4 (5.1) | 52.2 (5.0) |
| BMI, kg/m2 (mean [SD]) | 20.6 (2.0) | 20.5 (1.9) |
| Age at menarche, years (mean [SD]) | 12.4 (1.3) | 12.4 (1.3) |
| Last menses type (N [%]) | ||
| Induced | 48 (39.0) | 46 (40.0) |
| Spontaneous | 75 (61.0) | 69 (60.0) |
| Infertility (N [%]) | ||
| Primary | 85 (69.1) | 80 (69.6) |
| Secondary | 38 (30.9) | 35 (30.4) |
| Duration of infertility, months (mean [SD]) | 40.7 (27.4) | 39.4 (27.0) |
| Type of infertility (N [%]) | ||
| Female and male | 9 (7.3) | 6 (5.2) |
| Female only | 57 (46.3) | 54 (47.0) |
| Male only | 2 (1.6) | 2 (1.7) |
| Unexplained | 55 (44.7) | 53 (46.1) |
| Causes of female infertility (N [%]) | ||
| Tubal factor | 39 (31.7) | 35 (30.4) |
| Endometriosis | 6 (4.9) | 6 (5.2) |
| Ovulatory dysfunction | 6 (4.9) | 6 (5.2) |
| Other | 19 (15.4) | 17 (14.8) |
- BMI, body mass index; ITT, intention-to-treat; PP, per-protocol; SD, standard deviation.
3.2 Efficacy evaluation
In the ITT population, the clinical pregnancy rate per ET (95% CI) was 28.5% (20.7%-37.3%), compared with 24.3% in the historical control population (Table 2).14 The lower bound of the 95% CI minus 24.3% for the prospective arm (−3.6%) was above the predefined −10% limit for non-inferiority to the historical control. In the PP population the clinical pregnancy rate per ET was 27.8% (19.9%-37.0%) and the lower bound of the 95% CI was also above the predefined −10% limit for non-inferiority to the historical control (Table 2). The biochemical pregnancy rate per ET (95% CI) was 7.3% (3.4%-13.4%) in the ITT population and 7.8% (3.6%-14.3%) in the PP population.
| Variable | ITT Population (n=123) | PP Population (n=115) |
|---|---|---|
| Total number of embryos transferred | 141 | 133 |
| Number of embryos transferred per participant (median [range]) | 1 (1–2) | 1 (1–2) |
| Number of participants who were clinically pregnant (N [%]) | 35 (28.5) | 32 (27.8) |
| Clinical pregnancy rate per embryo transfer, % (rate [95% CI]) | 28.5 (20.7–37.3) | 27.8 (19.9–37.0) |
| Clinical pregnancy rate per embryo transfer (lower limit of 95% CI minus 24.3% [historical standard value14]), % | –3.6 | –4.4 |
- CI, confidence interval; ITT, intention-to-treat; PP, per-protocol.
3.3 Safety evaluation
The safety profile of the vaginal progesterone gel was as expected during this 12 week trial (Table 3). In the safety population, the most frequently reported TEAE was OHSS, which was reported by 32 (21.5%) participants, with mild, moderate, and severe OHSS reported by 24 (16.1%), four (2.7%), and four (2.7%) participants, respectively. None of the cases of OHSS reported was assessed as being related to the study drug.
| Number of Participants,N (%) | |
|---|---|
| At least one TEAE | 92 (61.7) |
| At least one TEAE related to the vaginal progesterone gel | 15 (10.1) |
| At least one serious TEAE | 5 (3.4) |
| At least one serious TEAE related to the vaginal progesterone gel | 1 (0.7) |
| At least one TEAE leading to trial termination | 10 (6.7) |
| At least one TEAE related to the vaginal progesterone gel, leading to dose modification or discontinuation of the vaginal progesterone gel | 11 (7.4) |
| At least one TEAE leading to death | 0 (0.0) |
- TEAE, treatment emergent adverse event.
TEAEs related to the vaginal progesterone gel were reported by 15 (10.1%) participants, with one (0.7%) participant reporting a serious TEAE. The most common TEAEs related to the vaginal progesterone gel were vaginal hemorrhage (2.7%), the presence of a foreign body (1.3%), and abdominal pain (1.3%), and the majority of TEAEs were mild in intensity. The only serious TEAE related to vaginal progesterone gel use was threatened abortion. This occurred after treatment with the vaginal progesterone gel was complete, 6 days after the final administration and 92 days after the first administration.
4 Discussion
This open-label, single-arm trial of vaginal progesterone gel demonstrated that the clinical pregnancy rate per ET was non-inferior to that observed in the historical registry data in Japan (28.5% vs 24.3%, respectively). Furthermore, a clinical pregnancy rate of 22.7% was observed in more recent (2013) Japanese registry data.16 The higher clinical pregnancy rate that was observed in this study, compared with the registry data, is probably related to the younger mean age of the women who were enrolled in the study, compared with the registry associated with the historical data. Nonetheless, the clinical pregnancy rate that was observed in the present trial was similar to that observed in a clinical trial in Japan of a 100 mg vaginal progesterone tablet (22.2%),17 as well as to that of a trial comparing an oral progestogen (chlormadinone acetate) and intramuscular progesterone for luteal phase support in Japan (20% and 25%, respectively).18 Moreover, these data are in line with the results of a meta-analysis by Polyzos et al,11 which demonstrated that there is no difference in clinical pregnancy rates following luteal phase support with vaginal progesterone gel compared with all other vaginal progesterone forms. The observed clinical pregnancy rate in the current study is also comparable with that observed in European studies of vaginal progesterone gel in women undergoing IVF/intracytoplasmic sperm injection cycles with oocyte retrieval (32.3%-32.9%).9, 19 The biochemical pregnancy rate in the present trial (7.3%) was low and similar to that reported for the trial comparing an oral progestogen and intramuscular progesterone for luteal phase support in Japan (5% and 10%, respectively).18
The safety profile of the vaginal progesterone gel was as expected during the trial, with no new safety issues identified. The most frequently reported TEAE was OHSS, with mild-to-severe OHSS reported by 21.5% of the safety population. None of the cases of OHSS were assessed as being related to the study drug by the investigator. This rate is similar to that observed in a trial of a vaginal progesterone tablet containing 100 mg of progesterone that was given either twice or three-times daily, during which OHSS was observed in 20.4% of the participants.17 Furthermore, the incidence of moderate-to-severe OHSS (5.4%) was within the range that is typically observed during IVF cycles (3.1%-8.0%).20 In addition, it should be noted that the underlying etiology of OHSS is gonadotropin rather than progesterone exposure, suggesting that its occurrence was not related to the vaginal progesterone gel.21
Previous studies, outside of Japan, comparing vaginal progesterone gel and vaginal progesterone tablets have indicated that, owing to the treatment regimen (once daily with vaginal gel, compared with twice or three-times daily with a vaginal tablet), the gel is considered to be more convenient to use.9-13 Furthermore, when treatment satisfaction with different progesterone formulations and routes of administration has been compared, satisfaction is significantly greater with the gel formulation, compared with an intramuscular injection (scale of 1-5, with 5 being the most satisfied: 4.4±0.9 vs 2.8±1.2, respectively; OR 13.7, P<.01),22, 23 and the vaginal gel also was reported to be more convenient than a vaginal tablet (scale of 1-10, with 1 being “very convenient” and 10 being “very inconvenient”: 2.9 [2.7-3.0] vs 4.8 [4.7-5.0], respectively; P<.0001).9, 23 In addition, in clinical trials, progesterone as a vaginal gel has been shown to be significantly more acceptable to patients, based on the ease of administration, convenience, and patient preference (P<.05 in all categories), compared with vaginal progesterone capsules.10, 13
The potential limitations of this study include its open-label nature and the fact that it compared outcomes against historical data, which might not reflect any recent changes in clinical care. However, it should be noted that the clinical pregnancy rate per ET was similar in both the current trial and the historical data to that observed in a recent clinical trial of luteal phase support in Japan with vaginal progesterone tablets.14, 17 This suggests that the historical data are still a valid comparator.
In conclusion, this single-arm, open-label, phase III trial demonstrated the efficacy of vaginal progesterone gel as luteal phase support for IVF/ET cycles in Japanese women, with the clinical pregnancy rate per ET observed to be non-inferior to the historical standard value from the JSOG registry. Furthermore, the vaginal progesterone gel demonstrated the expected safety profile, with no new clinical safety issues identified. The once-per-day application makes progesterone gel a highly convenient option for luteal phase support in women undergoing ART.
Acknowledgements
The trial was sponsored by Merck KGaA, Darmstadt, Germany. The authors would like to thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centers and at Merck KGaA, Darmstadt, Germany. The study investigators were: Yoshiaki Sato, Hideyuki Ikenaga, Kohzo Aisaka, Koji Yoshida, Hirotsugu Oku, Yuji Abe, Masanori Yamashita, and Naomi Yano. Medical writing assistance was provided by Alexander Jones, inScience Communications, London, UK, and was funded by Merck KGaA, Darmstadt, Germany.
Disclosure
Conflicts of interest: Daniela Rogoff is a former employee of EMD Serono Research & Development Institute, Inc., a business of Merck KGaA, Darmstadt, Germany. Shin Shimizu is an employee of Merck Serono Company, Ltd, an affiliate of Merck KGaA, Darmstadt, Germany. Shoji Kokeguchi, Naoki Hayashi and Osamu Ishihara declare that they have no relevant conflict of interest. Human rights statement and informed consent: All the procedures that were followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients to be included in the study. Animal rights: This article does not contain any studies with animals performed by any of the authors.




