Functionalized enamines - Part XXIV†. Reactions of β-amino-α,β-unsaturated esters with carbenes
H. Bieräugel
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Search for more papers by this authorJ.M. Akkerman
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Search for more papers by this authorJ. C. Lapierre Armande
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Taken in part from the forthcoming doctorate thesis of J. C. Lapierre Armande.
Search for more papers by this authorCorresponding Author
U.K. Pandit
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The NetherlandsSearch for more papers by this authorH. Bieräugel
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Search for more papers by this authorJ.M. Akkerman
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Search for more papers by this authorJ. C. Lapierre Armande
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Taken in part from the forthcoming doctorate thesis of J. C. Lapierre Armande.
Search for more papers by this authorCorresponding Author
U.K. Pandit
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The Netherlands
Organic Chemistry Laboratory, University of Amsterdam, Nieuwe Achtergracht 129, Amsterdam, The NetherlandsSearch for more papers by this author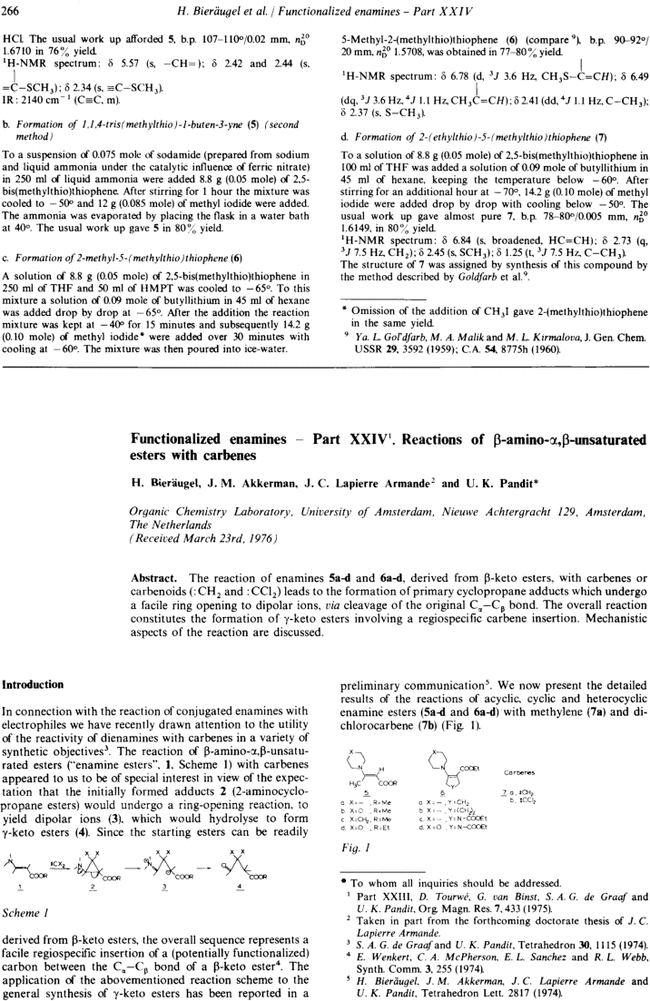
References
- 3 S.A.G. de Graaf and U.K. Pandit, Tetrahedron 30, 1115 (1974).
- 4
E. Wenkert,
C.A. McPherson,
E.L. Sanchez and
R.L. Webb,
Synth. Comm.
3,
255
(1974).
10.1080/00397917308065912 Google Scholar
- 5 H. Bieräugel, J.M. Akkerman, J. C. Lapierre Armande and U.K. Pandit, Tetrahedron Lett. 2817 (1974).
- 6 S. Sawada and Y. Inouye, Bull. Chem. Soc. Jap. 42, 2669 (1969).
- 7a W.M. Wagner, Proc. Chem. Soc. 229 (1959). b W.M. Wagner, H. Kloosterziel and S. v. d. Ven, Recl. Trav. Chim. Pays-Bas 80, 740 (1961).
- 8 M. Makosza and M. Wawrzywiewiez, Tetrahedron Lett. 4659 (1969).
- 9 E. Wenkert, R.A. Müller, E.J. Reardon Jr., S.S. Sathe, D.J. Scharf and G. Tosi, J. Amer. Chem. Soc. 92, 7428 (1970). * It is pertinent to add that in one experiment which was performed on a larger scale, we have been able to isolate compound 26, as a crystalline, stable compound. In the NMR spectrum, it can be seen from the shape of the morpholine protons, that there is restricted rotation about the C(5)-N bond.
- 10a H.C. Brown, J. Chem. Soc. 1248 (1956). b G.F. Hennion and F.X. Quinn, J. Org. Chem. 35, 3054 (1970).




