A case of pulmonary arterial hypertension with V/Q SPECT/CT that showed localized uptake of 99mTc just below the pleura and a unique distribution
Abstract
Pulmonary hypertension (PH) is a life-threatening disorder, which originates from various aetiologies. Ventilation–perfusion (V/Q) scanning is commonly used to evaluate the differential diagnosis of PH. Meanwhile, previous studies have shown that single-photon emission computed tomography (SPECT)/CT imaging can provide a more detailed analysis for the assessment of pulmonary blood flow. However, there is insufficient evidence supporting the merits of V/Q SPECT/CT image data in detecting pulmonary vascular disease. Here, we report a case of pulmonary arterial hypertension with localized accumulation and peculiar distribution just below the pleura on V/Q SPECT/CT. Our finding is unique, and it suggests that V/Q SPECT/CT image data might be useful to detect blood flow not only in cases of pulmonary embolism, but also in the more commonly encountered PH.
INTRODUCTION
Pulmonary hypertension (PH) is a progressive, fatal disease characterized by an increase in mean pulmonary artery pressure (PAP) and pulmonary vascular resistance. PH originates from diverse aetiologies and is categorized into five groups.1
Various imaging tests are recommended for the evaluation of PH, and they can be used for three main purposes: (1) suspecting the presence of PH, (2) determining the aetiology of the disease and (3) evaluating the prognosis and response to therapy.2
Considering the aetiology of PH, ventilation–perfusion (V/Q) scanning is often useful for screening patients with chronic thromboembolic PH (CTEPH) and distinguishing it from others.3 According to the 6th World Symposium on Pulmonary Hypertension, it is recommended that V/Q scanning should be performed in patients with high or intermediate risk of PH.4
V/Q single-photon emission computed tomography (SPECT) lung scanning could make it easier to avoid segmental overlap and define the size and location of perfusion defects in each segment.5 Moreover, dual-modality techniques with hybrid SPECT/CT pulmonary imaging can improve the specificity of V/Q SPECT by identifying lung disease in patients with perfusion abnormalities.4 However, there is insufficient evidence for the detection of pulmonary vascular disease using SPECT/CT image data.
Here, we report a case of PH with unique SPECT/CT imaging, showing localized subpleural perfusion.
CASE REPORT
A 46-year-old woman with chest pain was diagnosed with a right spontaneous pneumothorax at a former hospital. She was naturally healthy; however, 3 years before this presentation, she developed a right pneumothorax for the first time. Afterwards, she experienced recurrence during menstruation, several times. Wedge resectomy of the right upper lung was performed under general anaesthesia. Microscopically, endometrial stromal cells were observed in the visceral pleura, which led to the final diagnosis of catamenial pneumothorax. After the operation, the cardiac silhouette of the chest radiograph gradually enlarged. As ultrasonographic cardiography showed the trans-tricuspid pressure gradient as 75 mmHg and right ventricular systolic pressure as 88 mmHg, she was referred to our hospital on suspicion of PH. The patient had a history of endometriosis. She did not smoke tobacco or drink alcohol. There was no family history of pulmonary or cardiac disease.
The clinical cardiac functional status was New York Heart Association (NYHA) II. There was an accentuated pulmonic component of second heart sounds.
The plasma level of brain natriuretic peptide was 87.9 pg/ml (reference range, <18.4). Results of blood count and coagulation tests were normal. Other test results are shown in Table 1.
| Complete blood count | |
| WBC | 4400/μl |
| Neutrophil | 58.7% |
| Eosinophil | 0.5% |
| Basophil | 1.6% |
| Lymphocyte | 32.9% |
| Monocyte | 6.3% |
| RBC | 509 × 104/μl |
| Hb | 14.0 g/dl |
| Hematocrit | 42.6% |
| Plt | 29.7 × 104/μl |
| Coagulation | |
| Prothrombin time | 10.7 s |
| International normalized ratio of prothrombin time | 1.01 |
| Activated partial thromboplastion time | 33.4 s |
| d-dimer | 0.8 μg/ml |
| Biochemistry | |
| Total protein | 7.9 g/dl |
| Na | 139 mEq/L |
| K | 4.1 mEq/L |
| Cl | 108 mEq/L |
| Urea nitrogen | 11 mg/dl |
| Cre | 0.7 mg/dl |
| Uric acid | 4.3 mg/dl |
| T-Bil | 0.8 mg/dl |
| Aspartate aminotransferase | 20 IU/L |
| Alanine aminotransferase | 9 IU/L |
| Alkaline phosphatase | 173 IU/L |
| γ-Glutamyl transpeptidase | 13 IU/L |
| LDH | 217 IU/L |
| Creatinine kinase | 68 IU/L |
| CRP | 0.01 mg/dl |
| Endocrine | |
| FT4 | 1.05 ng/dl |
| Thyroid Stimulating Hormone | 1.97 μU/ml |
| BNP | 87.9 pg/ml |
| Immune system | |
| Antinuclear Antibody | ×80 |
| Anti-dsDNA antibody | <2.0 U/ml |
| Anti-SS-A antibody | 0.7 U/ml |
| Anti-SS-B antibody | 1.0 U/ml |
| Anti-ribonucleoprotein antibody | 0.7 U/ml |
| Anti-centromere antibody | 0.6 U/ml |
| Anti-Jo-1 antibody | (−) |
| Anti-Scl 70 antibody | (−) |
| Myeloperoxidase-anti-neutrophil cytoplasmic antibody | <1.0 EU |
| Proteinase-3-anti-neutrophil cytoplasmic antibody | <1.0 EU |
- Abbreviations: BNP, brain natriuretic peptide; CRP, C-reactive protein; Hb, haemoglobin; LDH, lactate dehydrogenase; Plt, platelet; RBC, red blood cell; WBC, White blood cell.
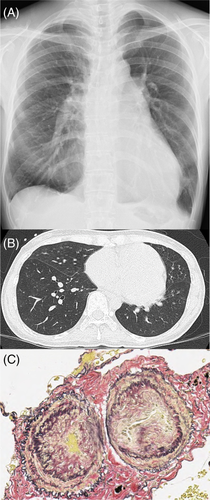
A frontal chest radiograph showed enlargement of the left and right pulmonary artery diameter and right ventricle (Figure 1A). Axial CT scans of the chest showed no notable findings in the lung parenchyma (Figure 1B). Histopathologically, the operated lung showed stenotic pulmonary arterioles with intimal thickening and medial hypertrophy, which is classified as Grade 3 in Heath–Edwards classification (Figure 1C).
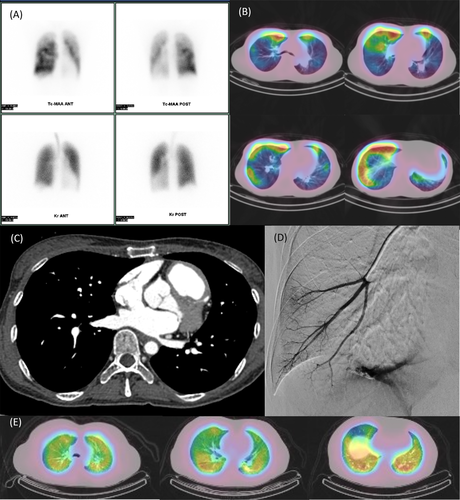
Perfusion lung scan was performed with technetium 99m (99mTc)-labelled macroaggregated albumin (MAA) to detect CTEPH, and it showed a mottled pattern in the planar view (Figure 2A). Ventilation scan was performed with krypton 81m, which revealed no notable findings. At the same time, SPECT/CT imaging was performed. Interestingly, the uptake of 99mTc was seen as localized accumulation just below the subpleura at the bilateral lungs (Figure 2B).
Right heart catheterization was performed, and it showed step-down of SaO2 (arterial oxygen saturation; superior vena cava 63.4% and right atrium 74.4%) and high PAP of 97/26 (51) mmHg. Pulmonary capillary wedge pressure was normal (6 mmHg). Supplemental results are shown in Table 2.
| On admission | Four months later | ||
|---|---|---|---|
| Room air | Room air | 100% O₂ | |
| PAP, mmHg | 97/26 (51) | 68/20 (38) | 62/11 (32) |
| Mean RAP, mmHg | 6 | ||
| Mean PCWP, mmHg | 6 | 5 | 5 |
| AoP, mmHg | 117/73 (83) | 119/69 (87) | |
| Qp, L/min | 8.22 | 8.40 | |
| Qs, L/min | 5.78 | 5.34 | |
| Qp/Qs | 1.423 | 1.572 | |
| PVR, Wood units | 8.44 | 4.01 | 3.21 |
| CO, L/min | 5.33 | 8.22 | 8.4 |
| Shunt ratio, % | 34.48 | 42.00 | |
| Oxygen saturation, % | |||
| Superior vena cava | 63.4 | ||
| Inferior vena cava | 54.2 | ||
| Right atrium | 74.4 | ||
| Right ventricle | 72.7 | ||
| Pulmonary artery | 72.6 | ||
- Abbreviations: AoP, aorta pressure; CO, cardiac output; Qp, pulmonary blood flow; Qs, systemic blood flow; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrium pressure.
Later, the patient underwent CT angiography, reporting a 16-mm defect hole in the atrial septum (Figure 2C). The shunt hole was relatively small, but we diagnosed that it led to pulmonary arterial hypertension (PAH) with an atrial septal defect (ASD).
Retrospectively, we performed a pulmonary wedge angiography, and hypoplasia of the right pulmonary artery was observed (Figure 2D).
We consulted a cardiac surgeon to look for any indications for repair of the shunt defect. Ahead of this, we had stared to administer vasodilators.
Four months later, we performed right heart catheterization again with macitentan 10 mg/day and riociguat 1.5 mg/day. Although the PAP went up to 68/20 (38) mmHg, the Qp/Qs (pulmonary blood flow/systemic blood flow) ratio by 100% O₂ inhalation was 1.572 (Table 2). Considering the value of the Qp/Qs ratio, Eisenmenger's syndrome was not detected. Therefore, we decided to continue with medical therapy, after which we would consider closure of the shunt hole using a catheter.
DISCUSSION
We encountered a case of PH which had unique SPECT/CT imaging findings, showing localized subpleural perfusion. To the best of our knowledge, this is the first report of such SPECT/CT imaging for screening for pulmonary vascular diseases. There has been a report of single-centre retrospective observational study evaluating V/Q SPECT findings in PAH patients. However, this pattern is completely different from that in our case.6 Furthermore, there are no other typical diseases that manifest with these findings.7 For reference, we present the SPECT/CT images of a typical case of idiopathic PAH patient without ASD, endometriosis and the history of pneumothorax or thoracic surgery (Figure 2E).
First, lobectomy might have affected SPECT findings: The operation for catamenial pneumothorax was performed only on the right side, while the abnormal accumulation on SPECT was bilateral; hence, this finding was contradictory.
Second, the possibility of presence of abnormal blood vessels in the chest wall caused by pre-existing endometriosis might have affected SPECT findings: Blood flow of abnormal blood vessels due to endometriosis is usually supplied from the systemic circulation in the chest wall to the subpleural lung parenchyma. Therefore, it might not be a cause of increased accumulation of 99mTc-MAA. Furthermore, we might consider the possibility of presence of abnormal blood vessels due to other factors. However, contrast-enhanced CT could detect obvious chest wall abnormalities or collateral circulation from the systemic circulation.
Third, due to morphological developmental abnormalities associated with ASD, peripheral pulmonary circulation might have affected SPECT findings: The clinical course in this case showed that abnormal development of pulmonary arteries existed, which is consistent with the wedge angiography image and the current blood flow SPECT/CT findings. It might be suggested that SPECT/CT image or the pulmonary wedge angiography would be helpful in determining effectiveness of treatment with vasodilators. However, SPECT/CT image was difficult to evaluate immediately due to various circumstances, which might be a limitation to this report.
The planar V/Q lung scanning, a two-dimensional technique, has been commonly performed in many institutions. However, it has limitations, especially related to the overlap of anatomic segments.5 On the other hand, SPECT is routinely used in the field of nuclear medicine because of its ability to provide images in three dimensions. SPECT has higher sensitivity compared with only planar V/Q lung imaging, and its outcomes have confirmed a high negative predictive value regarding the exclusion of pulmonary embolism (PE).4 There are various diseases other than PE for which lung SPECT/CT is useful, for example, predicting post-operative lung function after lung resection in patients with lung cancer, modifying radiotherapy fields to minimize radiation exposure to functioning lungs, demonstrating regional changes of V/Q in asthma.5, 7
There are no reports with SPECT/CT image of accumulation just below the pleura so far. Further reports on SPECT/CT imaging findings in cases with similar findings are awaited.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Yuri Suzuki and Ayumi Sekine wrote the manuscript. Yuri Suzuki, Ayumi Sekine, Akira Nishiyama, Toshihiko Sugiura, Nobuhiro Tanabe, Yuri Isaka, Yaeko Hashimoto, Tadasu Okaya, Ayaka Kuriyama, Jun Nagata, Ayako Shigeta and Seiichiro Sakao were involved in the acquisition and interpretation of the data. Koichiro Tatsumi and Takuji Suzuki provided expertise and feedback. All authors drafted the article, revised it critically for important intellectual content and approved the final version to be submitted.
ETHICS STATEMENT
Appropriate written informed consent was obtained for publication of this case report and accompanying images.




