Successful Tepotinib Treatment in MET Exon 14 Skipping Mutation-Positive Lung Adenocarcinoma With Idiopathic Pulmonary Fibrosis Through Nintedanib Combination
Funding: The authors received no specific funding for this work.
Yoshiki Kato and Takunori Ogawa contributed equally to this work.
ABSTRACT
The mesenchymal-epithelial transition exon 14 (METex14) skipping mutation is a driver of non-small cell lung cancer (NSCLC). MET tyrosine kinase inhibitors (TKIs), such as tepotinib, are effective against METex14 skipping-positive NSCLC but carry a risk of acute exacerbation of interstitial lung disease (AE-ILD) in patients with idiopathic pulmonary fibrosis (IPF). Here, we present a case of a 54-year-old man with IPF and METex14 skipping-positive lung adenocarcinoma. Following first-line platinum-based chemotherapy, disease control was not achieved for either lung cancer or IPF. Therefore, the patient underwent second-line treatment with tepotinib and the antifibrotic agent nintedanib. This combination effectively controlled both lung cancer and IPF, resulting in a partial response without AE-ILD. Our case suggests that nintedanib treatment may offer a safe and effective option for progressive IPF during MET-TKI therapy, presenting a novel therapeutic approach for patients with lung cancer complicated by IPF.
1 Introduction
The mesenchymal–epithelial transition exon 14 (METex14) skipping mutation is an oncogenic driver in non-small-cell lung cancer (NSCLC). MET tyrosine kinase inhibitors (MET-TKIs), such as tepotinib, show significant efficacy in METex14 skipping-positive NSCLC and are now part of standard treatment [1]. However, their use in patients with interstitial lung disease (ILD), including idiopathic pulmonary fibrosis (IPF), raises concerns due to the potential risk of exacerbating pulmonary complications.
Nintedanib, an approved antifibrotic for IPF, has been shown to slow forced vital capacity (FVC) decline and reduce acute exacerbation risk [2]. However, the safety and efficacy of nintedanib in the treatment of IPF during MET-TKI therapy remain unclear.
We report the case of an IPF patient diagnosed with METex14 skipping-positive lung adenocarcinoma. Using a strategic combination of nintedanib and tepotinib, we successfully managed the patient's treatment without AE-ILD.
2 Case Report
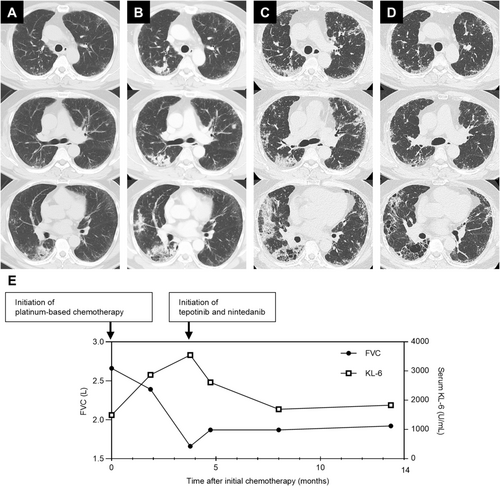
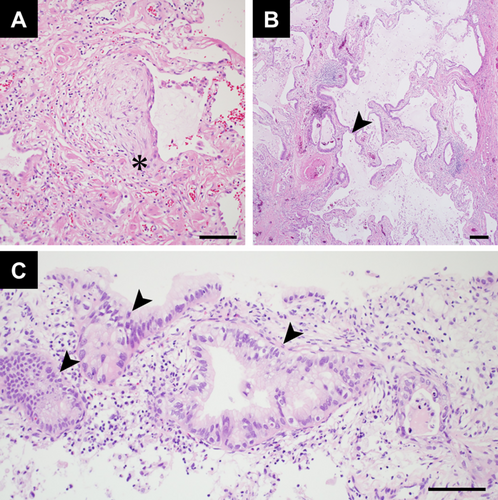
A 54-year-old Japanese man with a 25-pack-year smoking history and no prior history of respiratory disease presented with a three-month history of persistent cough. Computed tomography (CT) revealed a usual interstitial pneumonia (UIP) pattern, characterised by basal and peripheral reticular opacities, honeycombing, and traction bronchiolectasis (Figure 1A). CT also showed consolidations in the right lower lobe, raising suspicion of bacterial pneumonia. Despite antibiotic treatment, the consolidations did not improve. Video-assisted thoracoscopic surgery (VATS) was performed to evaluate the pulmonary lesions in the right lower lobe. Pathological findings from VATS showed a UIP pattern with honeycomb changes and fibroblast foci (Figure 2A,B), consistent with IPF, based on imaging and clinical history. However, the cause of the multiple consolidations remained unclear. As the consolidations progressed (Figure 1B), a CT-guided biopsy was performed on the right lower lobe lesion. Biopsy revealed invasive mucinous adenocarcinoma (Figure 2C). The consolidations in both lungs were interpreted as manifestations of this adenocarcinoma, resulting in a diagnosis of stage IVA lung adenocarcinoma with bilateral pulmonary metastases. Molecular biomarker testing using next-generation sequencing identified a METex14 skipping mutation, and programmed death ligand expression was negative. Pulmonary function testing showed restrictive ventilatory impairment. Serial CT imaging demonstrated gradual progression of IPF (Figure 1B). Therefore, MET-TKI was considered inappropriate as first-line chemotherapy due to high AE-ILD risk. The patient was initially treated with carboplatin, pemetrexed, and bevacizumab. After four cycles of chemotherapy, CT revealed progression of both invasive mucinous adenocarcinoma and reticular opacities associated with IPF (Figure 1C), along with further decline in lung function and increased serum Krebs von den Lungen-6 (KL-6) levels (Figure 1E). Given the risk of AE-ILD but aiming for long-term survival, tepotinib, a MET-TKI targeting the METex14 skipping mutation, was selected as second-line therapy. Nintedanib (150 mg twice daily) was started to prevent worsening of restrictive ventilatory impairment and AE-ILD, followed by tepotinib (250 mg/day) 1 week later. After initiating the combination, CT scan showed a significant reduction in bilateral pulmonary infiltrates consistent with lung adenocarcinoma (Figure 1D). Pulmonary function tests showed no decline in FVC after nintedanib administration, accompanied by a reduction in serum KL-6 levels (Figure 1E). No adverse events were observed with the tepotinib-nintedanib combination, except for mild diarrhoea. At 9 months, the patient maintained a partial response without AE-ILD and continued the same treatment.
3 Discussion
This case presents a rare approach to managing METex14 skipping-positive lung adenocarcinoma in a patient with IPF.
In this case, pulmonary function, which had been gradually declining, showed slight improvement after initiating nintedanib and remained stable. This suggests that nintedanib may have helped suppress the decline in pulmonary function associated with interstitial lung disease. Alternatively, the decline in lung function could have been caused by invasive mucinous adenocarcinoma, and its control with tepotinib may have contributed to the stabilisation of pulmonary function. Nevertheless, the stabilisation of subpleural reticulations on CT imaging further supports the potential efficacy of nintedanib in this case.
Most patients with chemotherapy-induced AE-ILD experience a decline in performance status, which hinders continued treatment. AE-ILD has been reported in patients treated with MET-TKIs, including tepotinib [3]. The incidence of ILD associated with tepotinib is reported to range from 3.8% to 10.5%, with a notably higher tendency observed among Japanese patients [1, 4]. A randomised study evaluating the preventive effects of nintedanib on AE-ILD induced by cytotoxic chemotherapy did not show a clear preventive effect on AE-ILD onset [5]. However, its preventive effects on TKI-induced AE-ILD remain unclear. In this case, it remains uncertain whether the absence of AE-ILD during tepotinib treatment was due to the preventive effect of nintedanib. Nonetheless, despite disease progression after platinum-based chemotherapy with bevacizumab, combining the MET inhibitor tepotinib and the antifibrotic agent nintedanib successfully controlled both lung adenocarcinoma and IPF without causing AE-ILD.
Nintedanib, an inhibitor of multiple angiokinases and receptor tyrosine kinases, has demonstrated potential antitumor effects in NSCLC when combined with cytotoxic chemotherapy [6]. In this case, it was paired with MET-TKI instead, and while its antitumor role remains unclear, it might have contributed to tumour control.
To our knowledge, this is the first reported case suggesting that nintedanib treatment may be both safe and effective for managing progressive IPF in a patient undergoing MET-TKI therapy. It could serve as a pretreatment for lung cancer patients with coexisting progressive IPF before initiating TKI therapy.
Author Contributions
Takunori Ogawa was responsible for drafting the work; conception and design of the work; and acquisition, analysis, and interpretation of the data. Yoshiki Kato, Akira Matsukida, Ryosuke Nagaoka, and Kotoba Esaki collected the clinical data and drafted the original manuscript. Kimiya Sato and Akihiko Kawana critically revised the manuscript for important intellectual content. Yoshifumi Kimizuka was responsible for revising the manuscript critically for important intellectual content. All authors have confirmed the final manuscript and agreed to publication.
Consent
The authors declare that written informed consent was obtained for the publication of this manuscript and accompanying images using the consent form provided by the Journal.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




