Mycobacterium goodii pulmonary disease with organizing pneumonia: A case report and review of literature
Abstract
Mycobacterium goodii, a rapidly growing non-tuberculous mycobacterium, rarely causes pulmonary diseases. A patient was admitted to our hospital with a fever and cough. Chest radiography revealed consolidation in the right middle lung. As he had previously been treated for organizing pneumonia (OP), he was diagnosed with OP recurrence and administered systemic corticosteroids. Although initial improvement was observed, the pulmonary consolidations worsened. Transbronchial lung cryobiopsy revealed an OP pattern. M. goodii was identified in sputum acid-fast bacilli cultures. The patient was diagnosed with M. goodii pulmonary disease and secondary OP. Although intravenous imipenem-cilastatin, amikacin, and ciprofloxacin led to initial improvement in pulmonary consolidations, the consolidations re-worsened. Systemic corticosteroids were initiated, resulting in improvement in the consolidations. The dose of systemic corticosteroids was tapered; oral antimycobacterial therapy was continued. M. goodii can cause pulmonary disease and induce OP; antimycobacterial therapy and systemic corticosteroids can be effective.
INTRODUCTION
Mycobacterium goodii is a rapidly growing non-tuberculous mycobacterium (NTM). Brown et al. have reported that M. goodii is a member of the Mycobacterium smegmatis group.1 M. goodii has been reported to be isolated from soil and water.2, 3 M. goodii infections occur through exposure to environmental sources and are commonly observed in patients with post-traumatic wound infections.1 Only a few reports on M. goodii pulmonary disease have been published.1, 4-7
Organizing pneumonia (OP) is a patchy process of lung tissue repair after injury.8 The OP pattern is a histological finding characterized by polypoid plugs of loose connective tissue in distal airspaces, mostly the alveoli and alveolar ducts and seldom the bronchioles.8 OP is usually caused by bacterial infection, drugs, or connective tissue diseases.9, 10 Although NTM pulmonary disease can induce OP, there are limited reports on such cases.7, 11-18
The clinical features of pulmonary disease caused by M. goodii remain unclear. Herein, we present a case wherein M. goodii pulmonary disease induced secondary OP.
CASE REPORT
A man in his 80s with a history of gastrectomy visited a nearby hospital with a productive cough and fever. Chest radiography revealed consolidation in the right middle lung; chest computed tomography revealed consolidation in the right upper lung lobe (Figure 1A,I). Seven years before symptoms developed, the patient had been treated for OP; thus, he was diagnosed with OP recurrence. Therefore, oral prednisolone (30 mg/day) was administered. Although initial improvement was observed (Figure 1B), consolidations exacerbated upon prednisolone dose reduction. Despite administering antibiotics and methylprednisolone (1000 mg/day for 3 days), no improvement was observed (Figure 1C,J). Eventually, the patient developed hypoxemia and was hospitalized.
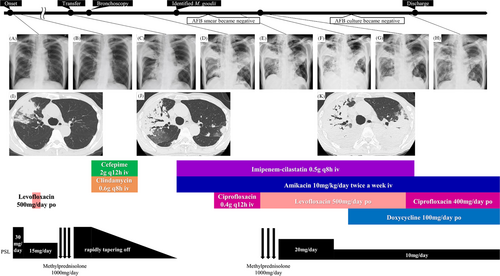
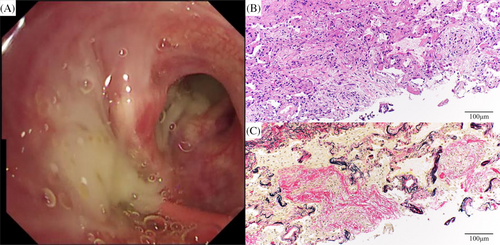
The patient was transferred to our department for evaluation and treatment of refractory pneumonia. Upon transfer, his percutaneous oxygen saturation was 95% under oxygen administration via a nasal cannula at 2 L/min. Blood test results revealed elevated white blood cell count and C-reactive protein levels (Table 1). No specific causes of pneumonia, such as serological autoantibodies or drugs, were identified. In the sputum culture collected before transfer from a nearby hospital, no specific bacteria were detected. Although the sputum acid-fast bacilli (AFB) smear showed negative results, the sputum AFB culture yielded positive findings; the species were unknown. Bronchoscopy was performed on the second day after hospitalization. A large amount of purulent sputum was observed in the bronchi (Figure 2A). Transbronchial lung cryobiopsy of the right upper lung lobe revealed an OP pattern (Figure 2B,C). Analysis of bronchoalveolar lavage fluid obtained from the right middle lung lobe showed a slightly elevated lymphocyte ratio and no evidence of microbial infection, including AFB (Table 1). Based on the results of bronchoscopy, the consolidations were considered to be bacterial pneumonia and secondary OP. Despite administering antibiotics and tapering of the oral prednisolone dose, the consolidations did not improve (Figure 1D). His respiratory condition worsened, developing respiratory failure. Sputum AFB tests were repeated twice, immediately after bronchoscopy and on the day following bronchoscopy. Consecutive sputum AFB smears and cultures revealed positive results. On day 16 after hospitalization, M. goodii was identified in all sputum AFB cultures, obtained prior to the transfer from a nearby hospital, immediately after bronchoscopy, and on the day following bronchoscopy (Table 1). M. goodii were identified by analysing the homology of rpoB, hsp65, secA1, and sodA genes, using the basic local alignment search tool (BLAST). Ultimately, refractory pneumonia was diagnosed as M. goodii pulmonary disease and secondary OP. On the same day, intravenous amikacin (10 mg/kg/day, twice weekly) and imipenem-cilastatin (0.5 g q8h) were administered. Additionally, intravenous ciprofloxacin (0.4 g q12h) was administered. Subsequently, respiratory failure and consolidations on chest radiography improved (Figure 1E). On day 22 after hospitalization, although the sputum AFB smear showed negative results, the culture findings remained positive. On day 29 after hospitalization, respiratory failure worsened. Chest radiography and computed tomography revealed exacerbation of the consolidations (Figure 1F,K), which was considered worsened OP. Therefore, methylprednisolone (1000 mg/day for 3 days) was administered. Furthermore, considering the complications of bacterial pneumonia, ciprofloxacin was substituted with oral levofloxacin (500 mg, q24h). Subsequently, consolidations on chest radiography and respiratory failure improved (Figure 1G). Methylprednisolone was switched to oral prednisolone (20 mg/day). Subsequently, the oral prednisolone dose was tapered; consolidations did not worsen (Figure 1H). On day 52 after hospitalization, the patient was discharged because he had been administered intravenous imipenem-cilastatin for approximately 1 month.
| Blood examinations | Result | Blood examinations | Result | BALF | Result | Sputum | Result |
|---|---|---|---|---|---|---|---|
| Complete blood count | Total bilirubin (mg/dL) | 0.6 | Total cell count (/mL) | 78,000 | AFB smear | (+) | |
| White blood cells (/μL) | 17,700 | Sodium (mmol/L) | 135 | Neutrophil (%) | 11 | AFB culture | M. goodii |
| Neutrophil (%) | 98.0 | Potassium (mmol/L) | 4.6 | Lymphocyte (%) | 23 | Drug susceptibility of M. goodii | MIC |
| Monocyte (%) | 2.0 | Chloride (mmol/L) | 96 | Monocyte (%) | 62 | Imipenem-cilastatin | <2 |
| Lymphocyte (%) | 0.0 | C-reactive protein (mg/dL) | 9.91 | Eosinophil (%) | 4 | Meropenem | <2 |
| Haemoglobin (g/dL) | 12.9 | KL-6 (U/mL) | 656 | CD4/8 ratio | 5.55 | Amikacin | <4 |
| Platelets (×104/μL) | 34.3 | Rheumatoid factor (IU/mL) | (−) | Bacterial culture | (−) | Clarithromycin | 32 |
| Blood chemistry | Anti-CCP antibody (U/mL) | (−) | AFB culture | (−) | Doxycycline | <0.5 | |
| Aspartate aminotransferase (U/L) | 21 | Anti-Nuclear antibody | (−) | Faropenem | 4 | ||
| Alkaline phosphatase (U/L) | 18 | Anti-ARS antibody | (−) | Tobramycin | 2 | ||
| Lactate dehydrogenase (U/L) | 184 | Anti-SS-A antibody (U/mL) | (−) | Azithromycin | >128 | ||
| Alkaline phosphatase (U/L) | 80 | Anti-SS-B antibody (U/mL) | (−) | Linezolid | <1 | ||
| γ-glutamyl transpeptidase (U/L) | 19 | Anti-U1-RNP antibody | (−) | Levofloxacin | <1 | ||
| Total protein (g/dL) | 6.4 | Anti-Scl70 antibody | (−) | Sitafloxacin | <0.25 | ||
| Albumin (g/dL) | 3.3 | Anti-centromere antibody | (−) | Moxifloxacin | <0.25 | ||
| Urea nitrogen (mg/dL) | 18 | PR3-ANCA | (−) | Sulfamethoxazole-trimethoprim | <0.25 | ||
| Creatinine (mg/dL) | 0.71 | MPO-ANCA | (−) | Clofazimine | 0.25 |
- Abbreviations: AFB, acid-fast bacilli; ANCA, antineutrophil cytoplasmic antibody; ARS, aminoacyl tRNA synthetase; BALF, bronchoalveolar lavage fluid; CCP, cyclic citrullinated peptide; CD, cluster of differentiation; DNA, deoxyribonucleic acid; KL-6, Krebs von den Lungen-6; M. goodii, Mycobacterium goodii; MIC, minimum inhibitory concentration; MPO, myeloperoxidase; PR3, proteinase3; RNP, ribonucleoprotein; SS, Sjogren's syndrome.
The regimen was changed to intravenous amikacin (10 mg/kg/day, twice weekly), oral ciprofloxacin (400 mg/day), and oral doxycycline (100 mg/day). Three months after antimycobacterial therapy initiation, three consecutive sputum cultures revealed negative results. Therefore, intravenous amikacin administration was discontinued. The prednisolone dose was gradually tapered; oral ciprofloxacin and doxycycline were continued for 11 months. Results on sputum cultures remained consistently negative; consolidations on chest radiography improved.
DISCUSSION
This case report presents three notable clinical findings. First, M. goodii rarely can cause pulmonary disease. Second, M. goodii pulmonary disease can be treated with a multidrug regimen based on in vitro susceptibility test results. Third, M. goodii pulmonary disease can induce secondary OP.
M. goodii, a rare pathogen, causes pulmonary disease. A literature review reporting 10 cases of M. goodii pulmonary disease, including the present case, is presented in Table 2.1, 4-7 In these cases, age distribution comprised all age groups (median age, 54.5 years; range, 15–80 years), with a male predominance (men:women, 7:3). Either productive or dry cough; fever; dyspnea; and chest pain have been reported. Thus, the symptoms were not specific to M. goodii pulmonary disease and were similar to those of other NTM pulmonary diseases.19 Regarding the cases of M. goodii pulmonary disease, lipoid pneumonia, achalasia, and a history of gastrectomy were reported as comorbidities in 3, 2, and 3 cases, respectively.1, 4, 6, 7 Chronic aspiration due to exposure to mineral oil or difficulty ingesting oily substances linked to oesophageal or swallowing disorders, subsequently resulting in lipoid pneumonia, have been identified as risk factors for pulmonary diseases caused by rapid growing NTM, including M. goodii.1, 20 Therefore, oesophageal and gastric anatomical or functional abnormalities may be risk factors for developing M. goodii pulmonary disease. Considering chest image findings of M. goodii pulmonary disease, consolidations were observed in four of 10 cases.5-7 NTM pulmonary disease related to achalasia commonly showed patchy bilateral consolidations resembling aspiration pneumonia.21 Thus, chest image findings of M. goodii pulmonary disease may be the features of M. goodii itself or involve patients' comorbidities.
| Case | Author | Year | Age and Sex | Specimen in which M. Goodii detected | Symptoms | Chest image findings | Comorbidities | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Wallace et al.4 | 1988 | 58, M |
Pleural fluid, lung biopsy |
N/A | N/A | Lipoid pneumonia | N/A |
| 2 | Brown et al.1 | 1999 | 76, M |
Lung biopsy | N/A | N/A | N/A | N/A |
| 3 | 56, M |
BALF | N/A | N/A | N/A | N/A | ||
| 4 | 34, F |
Lung biopsy | N/A | N/A | Lipoid pneumonia, prior gastrectomy | N/A | ||
| 5 | 18, M |
Lung biopsy | N/A | N/A | Lipoid pneumonia | N/A | ||
| 6 | 53, M |
Sputum | N/A | N/A | Achalasia | N/A | ||
| 7 | Buijtels et al.5 | 2005 | 66, M |
Pleural fluid | Productive cough, chest pain | Pleural effusion, peribranchial pathology, and consolidation on the CXR | N/A | N/A |
| 8 | Martinez-Gonzales et al.6 | 2011 | 15, F |
Sputum | Productive cough, fever | Bilateral GGO with consolidations on the chest CT | Achalasia | 12 months of oral ciprofloxacin and doxycycline |
| 9 | Waldron et al.7 | 2019 | 51, F |
Lung biopsy | Dry cough, dyspnea, and chest pain | Consolidations on the chest CT | Prior gastrectomy | 6 weeks of IV amikacin, IV meropenem, oral ciprofloxacin, and oral doxycycline Subsequently, 2 years of oral sulfamethoxazole-trimethoprim and ciprofloxacin |
| Our case | 2023 | 80, M |
Sputum | Productive cough, fever | Consolidations on the chest CT | Prior gastrectomy | 4 weeks of IV imipenem-cilastatin, 3 months of IV amikacin, being continued oral doxycycline and oral ciprofloxacin |
- Abbreviations: BALF; bronchial alveolar lavage fluid; CT, computed tomography; CXR, chest radiograph; F, female; GGO, ground glass opacity, IV, intravenous; M, male; M. goodii, Mycobacterium goodii; N/A, not available.
Evidence regarding the regimen and treatment duration for M. goodii pulmonary disease remains insufficient. The official clinical practice guidelines recommend treating common NTM, such as Mycobacterium avium complex (MAC), with a multidrug regimen based on in vitro drug susceptibility test results.22 In regard to relatively uncommon NTM pulmonary diseases, a consensus statement recommends that the treatment regimen should be chosen based on in vitro drug susceptibility test results.23 Regarding both common and relatively uncommon NTM pulmonary diseases, the treatment duration should be at least 12 months after negative culture results. Furthermore, the duration of treatment of rapidly growing NTM pulmonary diseases should be divided into an initial phase of at least 1 month with >3 drugs containing at least one intravenous drug and a continuation phase of >2 oral drugs.22, 23 Treatment regimens for M. goodii pulmonary disease have been reported in two cases (cases 8 and 9, Table 2).6, 7 One patient was treated with two oral antibiotics.6 Another patient was treated with a multidrug regimen involving two intravenous antibiotics, in accordance with clinical guidelines and consensus statements for other rapidly growing NTM pulmonary diseases.7 In our case, a multidrug regimen was initiated. The treatment regimen resulted in negative culture results within 3 months. Thus, two-phase treatment based on in vitro drug susceptibility test results may be effective in M. goodii pulmonary disease. However, the optimal regimen and treatment duration for M. goodii pulmonary disease remain unknown.
OP may be caused by M. goodii pulmonary disease. OP may be induced by NTM pulmonary disease as with other microorganism infections, or it may be one of the clinicopathological features of NTM pulmonary disease itself. Proinflammatory cytokines, such as tumour necrosis factor-α and interleukin-6, may be involved in OP development.24-27 Conversely, MAC pulmonary disease may be involved in the release of tumour necrosis factor-α and interleukin-6.28, 29 A similar response during OP development may occur in patients with MAC pulmonary diseases. However, whether similar mechanisms occur in other NTM pulmonary diseases has not been verified. A literature review of previous cases of pathologically proven OP induced by NTM pulmonary disease is presented in Table 3. OP caused by NTM pulmonary disease was improved by antimycobacterial therapy with systemic corticosteroids and sometimes antimycobacterial therapy alone, except in resection cases.7, 11-18 In the three cases, including the present case, administering antimycobacterial therapy alone showed insufficient therapeutic effect on OP caused by NTM pulmonary disease. The addition of systemic corticosteroids to the antimycobacterial therapy regimen resulted in improved pulmonary consolidations.15, 17 On the other hand, in three cases wherein only systemic corticosteroids were initially administered, pulmonary consolidations responded poorly to systemic corticosteroids. The addition of antimycobacterial agents to the systemic corticosteroids regimen resulted in improved pulmonary consolidations.7, 12, 18 Thus, OP caused by NTM pulmonary disease should be treated with antimycobacterial therapy; however, the addition of systemic corticosteroids is often likely to be effective. Similarly, in cases of OP caused by M. goodii pulmonary disease, administering antimycobacterial therapy and systemic corticosteroids may be effective.
| Case | Author | Year | Age and Sex | Specimen in which OP was proven | Pathogen | Treatment for NTM pulmonary disease and secondary OP |
|---|---|---|---|---|---|---|
| 1 | Marchevsky et al.11 | 1982 | 31, F |
SLB | MAC | N/A |
| 2 | 75, F |
SLB | MAC | N/A | ||
| 3 | 79, M |
SLB | MAC | N/A | ||
| 4 | 60, F |
SLB | MAC | N/A | ||
| 5 | 68, M |
SLB | M. gordonae | N/A | ||
| 6 | 55, F |
SLB | M. fortuitum | N/A | ||
| 11 | Hamada et al.12 | 2006 | 67, F |
TBLB | M. intracellulare | Systemic corticosteroids only, subsequently systemic corticosteroids + antimycobacterial therapy |
| 12 | Jones et al.13 | 2009 | 58, M |
TBLB | MAC | Systemic corticosteroids + antimycobacterial therapy |
| 13 | Starobin et al.14 | 2011 | 85, F |
TBLB | M. kansasii | Systemic corticosteroids + antimycobacterial therapy |
| 14 | Nakahara et al.15 | 2015 | 66, F |
SLB | M. avium | Resection |
| 15 | 74, M |
SLB | M. avium | Resection | ||
| 16 | 65, M |
TBLB | M. avium | Antimycobacterial therapy | ||
| 17 | 73, M |
TBLB | M. avium | Systemic corticosteroids + antimycobacterial therapy | ||
| 18 | 66, F |
TBLB | M. abscessus | Antimycobacterial therapy only, subsequently systemic corticosteroids + antimycobacterial therapy | ||
| 19 | Hong et al.16 | 2017 | 67, F |
Percutaneous lung biopsy | M. abscessus | Systemic corticosteroids + antimycobacterial therapy |
| 20 | Watanabe et al.17 | 2019 | 59, M |
TBLB | M. abscessus | Antimycobacterial therapy only, subsequently systemic corticosteroids + antimycobacterial therapy |
| 21 | Waldron et al.7 | 2019 | 51, F |
SLB | M. goodii | Systemic corticosteroids only, subsequently systemic corticosteroids + antimycobacterial therapy |
| 22 | Fernandes et al.18 | 2021 | 64, F |
Percutaneous lung biopsy | M. avium | Systemic corticosteroids only, subsequently systemic corticosteroids + antimycobacterial therapy |
| Our case | 2023 | 80, M |
TBLC | M. goodii | Systemic corticosteroids only, subsequently antimycobacterial therapy only, finally systemic corticosteroids + antimycobacterial therapy |
- Abbreviations: F, female; M, male; MAC, Mycobacterium avium complex; M. abscessus, Mycobacterium abscessus; M. avium, Mycobacterium avium; M. fortuitum, Mycobacterium fortuitum; M. goodii, Mycobacterium goodii; M. gordonae, Mycobacterium gordonae; M. kansasii, Mycobacterium kansasii; M. intracellulare, Mycobacterium intracellulare; NTM, non-tuberculous mycobacterium; N/A, not available; OP, organizing pneumonia; SLB, surgical lung biopsy; TBLB, transbronchial lung biopsy; TBLC, transbronchial lung cryobiopsy.
M. goodii causes pulmonary disease that can be treated with a multidrug regimen based on in vitro drug susceptibility test results. M. goodii pulmonary diseases might induce dysregulated immune responses in the lungs such as OP. Systemic corticosteroids combined with antimycobacterial therapy might be effective against immune responses caused by M. goodii pulmonary disease.
AUTHOR CONTRIBUTIONS
Yu Shionoya cared for the patient during hospitalization and wrote this manuscript, Hajime Kasai wrote and revised the manuscript, Reiya Kono helped writing the manuscript, Ryutaro Hirama cared for the patient during hospitalization and supervised this case report, Masayuki Ota made pathological diagnosis and provide pathological images, Akira Naito cared for the patient during hospitalization and supervised this case report, Mitsuhiro Abe cared for the patient during hospitalization and supervised this case report, Jun-ichiro Ikeda supervised pathological diagnosis, Takeshi Kawasaki cared for the patient during hospitalization and supervised this case report, Takuji Suzuki supervised this case report.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.




