Muscle atrophy in osteoarthritis: clinical features, pathological changes, underlying mechanisms, and exercise-based interventions
Tongyi Zhang, Bin Zhang, and Jinhui Wu contributed equally.
Funding information: This work was supported by the National Natural Science Foundation of China (No. 82372495, No. 81871817, No. 82202770) and the Natural Science Foundation of Chongqing (No. CSTB2022NSCQ-MSX1267, No. CSTB2022NSCQ-MSX0863).
Abstract
Background
Osteoarthritis (OA) is a common degenerative disease with cartilage injury as the core pathological phenotype, which has become the leading cause of disability in the elderly. Skeletal muscle is an important organ to maintain the structure and motor function of joints, which is highly related to OA progress. Patients with OA typically exhibit abnormalities in the skeletal muscles surrounding the joints, such as reduced muscle mass and strength. We refer to this condition as OA-related muscle atrophy (hereafter referred to as OAMA). The mechanisms of OAMA are multifactorial and unclear.
Methods
In this narrative review, we summarized relevant research progress of OAMA (i) to review the changes in skeletal muscle of patients with OA, (ii) to review the underlying biological mechanisms of OAMA, (iii) to review the effects of skeletal muscle on OA, and (iv) to provide perspectives on current and potential strategies of OA clinical treatment based on skeletal muscle, especially exercise training.
Results
OAMA may lead to the destruction of joint stability and cartilage homeostasis and then promote the occurrence and development of OA. Clinical manifestations of OAMA are a decline in muscle mass and strength and an abnormal movement pattern. The underlying biological mechanisms of OAMA include chronic inflammation, oxidative stress, ion metabolism, glycolipid metabolism, and epigenetics. The effects of skeletal muscle on OA are skeletal muscle-mediated cartilage damage via biomechanics, muscle-derived secretions, and muscle-derived stem cells.
Conclusions
More preclinical and clinical studies are imperative to study the mechanisms of OAMA and develop potential strategies. We hope that more studies can focus on the skeletal muscle during the OA process, which will be beneficial for delaying OA progression and improving the motor function of OA patients in the future.
Introduction
Osteoarthritis (OA) is a prevalent musculoskeletal disorder marked by the degradation of articular cartilage, abnormal remodelling of subchondral bone, the formation of osteophytes, and synovitis.1, 2 The incidence of OA has been increasing all over the world.3 Joint pain and deformity caused by OA not only seriously decrease motor function and affect the quality of life of the elderly but also bring a greater economic burden for families and society.4 However, the clinical treatment effect of OA is still not very satisfactory and needs to be further improved.
Skeletal muscle plays an important role in maintaining the mechanical stability and movement pattern of joints.5 Additionally, skeletal muscle participates in the functional regulation of various organs, including bones and joints.6, 7 Clinically, patients with OA generally have abnormalities in the skeletal muscles around the joints, including the decline of muscle mass and muscle strength, which we named OA-related muscle atrophy (hereafter referred to as OAMA). OAMA may lead to the destruction of joint stability and damage cartilage homeostasis, which promotes the occurrence and development of OA.8, 9 At present, some muscle-dependent rehabilitation treatments, especially exercise training, have been applied to the clinical treatment of OA patients and achieved certain therapeutic effects. However, the existing strategies targeting OAMA still have some limitations, such as (1) difficulty adhering to long-term exercise training, (2) repeated pain reducing compliance with training protocols, and (3) lack of specific drugs for muscle regeneration against OAMA.
In this review, we summarized the clinical characteristics of OAMA in OA patients, including muscle mass, muscle strength, and movement patterns, as well as the correlation between changes in OAMA and the progression of OA. In addition, we retrospected the underlying mechanisms of OAMA. Moreover, we summed up the current clinical strategies of OA treatment based on skeletal muscle, especially exercise training. At last, we discussed the potential research directions of OAMA. We hope that this review can further enhance the understanding of the significance and mechanisms of skeletal muscles in OA patients among clinical physicians and basic researchers in order to improve and optimize the clinical treatment effect for OAMA in the future.
Clinical manifestations of skeletal muscle in osteoarthritis patients
Muscle atrophy is characterized by reduced muscle mass, decreased force production, increased fatigue, diminished exercise capacity, and decreased quality of life.10, 11 Previous studies have demonstrated that muscle atrophy is often related to aging, disuse, central nervous system diseases, inflammation, mitochondrial damage, and metabolic abnormalities11, 12 (Figure 1). In addition, excessive muscle atrophy is highly associated with poor prognosis in myopathies and muscular dystrophies, as well as in cancer, diabetes, and sepsis.
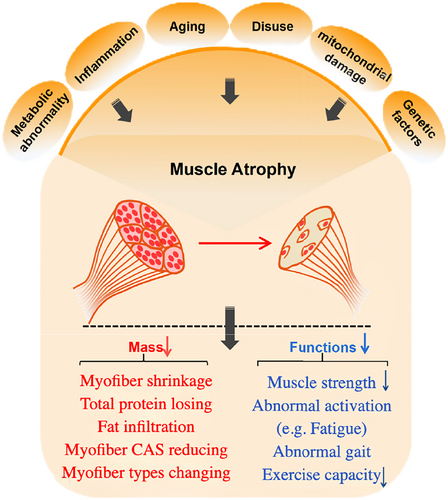
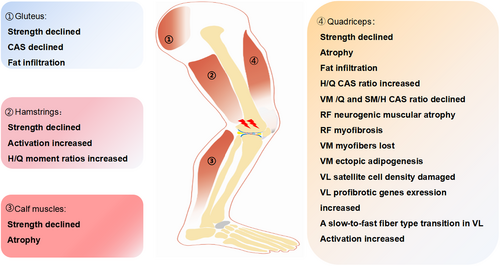
In OA patients, muscle atrophy commonly occurs around the degenerative joints and presents with several pathological changes in the skeletal muscles. These changes include myofiber shrinkage, alterations in fibre types, decreased fibre cross-sectional area, and fat infiltration at the histological level. Additionally, a reduction in muscle strength, abnormalities in muscle activation, and gait disturbances can be found in OA patients.18 Masashi Taniguchi et al. measured muscle thickness by ultrasonography and found that the muscle thickness of the mild knee OA (KOA) group was lower, suggesting that the skeletal muscles are significantly atrophic in KOA patients.19 The decrease in muscle mass of skeletal muscle around the joints is a common phenotype during OA progression. Another study suggested that knee extensor muscle weakness was not only associated with symptomatic OA but also associated with radiographic OA.9 In addition to the muscle mass reduction and muscle weakness, Shawn M. Robbins et al. found that muscle activation and knee mechanics were different between participants with non-traumatic and post-traumatic KOA.20 In summary, the changes in skeletal muscle of OA patients, including muscle mass, muscle strength, and movement pattern, are common phenotypes in OA patients that are highly related to clinical symptoms and OA progression (Figure 2).
The underlying mechanisms of osteoarthritis-related muscle atrophy
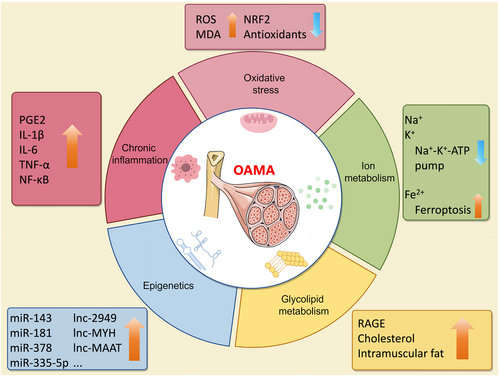
The pathological changes of OA are involved not only in cartilage loss, osteophyte, and synovitis but also in muscle atrophy.2 Unlike acute muscle injury, the pathological changes in OA-affected muscles include decreased muscle mass, inflammation, aging, fat deposition, and proprioception abnormalities. To date, scholars have observed the changes of skeletal muscles in different OA models, including the spontaneous OA in aging animals, the surgical-induced OA model, and the chemical factor-induced OA models.26, 27 However, the underlying mechanisms of pathological changes in OA muscle abnormalities are still largely unknown. Thus, we summarized the current underlying mechanisms of skeletal muscle in OA, including chronic inflammation, oxidative stress, ion metabolism, glycolipid metabolism, and epigenetics (Figure 3).
Chronic inflammation
Chronic inflammation contributes to aberrant muscle homeostasis in several diseases, such as rheumatoid arthritis,32 polymyositis,33 and chronic heart failure.34 In addition, chronic inflammation plays a key role in the occurrence and development of OA.35 Studies have shown that the levels of pro-inflammatory mediators such as prostaglandin E2 (PGE2) and interleukin (IL)-1β in the blood of patients were significantly increased during OA progression.36 Previous studies have focused on inflammation in cartilage and synovium, while inflammatory changes in OA-related muscles have also received increasing attention in recent years. Inflammation is associated with lower muscle strength in patients with KOA, and muscle inflammation is correlated with altered knee function.37, 38 A 2-year follow-up study found that elevated serum C-reactive protein values are related to a lower gain in muscle strength over time in OA patients.39 Exercise could reduce circulating inflammatory factors like IL-6 and tumour necrosis factor alpha (TNF-α), which are associated with increased quadriceps strength in OA patients.40 In addition to the clinical studies, rodent experiments have been used to research the underlying mechanisms of skeletal muscle in OA. The anterior cruciate ligament transection (ACLT)-induced rodent model promotes significant joint cartilage damage, which is a classical surgical-induced OA model.41 Silva et al. found that the content of IL-1β in muscle tissue increased in the ACTL-induced rat OA model, which was accompanied by increased expression of myostatin and myogenin and a 10% reduction in the cross-sectional area of the gastrocnemius muscle.23 Moreover, Wei-Yu Fang et al. found that inhibition of inflammatory factors in lipopolysaccharide (LPS)-treated C2C12 myotubes could prevent muscle atrophy.42 In summary, chronic inflammation in skeletal muscle is a specific pathological change for OA patients. The preclinical studies suggested that the chronic inflammatory response of muscle tissue may contribute to muscle atrophy during the OA process.
Oxidative stress
In addition to inflammation, the pathogenesis of OA is characterized by oxidative stress.43 Oxidative stress related to aging significantly contributes to chronic muscle injury. However, the connection between oxidative stress and OAMA has not yet been explored.44 Patients who have undergone knee meniscectomy (MNX) surgery show muscle weakness in the injured limb.45 Muscle dysfunction can be detected in an MNX-induced OA rat model.28 In the MNX-treated rat OA model, the expression level of the antioxidant-related gene nuclear factor erythroid 2-related factor 2 (NRF2) and the contents of antioxidant substances were significantly decreased in the OA group.28 Indeed, supplementation with antioxidants resulted in a significant improvement in muscle function, along with a significant reduction in joint pain and less loss of physical function in OA patients.46, 47 In addition, the administration of two plant extracts with antioxidant functions, Baicalin and Crocin, can also significantly improve muscle dysfunction and reduce OA symptoms in an MNX-induced OA rat model.48, 49 The above results indicate that the imbalance between oxidizing substances and antioxidant substances in skeletal muscles mediates OA-induced muscle atrophy. As oxidative stress has negative effects on OA-related muscle, it is possible that inhibition of oxidizing substances benefits OAMA. Research has shown that oxidative stress is a key pathological factor in OA-induced muscle atrophy and that reducing oxidative stress in muscles can help alleviate OA symptoms. However, the precise mechanisms underlying the disruption of redox balance in OA-affected muscles remain unclear.
Ion metabolism
Ion metabolism plays a crucial role in the symptoms and progress of OA patients.29 Limited studies have identified that disruptions in ion metabolism within muscle tissue are significant contributors to OA. The sodium-potassium transporter, the Na+-K+-ATP pump, is a heterodimer consisting of α-isoform and β-isoform, which is essential for skeletal muscle excitability and function.50 In addition, calcium-dependent potassium channels mediate muscle differentiation and myogenesis, which have been well reviewed by Roland Takács et al.51 Iron homeostasis is critical for joint health and synovial alterations, which are closely related to synovial alteration and cartilage degeneration in OA.52 Ferroptosis can trigger OA, including the promotion of cartilage degradation and subchondral bone remodelling.53 From the reported studies, we can conclude that Na+, Ca2+, and K+ play an important role in the maintenance of muscle function. However, further investigation is warranted to uncover the effects and mechanisms of iron on muscle, especially for OAMA. It is a potential strategy to manage OA by targeting the ion-related signal pathway after the elaboration of ion metabolism in OA muscle.
Glycolipid metabolism
Metabolic OA has been characterized as a subtype of OA patients.54 Abnormal glycolipid metabolism greatly contributes to the pathological process of OA, including synovial inflammation and chondrocyte destruction.55, 56 The formation and accumulation of receptor for advanced glycation end products (RAGE), particularly in collagen, can influence the quality of skeletal muscle, reducing the muscle function of men with KOA.57 Moreover, excess cholesterol accumulation may induce OA.58 Thigh muscle intramuscular fat fraction and area rather than muscle size are associated with the symptomatic and structural severity of KOA.59 Diabetes-associated longitudinal thigh muscle degeneration partially mediates the worsening of symptoms in KOA patients.60 Musculoskeletal impairment and OA-related symptoms were related to insulin resistance.61 In summary, skeletal muscle affected by OA exhibits abnormal glycolipid metabolism, characterized by the accumulation of RAGE and cholesterol, an increased intramuscular fat fraction, and insulin resistance. Further research is necessary to elucidate the specific mechanisms of glycolipid metabolism affecting OA-induced muscle atrophy.
Epigenetics
Epigenetic factors include non-coding RNAs, such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and m6A methylation, that function to regulate targeted genes and have important effects on joint health and disease.62 miRNAs, such as miR-143, miR-181, and miR-378, are important regulators of muscle atrophy during the progression of OA.63 Thomas G. Wilson et al. found high expression of miR-335-5p in the vastus medialis oblique muscle of late-stage KOA patients.64 The interaction between miR-181 and sirtuin1 regulates myotube size, relating to the pathophysiological changes of skeletal muscle during aging.65 LncRNAs, including lnc-2949/lnc-1369, lnc-MYH, lnc-MAAT, lnc-SMUL, lnc-mir22hg, and lnc-ORA, may be new regulators of chronic musculoskeletal disorder by inducing myoblast proliferation or differentiation.66 CircRNAs acting as miRNA sponges regulate OA progression via modulating inflammation and extracellular matrix (ECM) metabolism.67 Moreover, the m6A methylation of non-coding RNAs during skeletal muscle differentiation and development could be a targeted therapy for musculoskeletal disorders.68 Taken together, several miRNAs, including miR-143, miR-181, miR-335-5p, and miR-378, may contribute to OAMA. Even though there were lots of studies that reported the role of epigenetic factors like lncRNAs, circRNAs, and m6A methylation on musculoskeletal disorders, there is no direct evidence indicating the bioeffects and mechanisms of those factors on OAMA.
The effects of skeletal muscle on osteoarthritis
Skeletal muscle-mediated cartilage damage via biomechanics
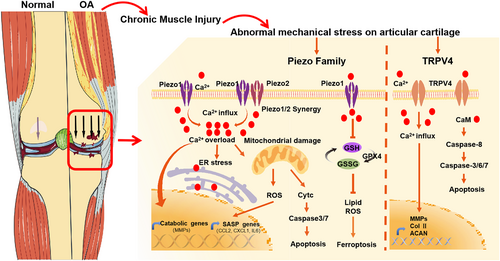
Skeletal muscle plays an important role in maintaining joint stability, which is closely related to the pathogenesis of OA by affecting joint biomechanics69 (Figure 4). Pathological mechanical loading can accelerate cartilage degeneration. The muscle strength of the quadriceps or hamstrings is closely associated with early changes in cartilage, which suggests that skeletal muscle plays a crucial role in the progression of OA.74 Mechanical stress can affect the behaviour and function of chondrocytes, which is related to different pressure-sensing receptors and their downstream signal pathways.75 The piezo1 and piezo2 are classical mechanosensitive channels that could transform mechanical signals into biological transduction signals such as Ca2+ influx and participate in a variety of physiological and pathological processes.76 Activation of piezo1 by abnormal mechanical stress can increase the release of cytochrome c from the mitochondrion and promote the activation of caspase-3/7 via Ca2+ overload-activated endoplasmic reticulum stress, eventually leading to chondrocyte apoptosis.76 An explant-cartilage injury model can be used as an in vitro model to research the pathological change of cartilage in OA. Whasil Lee et al. revealed that the piezo-blocking peptide GsMTx4 can alleviate chondrocyte death through repressing Ca2+ transients after mechanical injury in an explant-cartilage injury model, which indicates a potential treatment strategy for post-traumatic OA by targeting piezo-mediated cartilage mechanotransduction.77 Destabilization of the medial meniscus (DMM) surgery is performed by surgical sectioning of the medial meniscotibial ligament of the rodent knee, another classical OA animal model, altering joint biomechanics and inducing pathological changes similar to human OA after anterior cruciate ligament (ACL) or meniscal injury.78 The DMM model provided a reliable and reproducible progression of OA.79 In DMM-induced OA mice, researchers found that GsMTx4 treatment can lead to increased expression of anabolic-related molecules and decreased expression of catabolic-related genes in articular cartilage.70 In addition to piezo channels, excessive mechanical stress can increase the expression of transient receptor potential vanilloid 4 (TRPV4), which significantly increased the concentration of intracellular Ca2+ and then up-regulated Fas-associated protein with death domain and cleaved caspase-3, 6, 7, and 8 levels, ultimately leading to chondrocyte apoptosis in an ACLT rat model.80 In addition to surgery-induced OA models, aging can also induce OA through damage to articular cartilage homeostasis.81 Knockout of TRPV4 significantly reduced the severity of spontaneous aging in OA mice.82 The activation of TRPV4 will increase the concentration of intracellular Ca2+, thereby stimulating the gene expression of follistatin, which inhibits the bone morphogenetic protein (BMP) signal pathway, thereby affecting the quality of cartilage.83 These studies indicate that weakness or imbalance in the skeletal muscles surrounding joints can cause stress overload on the articular cartilage, whose mechanisms involve activation of piezo1, piezo2, and TRPV4 mechanosensitive channels, leading to chondrocyte death and cartilage damage, thereby accelerating the progression of OA.
The potential roles of skeletal muscle-derived secretions in osteoarthritis
In addition to the regulatory effect of mechanical stress on cartilage, the skeletal muscle can also regulate the related functions of cartilage through its secretions, including cytokines, metabolites, and exosomes84 (Figure 5). Cytokines secreted by skeletal muscle, including TNF-α, insulin-like growth factor 1 (IGF-1), IL-6, fibroblast growth factor 2 (FGF-2), and matrix metalloproteinase (MMP)-2, can get into multiple organs and tissues in the body, such as the brain, bone, heart, pancreas, liver, and kidneys, regulate their functions, and participate in physiological and pathological processes such as development and damage repair.6, 7 Among them, TNF-α is a classical pro-inflammatory factor, and there is high expression of TNF-α in the peripheral blood of OA patients. TNF-α can induce apoptosis of chondrocytes, inhibit the synthesis of the ECM of chondrocytes, and promote ECM decomposition.89 IGF-1, which is mainly derived from liver and skeletal muscle, can protect against OA pathogenesis via inhibition of nuclear factor kappa B (NF-κB) signal transduction by regulating mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI-3K)/protein kinase B (Akt) pathways, while it inhibits reactive oxygen species (ROS) production and finally reduces chondrocyte apoptosis.90 In addition, FGF-2 can up-regulate the expression of cartilage matrix-degrading enzymes MMP-1 and MMP-13 and down-regulate the expression of cartilage matrix components aggregator and collagen II, thereby promoting the catabolism of chondrocytes.91 Knockout of IL-6 decreased cartilage catabolism and nociceptive signalling via downstream mediators signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) in post-traumatic OA mice.92 Cytokines, including TNF-α, IGF-1, FGF-2, and IL-6, secreted from skeletal muscle may contribute to cartilage degradation. We need more direct evidence to confirm the effects of cytokines on different tissues of the joints.
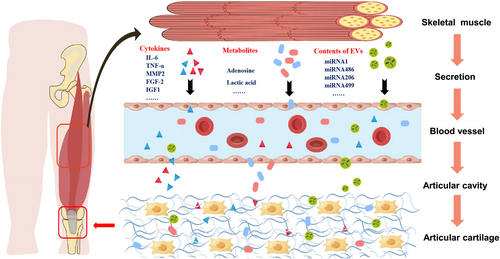
Metabolites like adenosine are key components of skeletal muscle secretion and play a significant role in physiological and pathological processes in OA, including the maintenance of articular cartilage homeostasis.93 Adenosine can activate the adenosine A2A receptor (A2AR), increase the expression of cyclic adenosine monophosphate (cAMP) in chondrocytes, reduce the levels of nitric oxide (NO) and PGE2, increase the level of IL-10, and finally reduce inflammation-mediated cartilage damage.85 Mice lacking adenosine A2AR had intact articular cartilage at birth, but they were prone to spontaneous OA at 16 weeks of age, indicating that extracellular adenosine levels are important for cartilage homeostasis maintenance and targeting adenosine A2AR may prevent age-associated OA.86, 94 Thus, skeletal muscle-derived metabolites, such as adenosine, have positive bioeffects on OA.
Moreover, skeletal muscle can also secrete extracellular vesicles such as exosomes, which could participate in the functional regulation of other organs and tissues, including the lung, liver, spleen, brain, heart, and pancreas, via signal transduction.95 The exosome is a type of extracellular vesicle with a diameter of 30–150 nm that plays an important role in various physical and pathological processes by regulating intercellular communication by transporting lipids, nucleic acids, and proteins.96 The exosomes secreted by muscle contain abundant micro RNAs (miRNAs), including miR-1, miR-206, miR-486, and miR-499, which can participate in the physiological and pathological regulation of multiple organs and tissues, such as liver, gut, and adipose tissue, through endocrine and paracrine mechanisms.87, 88 Based on the close vascular connection between the joint synovium and the muscles around the joint, the influence of exosomes and their contents derived from skeletal muscle on the OA synovium and cartilage deserves further exploration. In summary, different skeletal muscle-derived secretions have a specific role in OA. Skeletal muscle-derived cytokines (TNF-α, IGF-1, FGF-2, and IL-6) deteriorate OA, and metabolites (adenosine) ameliorate OA, while the role of skeletal muscle-derived extracellular vesicles is not clear.
Muscle-derived stem cells
The muscle-derived stem cells (MDSCs) have great potential for chondrogenic differentiation and cartilage repair in different OA models. Skeletal MDSCs can promote cartilage repair and reduce the pathological damage of OA. Chemically induced OA is another rodent model for the study of OA. In brief, OA-like arthritis can be induced by the intra-articular injection of a chemical reagent like mono-iodoacetate (MIA). In a study of an MIA-induced OA rat model, skeletal MDSCs with co-transfection of sFlt1 and BMP-4 presented a potent ability for cartilage repair.97 The injection mixture of muscle-derived BMP-4+/sFlt1+ stem cells and platelet-rich plasma effectively promotes cartilage repair in an MIA-induced OA rat model.98 Another study found that lenti-BMP-2/GFP transduction can further enhance the chondrogenic differentiation of human MDSCs in the same animal model.99 To summarize, MDSCs, especially BMP-4+, BMP-4+/sFlt1+, and BMP-2+ MDSCs, can promote the regeneration and repair of cartilage, which can be used as a potential strategy for OA treatment.
Exercise-based interventions for osteoarthritis-related muscle atrophy
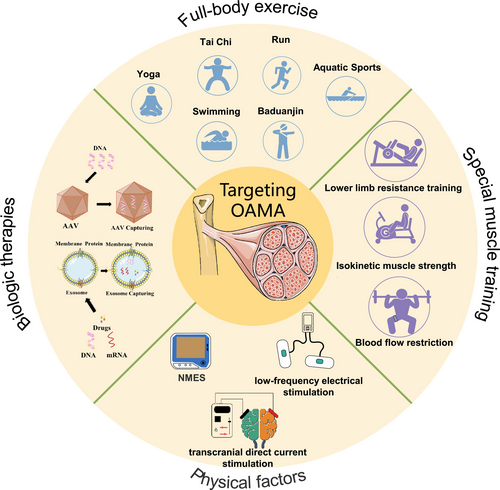
As muscle atrophy is a fundamental pathological change during the process of OA, many therapies of exercise targeting skeletal muscle are effective and non-invasive for the rehabilitation of OA. A network meta-analysis found that exercise has similar positive effects on the symptoms and function of KOA or hip OA compared with oral non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol.100 Among OA rehabilitation plans, skeletal muscle-strengthened exercise is recommended as the first-line treatment by different guidelines, such as those from the American College of Rheumatology/Arthritis Foundation101 and the OA Research Society International (OARSI).102 These guidelines recommend that exercise therapy should be the core strategy for conservative treatments of OA. Abundant clinical trials indeed showed that skeletal muscle-related therapies, including full-body exercise and specific muscle exercise, can effectively improve the clinical symptoms of OA patients, like pain and function of joints (Table 1). Exercise training can help prevent atrophy of the skeletal muscles around joints, maintain joint proprioception, and alleviate neurogenic pain. Future research should focus on determining the optimal exercise parameters for OA patients, including the most effective mode, duration, intensity, and frequency of exercise.
| Exercise modes | Exercise protocols | Pain | Function | Quality of life | Ref. | |
|---|---|---|---|---|---|---|
| Aerobic and/or strength exercises | Aerobic, multisegmental, and joint-specific exercises |
High dose (11 exercises, 70–90 min/time, 3 times/week, 12 weeks) Low dose (5 exercises; 20–30 min/time, 3 times/week, 12 weeks) |
KOOS pain↓ |
KOOS score↑ Sports and recreation of the high-dose group were superior. |
Quality of life of high-dose exercise was superior | 103 |
| Aerobic exercises |
Aerobic walking (15 min), resistance training (20 min), a second walking phase (15 min), and cool-down (10 min) (60 min/time, 3 times/week, 18 months) |
WOMAC pain↓ |
WOMAC function score↑ Mean 6-min walk distance↑ Short Form 36 physical subscale mean scores↑ |
— | 104 | |
| Isokinetic and aerobic exercises | 3 times/week, 6 weeks | KOOS pain↓ | WOMAC score↑ | — | 105 | |
| Quadriceps-strengthening exercise | Leg extension, leg press, and forward lunge exercises each performed in 3 sets of 10 repetitions with loads (3 times/week) | WOMAC pain↓ | WOMAC score↑ | — | 106 | |
| Strength or aerobic exercise |
Strength exercise: balance and resistance Aerobic exercise: stationary cycle (warm-up 10 min, moderate intensity 30 min, low intensity 5 min) (2–3 times/week, 12 weeks) |
NPRS↓ |
Quadriceps muscle strength↑ Maximal oxygen uptake↑ |
KOOS quality of life↔ | 107 | |
| Quadriceps-strengthening exercises | At least 20 min twice a week | WOMAC pain↓ |
WOMAC score↑ Physical function↑ Physical and mental health status↑ |
— | 108 | |
| Quadriceps-strengthening exercise |
Quadriceps strengthening: isometric quadriceps contraction and quadriceps extension (2 times/week, 6 weeks) |
NPRS↓ |
30-s chair stand test↑ Single leg stance↑ |
— | 109 | |
| Neuromuscular exercise | Neuromuscular joint facilitation exercise | Neuromuscular joint facilitation: D1/D2 flexion and extension (2 times/week, 6 weeks) | NPRS↓ |
30-s chair stand test↑ Single leg stance↑ |
— | 109 |
| Neuromuscular quadriceps-strengthening exercise | 14 individually supervised exercise sessions (14 individually supervised exercise sessions) | VAS scores↓ |
WOMAC score↑ Physical function↑ |
— | 110 | |
| Traditional Chinese exercises |
Tai Chi Standard exercise therapy |
Tai Chi (2 times/week, 12 weeks) Standard physical therapy (2 times/week, 6 weeks, followed 6 weeks of monitored home exercise) |
WOMAC pain↓ Between-group difference↔ |
WOMAC score↑ Depression symptoms of the Tai Chi group were superior |
Between-group difference↔ | 111 |
| Tai Chi Qigong training programme | 8 weeks of group Tai Chi Qigong sessions, with 60 min per session twice a week | WOMAC pain↓ |
WOMAC score↑ Physical function↑ |
Quality of life↑ | 112 | |
| Baduanjin | 30-min classes five times a week for 8 weeks | WOMAC pain↓ |
WOMAC stiffness↑ WOMAC physical function↑ |
— | 113 | |
| Baduanjin qigong training | At least 20 min twice a week | WOMAC pain↓ |
WOMAC score↑ Physical function↑ Physical and mental health status↑ |
— | 108 | |
| Wuqinxi exercise |
Aerobic activity/warming up (10–15 min), Wuqinxi exercise (40–45 min), and cool-down (5 min) (60 min/time, 4 times/week, 12 weeks) |
WOMAC pain↓ |
Berg Balance Scale↑ Timed up and go test↑ 6-min walk test↑ 30-s chair stand test↑ Isokinetic muscle strength↑ |
— | 114 | |
| Water exercise | Water exercise |
Trunk, upper, and lower extremity exercise Warming up, stretching, and strengthening exercises (45–60 min/time, 5 times/week, 3 weeks) |
VAS scores↓ WOMAC pain↓ |
WOMAC pain, stiffness, and physical function values↑ | — | 115 |
|
Water exercise Patient-education programme |
Aquatic exercise (16 individual sessions, 2 times/week) Educational programme (group sessions, 1 time/week) |
WOMAC pain↓ |
WOMAC functional capacity values of the water exercise group were superior Depression symptoms↔ |
Quality of life↑ Between-group difference↔ |
116 | |
| Relaxation exercise | Relaxation exercise for total knee arthroplasty patients | Post-operative Days 1, 2, and 3 (1 h/day) | NPRS↓ | Functional outcomes, muscle strength↑ | — | 117 |
|
Graded motor imagery Progressive muscle relaxation |
Explicit and mirror therapy or Jacobson's progressive muscle relaxation (5 times/week, 2 weeks) | WOMAC pain↓ | WOMAC score↑ | — | 118 | |
| Other | Biomechanically based yoga exercise | One of three supervised intervention (1-h group classes/sessions each week) |
KOOS pain↓ Intermittent pain↓ |
Self-reported physical function↑ | Quality of life↑ | 119 |
| Low-intensity resistance exercise therapy based on myofascial chains |
Warm-up and stretching activities 10 min, eccentric movement 3 s, concentric movement 3 s, isometric action 3 s (60–90 min/time, 2–3 times/week, 12 weeks) |
Self-reported less knee pain | Physical function↑ | — | 120 | |
|
Telerehabilitation-based exercise Paper-based exercise |
Telerehabilitation group (home exercise and education prescriptions via an online platform, 8 weeks) Paper group (home exercise and education prescriptions via an instruction manual, 8 weeks) |
Pain of telerehabilitation group was superior |
Knee function and left extremity proprioception of the telerehabilitation group were superior Muscle strength and performance tests↔ |
Quality of life index score↑ | 121 | |
- Abbreviations: KOOS, Knee Injury and Osteoarthritis Outcome Score; NPRS, Numeric Pain Rating Scale; VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; ↑, alleviate or increase; ↓, deteriorate or decrease; ↔, no change.
Perspectives and conclusion
In clinic, the current treatments, including training or exercise targeting the skeletal muscles, have been proven to be partially effective in relieving the symptoms of OA and delaying the progression of OA. However, some factors may negatively influence the effect of training on the skeletal muscles of OA, such as compliance, aging, obesity, chronic inflammation, abnormal glucose, and lipid metabolism. Therefore, we need to explore more strategies, such as exosome therapy, to optimize existing therapeutic models and alleviate OAMA.
A large number of studies have shown that exosomes derived from stem cells have the ability for immune regulation and tissue regeneration.122 Our group previously reported that the exosomes derived from mesenchymal stem cells (MSCs) of infrapatellar fat pads can improve the severity of cartilage pathological damage in DMM mice.123 However, we still do not know whether exosomes derived from stem cells have therapeutic effects on skeletal muscle around OA joints.124 We speculate that exosomes derived from stem cells may serve as a potential treatment strategy for OAMA. In addition to exosomes, more biomaterials that can target skeletal muscle also need to be further studied for the treatment of OAMA.
Overall, OAMA is very common in the clinic and is highly related to the clinical symptoms and pathological progress of OA. However, the mechanisms of OAMA have been largely unknown until now. Interestingly, some signalling pathways, such as transforming growth factor beta (TGFβ)/drosophila mothers against decapentaplegic 3 (SMAD3) and AKT/mammalian target of rapamycin (mTOR), can not only regulate the progression of OA disease125 but also affect the physiological and pathological functions of skeletal muscle.126 However, previous studies mainly focused on the regulation of these signalling pathways on the intra-articular tissues during OA, such as cartilage, subchondral bone, and synovium, which rarely involved the studies of skeletal muscle in an OA model or their effects on skeletal muscle around the joints. Therefore, more studies are needed to focus on the pathological characteristics and specific mechanisms of skeletal muscle during the OA process so as to facilitate the development of comprehensive anti-OA strategies targeting OAMA (Figure 6).
Now, it is generally accepted that OA is a total joint disease that can even affect the tissues outside the joint, including the surrounding muscles. For example, the pathological injuries of KOA involve cartilage, meniscus, synovium, subpatellar fat pad, joint capsule, subchondral bone, and the surrounding quadriceps femoris, hamstring, and calf triceps.2 Most of the previous studies mainly focused on how to improve the quality of articular cartilage, prevent the degeneration of OA joints, and prepare engineered articular cartilage. In recent years, scholars in the field have also begun to study the pathological changes and molecular mechanisms of other tissues inside the joint, such as subchondral bone, synovium, and subpatellar fat pad, during the OA process. However, current studies on the mechanisms of tissues outside the OA joint are very limited. The tissues outside of the joint, such as surrounding muscles, are very important for joint stability and motor function, which are the major targets of rehabilitation treatment in the clinic. In the future research agenda of OAMA, more new modern technology methods such as single-cell sequencing and multi-omics could be used to study the pathological changes and detailed mechanisms of skeletal muscle in OA so as to improve the clinical therapeutic effect of OA.
There are several rodent models that can be used to study the pathological mechanisms of human OA. Surgery trauma such as MNX, DMM, and ACLT surgery alters joint biomechanics and induces pathological changes similar to human OA after ACL or meniscal injury.78 In addition to surgery-induced OA models, aging can induce OA via damage to articular cartilage homeostasis.81 The most common and mature rodent OA models include DMM-induced and aging-associated OA models. Furthermore, surgically induced OA like DMM surgery appears qualitatively similar to aging-associated OA in mice.127 The pathological changes and mechanisms of different animal models are dissimilar in many respects,128 but all of them could partially mimic the progress of OA, especially for the cartilage damage. Besides, different rodent models lead to different pathological changes of OA, which is similar to the different types of human OA induced by different inducements. Several studies have used different rodent OA models to illustrate the same research question.129 Further studies of OAMA with different animal models are needed.
OAMA is a common pathological change during the progression of OA. Different influence factors induced specific biological changes in skeletal muscle. However, there are several factors that contribute to muscle atrophy, including but not limited to aging13, 130 and disuse.14, 131 In addition to the effects of aging and disuse on muscle atrophy, the OAMA may be induced by other specific factors, such as an imbalance of the force line, an aberrant gait, heightened fatigue, and inadequate activation.2 The synergism between the above-specific factors and common factors like aging and disuse contributes to the progress of OAMA. The pathological changes in OAMA, including chronic inflammation, oxidative stress, and disruptions in ion and glycolipid balance, may be caused by OA-related insufficient movement, force line imbalance, and abnormal gait. Conversely, muscle atrophy can contribute to the onset and progression of OA. There are bidirectional relationships between OA and muscle atrophy that need to be explored in future research.
Exercise is the most important method to enhance muscle mass in clinical practice, but there are still some problems during the treatment of OAMA. Repeated joint pain seriously limits OA patients' ability to follow appropriate exercise prescriptions, resulting in poor compliance and prognosis. In addition, due to the complex pathogenesis of OAMA, the therapeutic effect of exercise training alone is still limited. Therefore, we need to explore more effective and convenient methods, including drugs, physical factors, and biological therapy, combined with exercise, to improve the clinical symptoms and outcomes of OAMA in the future.
Conflict of interest statement
The authors declare that there are no personal, organizational, or financial conflicts of interest.
Ethics statement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship.




