Mechanisms of chemotherapy-induced muscle wasting in mice with cancer cachexia
Abstract
Background
Cachexia is a debilitating complication of cancer characterized by progressive wasting and weakness of skeletal muscles that reduces quality of life and can compromise survival. Many anticancer treatments, such as chemotherapy, also cause muscle wasting, which impairs the response to treatment. Given that many cancer patients present with cachexia at the initiation of treatment, we investigated whether cachectic mice were susceptible to chemotherapy-induced muscle wasting and to investigate contributing mechanisms, including the dysregulation of microRNAs (miRs).
Methods
Cachectic colon-26 (C-26) tumour-bearing mice were given 5-fluourouracil (5-FU) chemotherapy or vehicle treatment and analysed for muscle mass, fibre size and composition, and miR expression. Mechanisms were validated in vitro using C2C12 cell culture and miR mimics and inhibitors and were confirmed in vivo by injecting muscles of 5-FU-treated cachectic mice with recombinant adeno-associated viral (rAAV) vectors encoding a miR sponge.
Results
In cachectic tumour-bearing mice, 5-FU chemotherapy exacerbated the loss of skeletal muscle mass compared with vehicle treatment (by −12% to −20%, P < 0.05). miR expression profiling, quantitative real-time PCR, and in vitro analyses revealed contributing mechanisms including miR-351-3p-dependent ERK2 inhibition. Intramuscular injection of rAAV vectors encoding a sponge to reduce miR-351-3p expression in 5-FU-treated cachectic mice enhanced ERK phosphorylation (+18%, P < 0.05) and increased muscle fibre size (+15%, P < 0.01). Hsa-miR-125a-3p shares similar predicted gene targets as mmu-miR-351-3p, and its inhibition in human muscle cells in vitro prevented 5-FU-induced atrophy (P < 0.001) and increased ERK phosphorylation (P < 0.001).
Conclusions
The findings implicate miR-351-3p-mediated ERK2 inhibition as a contributing mechanism in chemotherapy-induced muscle wasting in mice with cancer cachexia and that its inhibition is a promising adjunct therapy for preserving muscles during cancer treatment.
Introduction
Cachexia is the profound weight loss, frailty, and fatigue that affects many cancer patients. It is a multifactorial syndrome that manifests as progressive wasting of skeletal muscle (and typically fat) and is associated with significant functional impairments.1 In the worst cases, cachexia leads to metabolic, respiratory (diaphragm), and/or cardiac (heart) muscle failure and accounts for 20–30% of all cancer-related deaths.2, 3 Indeed, weight loss and body mass index are predictors of survival in cancer patients.4, 5 Cachexia affects 40–80% of all advanced cancer patients with the highest prevalence in those with pancreatic, gastric, oesophageal, colorectal, and lung cancer.6, 7 It can also occur early in disease with weight loss present at diagnosis in >40% of patients with head and neck, gastrointestinal, pancreatic, colorectal, and lung cancers and is associated with reduced response to chemotherapy, increased treatment toxicity, and poorer survival.8-11
Anticancer treatments, such as chemotherapy, can also cause muscle wasting and weakness, with fatigue being one of the most debilitating side effects.12 In patients, cachexia that develops during chemotherapy is associated with poor prognosis.13 Preclinical studies in non-tumour-bearing mice have revealed that muscle wasting and weakness caused by 5-fluorouracil (5-FU)-based chemotherapy combinations result from muscle fibre atrophy, a shift from slow-to-fast fibre types, and mitochondrial dysfunction.14, 15 Furthermore, muscle wasting persisted in the colon-26 (C-26) mouse model of cancer cachexia despite cisplatin chemotherapy reducing tumour size by nearly four-fold.16 Whether existing cachexia alters the effects of chemotherapy on skeletal muscle is unknown, but because many cancer patients present with cachexia when chemotherapy begins,8-11 it is essential to determine whether they remain susceptible to chemotherapy-induced muscle wasting and, if so, to identify the mechanisms responsible. To this end, we focused mechanisms on dysregulated microRNAs (miRs) because they are important contributing regulators of muscle size17 and protein turnover,18 altered miR expression is associated with cancer cachexia19-21 and other conditions of muscle wasting,22, 23 and miR-based therapies are effective and safe to use in vivo.24 We tested the hypothesis that mice with cancer cachexia would remain susceptible to chemotherapy-induced muscle wasting as a consequence of dysregulated miR expression. This information has important implications for the design of adjuvant therapies and the development of strategies that can attenuate chemotherapy-induced muscle wasting, reduce the risk of recurrence, and improve survival for cancer patients.
Materials and methods
Experimental animals
All experiments were approved by the Animal Ethics Committee of The University of Melbourne and conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes as stipulated by the National Health and Medical Research Council (Australia). Male CD2F1 and Balb/c mice were obtained from the Animal Resources Centre (Canning Vale, Western Australia) and housed in the Biological Research Facility at The University of Melbourne under a 12:12 h light–dark cycle. Water and standard laboratory chow were available ad libitum, and consumption was recorded daily.
Effect of 5-fluorouracil chemotherapy in cachectic tumour-bearing mice
Frozen C-26 cells were kindly donated by Prof. Martha Belury (The Ohio State University, Columbus, OH, USA). We have previously described the procedures used to thaw and count the C-26 cells and have characterized the functional and metabolic impairments in mice injected with these cells in detail elsewhere.25
On the day of inoculation (Day 1), 11- to 12-week-old CD2F1 mice were allocated randomly into one of three experimental groups: a control group injected with phosphate-buffered saline (PBS) alone (n = 8); a C-26 tumour-bearing group treated with DMSO vehicle (n = 6); and a C-26 tumour-bearing group treated with 5-FU (n = 7). Mice were anaesthetized via an intraperitoneal (i.p.) injection of a mixture of ketamine (100 mg·kg−1) and xylazine (10 mg·kg−1; VM Supplies, Chelsea Heights, VIC, Australia), such that they were unresponsive to tactile stimuli. Mice were shaved on the dorsal side and given a subcutaneous (s.c.) injection of 5 × 105 C-26 cells suspended in 100 μL of sterilized PBS or 100 μL of sterilized PBS only (control). Mice recovered from anaesthesia on a heat pad and were given an s.c. injection of atipamezole (Antisedan; 1 mg·kg−1; VM Supplies) to partially reverse the effects of xylazine and promote faster recovery from sedation. On Days 15, 18, and 21, lean body mass was assessed (EchoMRI™, Houston, TX, USA) and mice received an i.p. injection of either 60% DMSO vehicle (3.3 μL·g−1 body mass; Sigma-Aldrich, Castle Hill, NSW, Australia) or 5-FU diluted in 60% DMSO (100 mg·kg−1 body mass, 3.3 μL·g−1 body mass; Sigma-Aldrich). The 5-FU dose was based on that used previously to reduce C-26 tumour size26 and predicted to be within the clinical range (50–154 mg·kg−1 lean body mass).27 On Day 22, mice were anaesthetized with sodium pentobarbitone (Nembutal; 60 mg·kg−1; Sigma-Aldrich) via i.p. injection and various hindlimb skeletal muscles [tibialis anterior (TA), extensor digitorum longus, soleus, and plantaris] as well as the epididymal fat, spleen, heart, and tumour were carefully excised, blotted on filter paper, and weighed on an analytical balance. The TA muscles were mounted in embedding medium and frozen in thawing isopentane, and the other tissues were frozen rapidly in liquid nitrogen. All tissues were then stored at −80°C for subsequent analyses. Mice were killed as a consequence of heart excision while still anaesthetized deeply.
Generation and intramuscular injection of rAAV6:eGFP-miR-351-3p sponge in 5-FU-treated mice with cancer cachexia
Methods for the design, cloning, and in vitro validation of the AAV:eGFP-miR-351-3p sponge and scrambled controls are provided in the Supporting Information. Transfection of the AAV:eGFP-miR-351-3p sponge and AAV:eGFP-Scrambled control plasmids with the pDGM6 packaging plasmid into HEK 293 cells generated type 6 pseudotyped viral vectors that were harvested and purified as described previously.28 Briefly, HEK 293 cells were plated on a 10 cm culture dish, 8–16 h prior to transfection with 10 μg of a vector genome-containing plasmid and 20 μg of the packaging plasmid pDGM6, using the calcium phosphate precipitate method to generate pseudotype 6 vectors. Seventy-two hours after transfection, the media and cells were collected and homogenized through a microfluidizer (Microfluidics) prior to 0.22 μm clarification (Millipore). Purified vector was generated from the clarified lysate by affinity chromatography over a HiTrap heparin column (Amersham) and ultracentrifuged overnight prior to resuspension in sterile Hank's balanced salt solution.
For this experiment, we used the more readily available Balb/c mouse model that has an endpoint of 18 days after the injection of C-26 cells, at which time mice exhibit a similar percentage decrease in tumour-free body mass and loss of muscle and heart mass as C-26 tumour-bearing CD2F1 mice at endpoint (21 days after C-26 injection). On the day of inoculation (Day 1), 15- to 16-week-old male Balb/c mice (n = 8) were anaesthetized with isoflurane (induction, 3–4% oxygen–isoflurane at 0.5 L·min−1; maintenance, 2–3% at 0.5 L·min−1), such that they were unresponsive to tactile stimuli. They were then shaved on the dorsal side and given an s.c. injection of 5 × 105 C-26 cells suspended in 100 μL of sterilized PBS. Four days later (Day 5), mice were again anaesthetized with isoflurane, both hindlimbs shaved and the right TA muscle was injected with rAAV6:eGFP-miR-351-3p sponge while the left TA muscle was injected with rAAV6:eGFP-Scrambled sponge at a dose of 5 × 109 vector genomes (determined by optimization pilot studies) in 40 μL of sterilized Hank's balanced salt solution. Mice were given an s.c. injection of buprenorphine (0.05 mg·kg−1) for analgesia and recovered on a heat pad. To match the timing of 5-FU treatments to the CD2F1 model where injections were given every 3 days with the final injection given the day before endpoint, on Days 12, 15, and 18, mice received an i.p. injection of 5-FU diluted in 60% DMSO (100 mg·kg−1 body mass). On Day 19, mice were anaesthetized with sodium pentobarbitone (Nembutal; 60 mg·kg−1) via i.p. injection and the right TA and left TA muscles were carefully excised, blotted on filter paper, and weighed on an analytical balance. The TA muscles were mounted in embedding medium, frozen in thawing isopentane, and stored at −80°C for subsequent analyses. Mice were killed by cardiac excision while still anaesthetized deeply.
Culture, transfection, and treatment of C2C12 cells
Murine C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA) were seeded in 12-well plates and cultured in growth media containing Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Scoresby, VIC, Australia) supplemented with 10% FBS and 1% l-glut (DMEM/10% FBS/1% l-glut) at 37°C + 5% CO2. Upon confluency, the media were changed to DMEM containing 2% (v/v) horse serum (HS, Life Technologies) and 1% l-glut (DMEM/2% HS/1% l-glut, differentiation media) for 4 days to induce differentiation during which time the media were changed every 48 h. For experiments assessing the effect of treatment with various chemotherapeutics, and as per our standard procedures,29, 30 treatment was initiated on Day 4 of differentiation, at which point we observe fully differentiated myotubes. Cells were then incubated for an additional 48 h at 37°C + 5% CO2 in fresh DMEM/2% HS/1% l-glut containing 5-FU (5 μM in 0.0013% DMSO) or vehicle (0.0013% DMSO). Pilot dose–response experiments showed that 5 μM was the lowest dose of 5-FU to induce significant and reproducible atrophy.
For miRNA mimic and inhibitor experiments, murine C2C12 myoblasts were seeded in six-well plates and cultured in growth media at 37°C + 5% CO2. When myoblasts reached ~75% confluency, they were transfected with 25 pmol of miRNA mimic negative control [Applied Biological Materials Inc. (ABM), Richmond, BC, Canada]; mmu-miR-351-3p mimic (ABM); miRNA inhibitor negative control (ABM); or mmu-miR-351-3p inhibitor (ABM) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, USA), according to manufacturer's instructions. At 24 h after transfection, growth media were changed. At 48 h after transfection, the media were changed to differentiation media for 6 days, with media refreshed every 48 h. In these experiments, myotubes were incubated at 37°C + 5% CO2 for 48 h (Days 4–6) in fresh differentiation media with vehicle control (0.0013% DMSO) or 5-FU (5 μM); vehicle control (0.2% DMSO), oxaliplatin (40 μM, Sigma-Aldrich), or cisplatin (50 μM, Sigma-Aldrich); and leucovorin (10 μg·ml−1, Sigma-Aldrich) or irinotecan (20 μg·ml−1, Sigma-Aldrich).
Statistics
All values are expressed as mean ± SEM unless stated otherwise. Groups were compared using Student's t-test or a one-way or two-way ANOVA, where appropriate. Bonferonni's post hoc test was used to determine significant differences between individual groups. The level of significance was set at P < 0.05 for all comparisons except global miRNA expression profiling where cut-offs for significant changes were a fold-change >1.5 and a P value ≤0.01.
Further details about the Materials and methods section are provided in the Supporting Information.
Results
Cachectic mice are susceptible to chemotherapy-induced muscle wasting
As depicted in Figure 1A, cachectic CD2F1 mice bearing C-26 tumours received three intraperitoneal injections of vehicle (DMSO) or 5-FU (Days 15, 18, and 21) and, 1 day after the final injection, were anaesthetized deeply, and the tumour, various skeletal muscles, and organs were carefully excised and weighed. Lean body mass was assessed prior to each treatment, and calculations revealed that mice received an average 5-FU dose of 117 ± 2 mg·kg−1 lean body mass. No signs of 5-FU-related toxicity such as diarrhoea, palmar plantar erythematosus, mucositis, or ataxia were observed. Cumulative food intake was not different between vehicle-treated and 5-FU-treated mice (Figure S1A), but 5-FU increased cumulative water intake from Day 16 (P < 0.0001; Figure S1B). 5-FU had no effect on the percentage change in tumour-free body mass from the start of the experiment (Figure S1C) but reduced relative tumour mass by 42% compared with vehicle (P < 0.001, Figure 1B). Compared with vehicle, 5-FU treatment reduced mass of the soleus (by −13%, P < 0.01), extensor digitorum longus (by −14%, P < 0.05), plantaris (by −9%, P < 0.05), TA (by −11%, P < 0.05, all Figure 1C), and heart (by −8%, P < 0.03, Figure 1D) but had no effect on mass of the epididymal fat or spleen (Figure S1D).
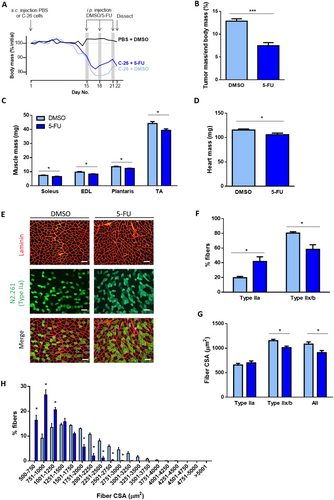
Tibialis anterior muscle cross-sections were reacted for myosin IIa (N2.261, green) and laminin (red) to identify type IIa fibres and to visualize all fibres (Figure 1E), respectively, for assessment of fibre-type proportions and cross-sectional area. Because mouse TA muscles contain very few detectable type I fibres,31 those fibres not reacting to N2.261 were assumed to be type IIx/b fibres. 5-FU induced a fibre-type shift towards more type IIa fibres (+22%, P < 0.05) and fewer type IIx/b fibres (−22%, P < 0.05, Figure 1F) and reduced cross-sectional area of the type IIx/b fibres (−15%, P < 0.05) and average of all muscle fibres compared with vehicle-treated controls (−16%, P < 0.05, Figure 1G). A histogram analysis confirmed that 5-FU reduced the proportion of larger muscle fibres and increased the proportion of smaller muscle fibres in cachectic mice (P < 0.05, Figure 1H).
Chemotherapy-induced muscle wasting is associated with miR-351-3p-dependent ERK2 inhibition
Given that recent studies have demonstrated that cancer cachexia is associated with dysregulated miR-regulated networks,20, 32, 33 we performed global miR expression profiling analysis to identify whether miR expression alters with chemotherapy-induced wasting in cachectic mice. Of the 641 miRNAs measured, 313 (49%) were considered not expressed in mouse plantaris muscle and were therefore excluded from further analysis. Using a cut-off for significant changes of a fold-change >1.5 and P value ≤0.01 (q value <0.05), five miRs were differentially expressed (three up-regulated and two down-regulated) in plantaris muscles from 5-FU-treated mice compared with DMSO vehicle-treated mice (Figure 2A). Of the five differentially regulated miRs, only miR-351-3p was significantly increased with 5-FU when analysed by quantitative real-time PCR (qPCR, by 7.6-fold, P < 0.05, Figure 2B). Gene expression of the three up-regulated miRs was also examined in PBS mice and compared with DMSO vehicle-treated C-26 mice to differentiate between effects of cancer cells and chemotherapy. There were no differences between groups, indicating that the increase in miR-351-3p mRNA expression in 5-FU-treated cachectic mice was due to the chemotherapy and not the cancer cells (data not shown).

The gene targets of mouse miR-351-3p (mmu-miR-351-3p) were predicted on the basis of 3′UTR sequence homology, with 2370 potential targets identified, and these were submitted to KEGG analysis of signalling pathways (Table S1). Of known pathways typically associated with regulation of muscle size,34 the MAPK signalling pathway was the top pathway enriched with predicted gene targets of mmu-miR-351-3p, including Mapk1 (ERK2), Mapk14 (p38), and Mapk8 (JNK1) (Figure 2C and Table S1), and so we investigated the phosphorylation status of these proteins in muscles from vehicle-treated and 5-FU-treated cachectic mice (Figure 2D). 5-FU reduced ERK1/2 phosphorylation by 37% (P < 0.05) but had no effect on phosphorylation of p38 or p54/p46 SAPK/JNK (Figure 2E).
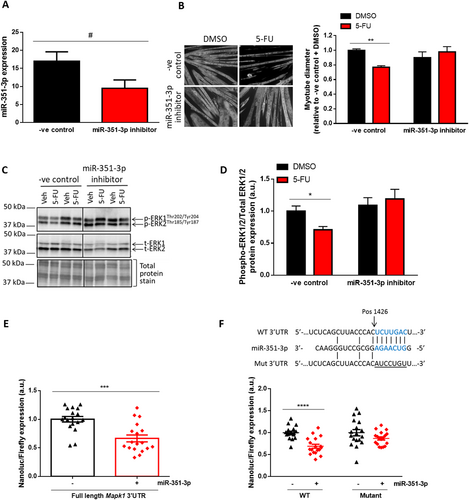
We used the C2C12 cell culture system to examine the direct effects of miR-351-3p modulation on myotube size and/or the phosphorylation status of ERK1/2. C2C12 myoblasts transfected with a miR-351-3p mimic and differentiated into mature myotubes resulted in a modest but significant increase in miR-351-3p expression (Figure 2F) and caused a 23% reduction in myotube size (P < 0.0001, Figure 2G) and a 43% decrease in ERK1/2 phosphorylation (P < 0.05, Figure 2H and 2I).
To further investigate the involvement of miR-351-3p in 5-FU-induced muscle atrophy, C2C12 myoblasts were transfected with a miR-351-3p inhibitor or negative control and, after 4 days of differentiation into myotubes, were treated for a further 48 h with 5-FU or vehicle control. The miR-351-3p inhibitor induced a trend for reduced miR-351-3p expression (P < 0.06, Figure 3A) and completely prevented atrophy induced by 5-FU (P < 0.01, Figure 3B). Furthermore, the miR-351-3p inhibitor prevented the 5-FU-induced decrease in ERK1/2 phosphorylation in C2C12 myotubes (P < 0.05, Figure 3C and 3D). To assess whether the findings reflected effects on proliferation, raw cell counts were taken at 24, 48, and 72 h after transfection and revealed very similar growth curves for C2C12 myoblasts transfected with the miR-351-3p inhibitor and inhibitor negative control (Figure S2A and S2B) and no difference between groups for the mean doubling time (Figure S2C). These findings demonstrate that miR-351 inhibition does not affect the proliferative capacity of C2C12 myoblasts.
Given that the MAPK pathway has been associated with increased expression of the ubiquitin ligases MuRF-1 and atrogin-1, their expression was investigated in the experiments described previously. There was no difference in MuRF-1 (P < 0.44) or atrogin-1 mRNA expression (P < 0.48) in muscles from vehicle-treated and 5-FU-treated cachectic mice (Figure S2D). There was also no effect on MuRF-1 and atrogin-1 mRNA expression of the miR-351-3p mimic (Figure S2E) or of either 5-FU or the miR-351-3p inhibitor (Figure S2F).
To determine whether miR-351-3p could directly bind and regulate the Mapk1 3′UTR, HEK 293 cells were co-transfected with a reporter plasmid containing the full-length mouse Mapk1 3′UTR alongside either a miR-351-3p mimic or an irrelevant miRNA lacking the predicted binding site on the Mapk1 3′UTR (negative control). HEK 293 cells were used for this purpose due to their high transfection efficiency. At 24 h after co-transfection, there was a 33% reduction in luciferase activity in the cells containing the miR-351-3p mimic compared with the negative control (P < 0.001, Figure 3E). We then investigated the miR-351-3p putative binding site on the Mapk1 3′UTR by co-transfecting HEK 293 cells with the miR-351-3p mimic or negative control as well as a reporter plasmid with a shorter version of the Mapk1 3′UTR containing only the putative miR-351-3p binding site (WT, Figure 3F). At 24 h after co-transfection, there was a 31% reduction in luciferase activity (P < 0.0001) in cells containing the miR-351-3p mimic compared with the negative control (Figure 3F). However, this reduction was completely prevented when cells were co-transfected with a shorter Mapk1 3′UTR reporter plasmid in which the miR-351-3p binding site was mutated (Mut, P < 0.13, Figure 3F). Taken together, these data indicate that Mapk1 is a direct target of miR-351-3p.
miR-351-3p inhibition increases muscle fibre size in chemotherapy-treated cachectic mice
To test the therapeutic potential of miR-351-3p inhibition for attenuating chemotherapy-induced muscle fibre atrophy, we generated a AAV:eGFP-miR-351-3p sponge (Figure S3A) and AAV:eGFP-Scrambled (control) sponge plasmid. Function of the AAV:eGFP-miR-351-3p sponge was confirmed with transduction of C2C12 myotubes preventing the 5-FU-associated decrease in myotube size observed with the scrambled sponge (−26%, P < 0.0001; Figure S3B). These findings also confirm that effects of the miR-351-3p inhibitor were directly on myotubes and not myoblasts.
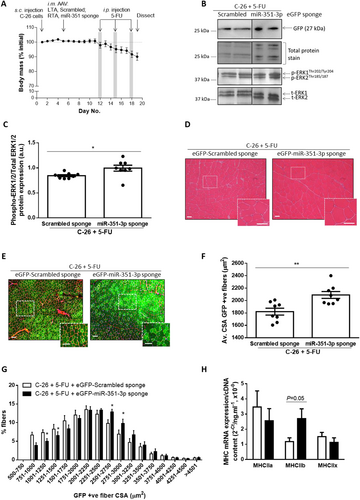
Having confirmed the function of the vector in vitro, we generated AAV6 preparations to test whether AAV6-mediated inhibition of miR-351-3p expression could increase muscle fibre size in 5-FU-treated mice with cancer cachexia. For this experiment, we used the more readily available Balb/c mouse model, which, after the injection of C-26 cells, develops tumours at a similar rate and size as the CD2F1 model (Figure S3C) and, at endpoint (Day 19), exhibits a similar percentage decrease in tumour-free body mass (Figure S3D) and loss of muscle and heart mass as C-26 tumour-bearing CD2F1 mice (Figure S3E). A schematic of the experimental timeline is shown in Figure 4A, with intramuscular injection of AAV6 on Day 5 (4 days after C-26 injection) and 5-FU treatments on Days 12, 15, and 18 to match the timing in the CD2F1 model where injections occurred every 3 days with the final injection given the day before endpoint. At 14 days after intramuscular injection into the TA muscles of cachectic C-26 tumour-bearing mice (Day 19), western blotting for GFP confirmed effective transduction of AAV6:eGFP-Scrambled sponge and AAV6:eGFP-miR-351-3p sponge vectors (Figure 4B), and injection of the AAV6:eGFP-miR-351-3p sponge increased ERK1/2 phosphorylation (+18%, P < 0.05, Figure 4B and 4C). H&E staining revealed no evidence of toxicity after intramuscular injection of AAV6:eGFP-miR-351-3p sponge or AAV6:eGFP-Scrambled control sponge (Figure 4D). Immunohistochemistry was used to differentiate between transduced (GFP positive) and non-transduced (GFP negative) fibres and revealed that the average size of fibres transduced with the AAV6:eGFP-miR-351-3p sponge was greater than those transduced with AAV6:eGFP-Scrambled sponge (+15%, P < 0.01, Figure 4E and 4F), due to a reduced proportion of smaller muscle fibres and an increased proportion of larger muscle fibres (P < 0.05, Figure 4G). qPCR was performed to examine changes in expression of the myosin heavy chain (MHC) isoforms and found an increase in MHCIIb mRNA expression (+130%, P = 0.05, Figure 4H) but no change in MHCIIa or MHCIIx mRNA expression with the AAV6:eGFP-miR-351-3p sponge.
Relevance to other chemotherapeutic drugs and human cancer
To gain a better understanding of the therapeutic potential of miR-351-3p inhibition, we examined the ability of the miR-351-3p inhibitor to attenuate muscle wasting in response to other chemotherapeutic agents. These included leucovorin and oxaliplatin, which are often used in combination with 5-FU (Folfox) as well as irinotecan, used in combination with 5-FU and leucovorin (Folfiri). Cisplatin was also examined as it is used to treat numerous human cancers including lung, ovarian, breast, bladder, head and neck, and testicular cancer. None of the chemotherapeutic agents increased endogenous miR-351-3p mRNA expression (Figure S4A and S4B). When added to C2C12 myotubes in vitro, oxaliplatin and cisplatin reduced myotube size by 23% (P < 0.0001) and 28% (P < 0.0001), respectively; effects were completely prevented by miR-351-3p inhibition (Figure S4C and S4D). Irinotecan increased ERK1/2 phosphorylation (by 1.77-fold, P < 0.01), but neither leucovorin nor irinotecan induced C2C12 myotube atrophy (Figure S4E and S4F) and so were not combined with the miR-351-3p inhibitor. Thus, miR-351-3p inhibition may have broad therapeutic potential across multiple and specific chemotherapy regimens.
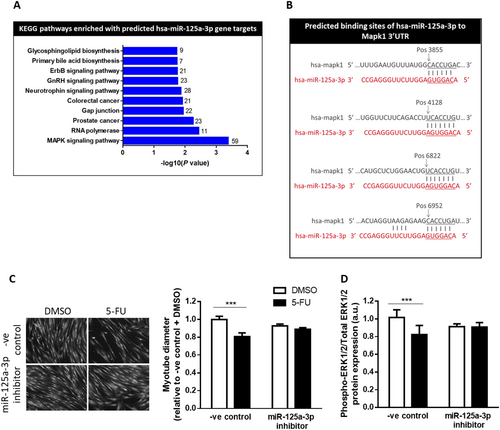
miR-351-3p is not expressed in the human genome, and so we sought to identify a human miRNA targeting similar genes and pathways. Using miRWalk software and KEGG pathway analysis, we found that 16 of the 27 pathways enriched in predicted gene targets of human miR-125a-3p (hsa-miR-125a-3p) were shared with mouse miR-351-3p (mmu-miR-351-3p, Table S2). Furthermore, the MAPK signalling pathway was the top-ranked pathway enriched in genes predicted to be targets of hsa-miR-125a-3p (Figure 5A and Table S2), including MAPK1, which contains four potential miR-125a-3p binding sites (Figure 5B). To assess the relevance of our findings to humans, we tested whether hsa-miR-125a-3p inhibition could attenuate 5-FU-induced atrophy of human primary skeletal muscle cells (Figure 5C and 5D). Transfection of the hsa-miR-125a-3p inhibitor had no effect on the size of DMSO-treated myotubes (P < 0.69) but completely prevented the 5-FU-induced reduction in muscle cell size and ERK1/2 phosphorylation (P < 0.001; Figure 5C and 5D). Therefore, inhibition of hsa-miR-125a-3p, which is predicted to target similar genes and pathways as mmu-miR-351a-3p, has therapeutic potential for attenuating chemotherapy-induced muscle wasting in patients with cancer cachexia.
Discussion
Given that many cancer patients present with cachexia at diagnosis and initiation of anticancer treatment, and because chemotherapy itself can cause muscle wasting and weakness, we investigated whether mice with existing cachexia were susceptible to chemotherapy-induced muscle wasting. Here, we show that in mice with cancer cachexia, 5-FU chemotherapy treatment increased loss of skeletal muscle and heart mass despite reducing tumour mass. As summarized in Figure 6, mechanisms mediating the chemotherapy-associated muscle wasting in cachectic mice include miR-351-3p-dependent ERK2 inhibition. Reducing miR-351-3p levels with an AAV-based sponge in skeletal muscle of 5-FU-treated mice with cancer cachexia enhanced ERK phosphorylation and increased muscle fibre size. These findings identify potential therapeutic targets and strategies to preserve muscle fibres during chemotherapy in patients with cancer cachexia.
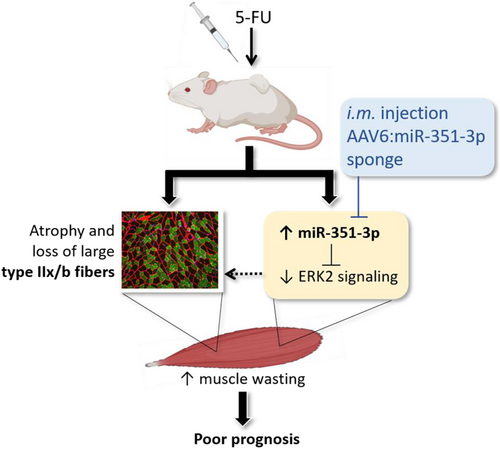
Because more than 40% of patients with gastrointestinal, pancreatic, colorectal, and lung cancer exhibit cachexia upon diagnosis and the initiation of anticancer treatment,8-11 it is clinically relevant to determine whether these patients are susceptible to chemotherapy-induced wasting and, if so, identify potential mechanisms and treatments to preserve skeletal muscle mass and quality of life in an already fragile population. We chose 5-FU-based chemotherapy as it is the common drug in combination therapies such as Folfox (5-FU, leucovorin, and oxaliplatin) and Folfiri (5-FU, leucovorin, and irinotecan) that are used frequently to treat cancers associated with early cachexia. Using 5-FU alone enables identification of its specific effects that could not be discerned with combination therapy. Importantly, the dose received normalized to lean body mass (∼117 mg·kg−1) was within the clinical range (50–154 mg·kg−1).27 We found that 5-FU treatment initiated after C-26 tumour-bearing mice had already began to exhibit cachexia exacerbated chemotherapy-induced wasting of skeletal muscles and the heart. Although cardiac function was not assessed in the present study, it is well known that chemotherapeutic agents cause cardiotoxic side effects,35 with >20% of patients receiving 5-FU exhibiting cardiotoxicity.36 The increased loss of heart mass in 5-FU-treated mice highlights the need for non-5-FU-based chemotherapeutics for cachectic patients in order to reduce toxic effects on the heart. Although 5-FU and other chemotherapeutics are frequently associated with anorexia, this was not observed in the present study, which is likely due to cachectic C-26 tumour-bearing mice already exhibiting anorexia compared with PBS controls.25
The mechanisms underlying chemotherapy-induced muscle wasting in cachectic mice remain largely unknown with most studies having been conducted in non-tumour-bearing rodents,14, 15, 37-41 limiting their clinical relevance. The few studies comparing chemotherapy treatment in tumour-bearing and non-tumour-bearing mice have found that despite treatment inducing similar reductions in muscle mass, mechanisms vary considerably between groups.16, 42 It was therefore essential in the present study to investigate mechanisms underlying the susceptibility of cachectic muscles to chemotherapy-induced wasting, and in this regard, we focused on dysregulated miRs because they are important contributing regulators of muscle size,17 altered miR expression is associated with numerous muscle wasting conditions22, 23 including cancer cachexia,19-21 and miR-based therapies are effective and safe to use in vivo.24 Global miR expression profiling and qPCR analyses revealed mechanisms of 5-FU-induced muscle wasting to include increased expression of miR-351-3p, and using luciferase reporter assays, we demonstrated that ERK2 is a direct gene target of miR-351-3p. Supporting these results, ERK1/2 phosphorylation was decreased in muscles from 5-FU-treated cachectic mice and associated with selective atrophy and loss of the large, glycolytic type IIx/b muscle fibres. These findings are consistent with previous reports of ERK1/2 inhibition inducing muscle atrophy43 and a fibre-type shift.44 However, they contrast with those implicating increased ERK1/2 phosphorylation in the pathophysiology of cachexia in tumour-bearing mice45-47 and those in healthy mice where ERK1/2 activation was associated with Folfox- and Folfiri-induced muscle wasting.15 The discrepancies provide further evidence that different mechanisms are responsible for chemotherapy-induced muscle wasting in tumour-bearing and non-tumour-bearing mice. They may also reflect effects of irinotecan, which increased ERK1/2 phosphorylation in the present study but did not induce C2C12 myotube atrophy. However, they are unlikely to involve effects of leucovorin (used in both Folfox and Folfiri), which did not affect myotube size or ERK1/2 phosphorylation.
This is the first report of miR-351-3p as a mediator of chemotherapy-induced wasting and adds to the growing body of evidence implicating dysregulated miR-regulated networks in cancer cachexia20, 32, 33 and the regulation of skeletal muscle MAPK signalling.48 There is a dearth of information on the role of miR-351-3p in skeletal muscle, but our in vitro experiments revealed that its overexpression reduced C2C12 myotube size and its inhibition did not alter the size of healthy myotubes but completely prevented myotube atrophy induced by chemotherapeutics including 5-FU, oxaliplatin, and cisplatin. Proliferation experiments confirmed that miR-351-3p inhibition did not affect C2C12 proliferation, and transduction of myotubes with an AAV:eGFP-miR-351-3p sponge confirmed direct effects on myotube size. Taken together, these findings suggest that endogenous miR-351-3p expression is low with little effect on the regulation of healthy muscle size but that its expression can increase significantly with some cytotoxic drugs such as 5-FU and contribute to muscle wasting in these conditions. Importantly, inhibition of miR-351-3p can also be beneficial for reducing the muscle wasting associated with other cytotoxic drugs such as oxaliplatin and cisplatin even without an increase in its endogenous expression.
We used intramuscular injection of an AAV-based therapeutic expressing a miRNA sponge to specifically inhibit miR-351-3p expression in a single skeletal muscle and investigate its therapeutic potential for protecting cachectic muscle from chemotherapy-induced wasting. Advantages of this system include that miRNA sponges are plasmid constructs containing multiple high-affinity miRNA antisense binding sites that can inhibit specific miRNAs and prevent their binding to endogenous target genes. Because of tissue limitations, we were unable to assess the unlikely possibility that other miRs were altered with the miR-351-3p sponge, but combining AAV with a tissue-specific promoter and a miRNA sponge limits regulation to a specific organ and avoids potential off-target effects typically associated with systemic delivery of miRNA sponges.49 These benefits have enhanced the likelihood of vector-based strategies manipulating miRNA activity entering the clinic. Because we used a dose of AAV that reduces miR-351-3p levels without inducing toxicity and not transducing all muscle fibres, the addition of a GFP tag to the sponge constructs revealed that transduced (GFP-positive) fibres were significantly larger with the miR-351-3p sponge compared with the scrambled control sponge in 5-FU-treated cachectic mice. These findings demonstrate the utility of our approach for inhibiting miR-351-3p activity in striated muscle and provide proof-of-principle evidence of its therapeutic potential for protecting cachectic muscles from chemotherapy-induced wasting. A limitation of the constructs containing a GFP tag is that we were unable to assess whether the miR-351-3p sponge attenuated the 5-FU mediated fibre-type shift because the filter for immunohistochemical detection of GFP is the same as that for identification of the type IIa fibres. However, qPCR analysis of the mRNA expression of the MHC isoforms expressed in mouse TA muscles supported this notion by revealing that the miR-351-3p sponge increased MHCIIb expression.
Although miR-351-3p is not expressed in the human genome, we identified that human miR-125a-3p shares similar predicted target genes and pathways, including MAPK1 (ERK2) and other members of the MAPK signalling pathway, and is part of the miR-125 family that has been implicated in carcinogenesis and chemoresistance.50 Furthermore, miR-125a-3p inhibition completely prevents 5-FU-induced decreases in ERK1/2 phosphorylation and atrophy of primary human skeletal muscle cells, establishing the relevance of our findings to humans. Thus, local inhibition of miR-125a-3p could help prevent chemotherapy-induced wasting in patients with cancer cachexia. Further development of the viral vector approach for manipulating miRNA activity could include intravascular injection to achieve widespread transduction of striated musculature, including the heart, which exhibits susceptibility to 5-FU-induced wasting in cachectic mice.
In conclusion, mice with cancer cachexia remain susceptible to chemotherapy-induced muscle wasting, with mechanisms including miR-351-3p-dependent ERK2 inhibition. We have identified inhibition of mmu-miR-351-3p/hsa-miR-125a-3p as a potential adjunct therapy for attenuating chemotherapy-induced muscle wasting in patients with cancer cachexia. These findings highlight the importance of initiating strategies to combat cachexia prior to treatment to enhance the effectiveness of chemotherapy and improve cancer prognosis.
Acknowledgements
The authors thank Prof. Martha Belury (Department of Human Nutrition, The Ohio State University) for donating the C-26 cells and Prof. Donna McCarthy (College of Nursing, The Ohio State University) for arranging the shipment of these cells, Dr Marissa Caldow (Centre for Muscle Research, Department of Anatomy and Physiology, The University of Melbourne) for donating the primary human skeletal muscle cells, and Associate Professor Peter Crack (Department of Biochemistry and Pharmacology, The University of Melbourne) for use of the FlexStation 3 machine. The MF 20 monoclonal antibody, developed by D. A. Fischman (Weill Cornell Medical College), and the N2.261 monoclonal antibody, developed by H. M. Blau (Standford University School of Medicine), were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Funding
This study was supported by project grants from the National Health and Medical Research Council (NHMRC, Australia, GNT1041865, to G.S.L. and K.T.M.), Victorian Cancer Agency (16852, to K.T.M.), and Cancer Council Victoria (1120752, to K.T.M. and G.S.L.). K.T.M. was supported by a Career Development Fellowship from the NHMRC (1023178). K.S. was supported by an Early Career Fellowship from the NHMRC (575580) and an Early Career Seed grant from The University of Melbourne.
Conflict of interest
None declared.




