Survival Outcomes and Impact of Targeted PAH Therapy in Portopulmonary Hypertension in the PVRI GoDeep Meta-Registry
Arun Jose, Athiththan Yogeswaran, Khodr Tello, Jean Elwing, and Werner Seeger contributed equally to this study.
ABSTRACT
Portopulmonary hypertension (PoPH), a type of pulmonary arterial hypertension (PAH) in patients with liver disease, is associated with high morbidity and mortality. The relationship between cardiopulmonary hemodynamics, PAH therapy, and survival in PoPH remains unclear. We performed a retrospective cohort study of PoPH patients from the international pulmonary hypertension (PH) meta-registry, PVRI GoDeep. PAH was defined by a mean pulmonary arterial pressure > 20 mmHg, pulmonary arterial wedge pressure ≤ 15 mmHg, and a pulmonary vascular resistance (PVR) > 2 Wood Units. PoPH diagnoses were assigned by each center's PH specialist based on international guidelines at the time of enrollment. 246 incident PoPH patients met eligibility criteria and were included in the analysis, equally split between males (51%) and females (49%), with a median age of 54 years. When compared to both patients with IPAH and those with other subtypes of PAH (not classified as PoPH or IPAH), those with PoPH had significantly lower 5-year survival rates (46% vs. 68% vs. 65%, log-rank p < 0.001). Amongst the PoPH patients, however, there was no significant difference in 5-year survival when dichotomized by disease severity, either by a PVR of 5 Wood Units or a CI of 2.5 L/min/m2. Treatment of the PoPH patients with PAH-targeted therapies was associated with significantly higher 5-year survival rates compared to those not receiving such treatments, as shown by Kaplan–Meier analysis. This survival benefit was observed for PDE5i (50% vs. 34%, log-rank p = 0.029), ERA (58% vs. 34%, log-rank p < 0.001), and the combination of PDE5i and/or ERA (51% vs. 22%, log-rank p < 0.001), as well as any PAH-targeting treatment (50% vs. 26%, log-rank p = 0.007). Corresponding survival advantage was noted when including only PoPH patients with MELD Score ≥ 13. PoPH is a disease with significantly worse long-term survival than other PAH subtypes, but targeted PAH therapy is associated with a robust survival benefit. Survival did not differ across high-risk PVR and cardiac index thresholds, suggesting the factors that influence prognosis and survival in PoPH may be unique as compared to other PAH subtypes, and warrant further investigation.
Research in Context
Evidence Before This Study
Portopulmonary hypertension (PoPH) is a subset of pulmonary arterial hypertension (PAH) that occurs in patients with underlying liver disease. Previous studies have documented the high morbidity and mortality associated with PoPH, but the relationship between hemodynamics, PAH-targeted therapy, and outcomes remains poorly defined. Moreover, while PAH therapies are well established for other PAH subtypes, their impact on long-term survival in PoPH is less clear.
Added Value of This Study
This study includes data from a large, multicenter international registry (PVRI GoDeep) to evaluate outcomes and treatment patterns in a well-characterized cohort of 246 incident PoPH patients. It highlights the distinct clinical trajectory of PoPH compared to other PAH subtypes, including a significantly lower 5-year survival rate. Importantly, these findings demonstrate that PAH-targeted therapies are associated with a survival benefit in PoPH patients, irrespective of the severity of hemodynamic impairment, such as pulmonary vascular resistance (PVR) or cardiac index. These results suggest that the prognostic factors in PoPH may differ from other PAH subtypes and underscores the critical role of PAH-specific therapies in improving outcomes in this population.
Implications of All the Available Evidence
The findings of this study suggest that PoPH represents a unique clinical and pathophysiological entity within the spectrum of PAH, with distinct prognostic factors that may not align with traditional markers of disease severity in other PAH subtypes. This underscores the need for further investigation into the mechanisms driving PoPH prognosis and survival. Clinically, these results support the use of targeted PAH therapies in PoPH patients to improve long-term outcomes, irrespective of initial hemodynamic severity.
1 Introduction
Portopulmonary hypertension (PoPH) is the third most common type of precapillary pulmonary arterial hypertension (PAH), afflicting 5%–6% of all patients with underlying portal hypertensive liver disease [1-3]. Despite exhibiting the same histopathologic changes of pulmonary vascular remodeling and arteriopathy as other PAH subtypes, PoPH patients exhibit higher mortality and confront greater healthcare disparities as compared to those with idiopathic PAH (IPAH) [3-5]. Unfortunately, despite this considerable burden of disease, data concerning the association between cardiopulmonary hemodynamics and clinical outcomes in PoPH is conflicting, even for hemodynamic variables (pulmonary vascular resistance, PVR, and cardiac index, CI) that predict survival in IPAH [6-10]. Additionally, PoPH patients have historically been excluded from the vast majority of randomized controlled trials of PAH therapeutics, and the precise value of treatment with these medications in PoPH remains uncertain [3, 11, 12]. To clarify the relationship between hemodynamic disease severity, targeted PAH therapy, and survival in PoPH, and place these findings in a global context, we performed an analysis of the PVRI GoDeep registry, a large international meta-registry that integrates and harmonizes multiple individual pulmonary hypertension (PH) registries.
2 Methods
2.1 PVRI GoDeep Registry
The PVRI GoDeep meta-registry integrates existing international pulmonary hypertension (PH) registries, ensuring continuous updates of both prospective and retrospective data from integrated local registries [1, 2, 13]. Patient data undergo rigorous harmonization and validation before inclusion, with strict adherence to diagnosis criteria and classification guidelines [1, 14, 15].
As of July 2024, 22 centers have entered a total of 32,533 patients into the meta-registry. Out of these, 246 PoPH patients with complete hemodynamic data were included in the final analysis (see patient flow chart, Figure 1), originating from the PH centers in Giessen (60 PoPH patients), Sheffield (40 patients), Stanford (31 patients), Baltimore (23 patients), London (21 patients), Cincinnati (18 patients), Houston (11 patients), Graz (9 patients), Abu Dhabi (7 patients), Heidelberg (7 patients), Thessaloniki (6 patients), Kiev (5 patients), Rochester (5 patients), and Pavia (3 patients). The University of Giessen/University Hospital Ethics Committee and the responsible local ethics committees have approved the PVRI-GoDeep central data repository (ClinicalTrials.gov; NCT05329714).
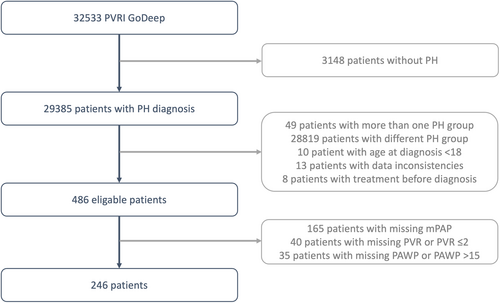
2.2 Patient and Data Selection
From GoDeep, patients were selected by the following criteria: age ≥ 18 years, PH group 1.4.3 as classified by each local center, PAH defined by mean pulmonary arterial pressure (mPAP) > 20 mmHg, PVR > 2 Wood Units (WU), and pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg [1].
The initial diagnosis time was defined as the first right heart catheterization confirming PH, corresponding to registry entry in incident patients. Baseline data covered the period from 1 month prior, to 6 months after, the initial diagnosis. If multiple data points for the same variable were available, the one closest to the initial diagnosis was chosen. PoPH and IPAH diagnoses were determined by each local center's PH specialist group according to international guidelines at the time of enrollment [1, 15]. In addition to the quality control mechanism established at the various PH referral centers, the central GoDeep team conducted plausibility and consistency checks, providing feedback to the referral centers.
2.3 Statistics
Data were extracted from the database on July 5, 2024, and analyzed with R version 4.3.3 using the package survival version 3.5.8 [16]. In this data set, missing values were calculated from other available data whenever possible. Continuous variables were summarized in tables by median [Q1, Q3]. Groups were compared using t-tests, while frequency distributions of categorical variables were compared by Chi-squared tests. A patient was considered to be treated with a medication if at least one report stated that the respective medication was prescribed to the patient.
The multivariable Cox proportional hazards models were fit with the function coxph using data for sex, center, diagnosis decade, World Health Organization (WHO) functional class, body mass index (BMI), PVR, mPAP, Model for End-Stage Liver Disease (MELD) score, sodium level, and the information about treatment with phosphodiesterase-5 inhibitors (PDE5i), endothelin receptor antagonists (ERA), agents addressing the prostacyclin pathway (PGI2, either inhaled, oral, subcutaneous, or intravenous), soluble guanylate cyclase stimulators, and prostacyclin receptor agonists. MELD score was calculated in the standard fashion using serum creatinine, serum bilirubin, and International Normalized Ratio (INR) [17]. Sodium was log-transformed before analysis. Age was included as a natural spline with 2 degrees of freedom and center, diagnosis decade, and sex were added as strata. We also dichotomized the cohort by disease severity, using previously identified thresholds for high-risk in PoPH (PVR > 5 Wood Units, CI < 2.5 L/min/m2), and fitted multivariable Cox proportional hazards models as described above [18, 19]. Missing data were imputed using the package mice version 3.16.0. All variables included in the Cox proportional hazards models were imputed with the exception of treatment information. The plausibility of the distribution of imputed values was verified using diagnostic plots (Figure S1).
The proportional hazards assumption was confirmed by diagnostic plots of the Schoenfeld residuals, and the fits were checked for influential data points by diagnostic plots of the deviance residuals. Statistical significance of the estimated effects of variables on survival was determined by Type III likelihood-ratio tests. The statistical significance of individual coefficient estimates is taken from Wald z-tests. Landmark analyses were utilized to mitigate potential immortal time bias [20]. Overall, the following models were calculated: (a) Base model for the unimputed data set, adjusted for center, diagnosis decade, and sex as strata and age, as natural spline with 2 degrees of freedom. (b) Base model, but additionally using the landmark approach. (c) Full model using the imputed data set, further adjusted for WHO functional class, body mass index, mPAP, PVR, MELD score, and sodium. (d) Full model, using the imputed data set but including additionally the landmark approach.
Sensitivity analyses were performed by estimating Heller Explained Relative Risk, as well as by adding and removing individual covariables and groups of covariables to the base and from the full Cox model, respectively.
3 Results
3.1 Baseline Characteristics
Out of 32,533 enrolled patients, a total of N = 246 incident PoPH patients met eligibility criteria and were included in the subsequent analyses (Figure 1). The median age of the cohort was 54 years, equally distributed between male (51%) and female (49%), with the majority exhibiting World Health Organization Functional Class (WHO-FC) III symptoms (66%) at time of study entry (Table 1). The enrollment period spanned from 1990 to 2024, with most patients (52%) being enrolled in the decade 2010 to 2020. The median body mass index (BMI) was elevated at 29 kg/m2. Functional capacity was impaired with a median 6-min walk test (6MWT) distance of 340 m. As expected of a PoPH cohort, baseline total bilirubin was elevated (median 1.6 mg/dL, interquartile range [0.94, 2.8]), MELD score was elevated (median 13, interquartile range [9.3, 18]), but transaminase levels were normal and renal function at baseline was only slightly diminished (median eGFR 88 mL/min, interquartile range [61, 99]). Among the included patients with available data on comorbidities or diagnoses, 31% had ascites, and alcohol-related liver disease was the most common etiology (25%), followed by viral hepatitis (13%). 211 patients (86%) received PH-targeted therapy, including 179 (73%) treated with PDE5 inhibitors and 124 (50%) with ERAs, while 56 (23%) received prostacyclins—administered inhaled, orally, or parenterally–and 15 (6.1%) sGC stimulators (15, 6.1%).
| Overall | Missing | |
|---|---|---|
| N | 246 | 246 |
| Age at diagnosis (years) | ||
| Median [Q1, Q3] | 54 [47, 61] | 0 (0%) |
| Sex | ||
| male | 125 (51%) | 0 (0%) |
| WHO FC | ||
| I | 4 (2%) | |
| II | 45 (22%) | |
| III | 133 (66%) | |
| IV | 19 (9.5%) | 45 (18%) |
| BMI (kg/m²) | ||
| Median [Q1, Q3] | 29 [25, 34] | 33 (13%) |
| 6MWD (m/6 min) | ||
| Median [Q1, Q3] | 340 [250, 410] | 76 (31%) |
| BNP (pg/mL) | ||
| Median [Q1, Q3] | 130 [38, 290] | 99 (40%) |
| eGFR (mL/min) | ||
| Median [Q1, Q3] | 88 [61, 99] | 17 (6.9%) |
| Sodium (mmol/L) | ||
| Median [Q1, Q3] | 140 [140, 140] | 20 (8.1%) |
| ALT (U/L) | ||
| Median [Q1, Q3] | 25 [17, 39] | 18 (7.3%) |
| Albumin (g/L) | ||
| Median [Q1, Q3] | 34 [27, 39] | 66 (27%) |
| AST (U/L) | ||
| Median [Q1, Q3] | 40 [28, 63] | 28 (11%) |
| Total bilirubin (mg/dL) | ||
| Median [Q1, Q3] | 1.6 [0.94, 2.8] | 23 (9.3%) |
| INR | ||
| Median [Q1, Q3] | 1.2 [1.1, 1.4] | 69 (28%) |
| DLCO %predicted (%) | ||
| Median [Q1, Q3] | 59 [49, 69] | 104 (42%) |
| mPAP (mmHg) | ||
| Median [Q1, Q3] | 47 [38, 55] | 0 (0%) |
| PAWP (mmHg) | ||
| Median [Q1, Q3] | 10 [8, 12] | 0 (0%) |
| PVR (WU) | ||
| Median [Q1, Q3] | 7.2 [5, 9.5] | 0 (0%) |
| CO (L/min) | ||
| Median [Q1, Q3] | 5.2 [4, 6.3] | 0 (0%) |
| CI (L/(min·m²)) | ||
| Median [Q1, Q3] | 2.7 [2.1, 3.3] | 13 (5.3%) |
| CVP (mmHg) | ||
| Median [Q1, Q3] | 7 [4, 11] | 111 (45%) |
| Heart rate (beats/min) | ||
| Median [Q1, Q3] | 76 [65, 85] | 57 (23%) |
| Ascites | ||
| No ascites | 100 (68%) | |
| Yes and refractory to treatment | 15 (10%) | |
| Yes but treatable | 31 (21%) | 100 (41%) |
| Underlying liver disease | ||
| Alcohol-related liver disease | 57 (25%) | |
| Autoimmune hepatitis | 10 (4.3%) | |
| MASH | 8 (3.5%) | |
| Viral hepatitis | 29 (13%) | |
| Other | 127 (55%) | 15 (6.1%) |
| Liver transplantation | ||
| Yes | 12 (4.9%) | 0 (0%) |
| MELD | ||
| Median [Q1, Q3] | 13 [9.3, 18] | 77 (31%) |
| PDE5i | ||
| Treated | 179 (73%) | 0 (0%) |
| ERA | ||
| Treated | 124 (50%) | 0 (0%) |
| sGC stimulators | ||
| Treated | 15 (6.1%) | 0 (0%) |
| PGI2 | ||
| Treated | 56 (23%) | 0 (0%) |
| PRA | ||
| Treated | 16 (6.5%) | 0 (0%) |
| PDE5i & ERA | ||
| Treated | 98 (40%) | 0 (0%) |
| ERA & PGI2 | ||
| Treated | 23 (9.3%) | 0 (0%) |
| PDE5i & PGI2 | ||
| Treated | 49 (20%) | 0 (0%) |
| PDE5i & ERA & PGI2 | ||
| Treated | 21 (8.5%) | 0 (0%) |
| One or more PH drugs | ||
| Treated | 211 (86%) | 0 (0%) |
- Note: Patient characteristics for PoPH patients at baseline.
- Abbreviations: 6MWD = 6-minute6-min walk distance, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BNP = B-type natriuretic peptide, CI = cardiac index, CVP = central venous pressure, CO = cardiac output, DLCO %predicted = diffusing capacity of the lung for carbon monoxide percent predicted, eGFR = estimated glomerular filtration rate, ERA = endothelin receptor antagonists, INR = International Normalized Ratio, MASH = metabolic dysfunction-associated steatohepatitis, MELD = Model of End Stage Liver Disease, mPAP = mean pulmonary arterial pressure, PAWP = pulmonary arterial wedge pressure, PDE5i = phosphodiesterase-5 inhibitors, PGI2 = prostaglandin I2 and its analogs (inhaled or parenteral), PoPH = portopulmonary hypertension, PRA = Prostacyclin receptor agonists, PVR = pulmonary vascular resistance, sGC = soluble guanylate cyclase, WHO FC = WHO functional class, WU = Wood Units.
The median follow-up time was 2.9 [0.7, 5.1] years. During the follow-up period, 12 patients (4.9%) underwent liver transplantation, at which point they were censored. Lung transplantation, considered an event (i.e., lung-transplant-free survival), was rare, occurring in four patients.
Consistent with a PAH cohort, baseline pulmonary function testing excluded obstruction (median ratio of forced expiratory volume at 1 s to forced vital capacity [FEV1/FVC] of 0.75, interquartile range [0.70, 0.81]) and restriction (median total lung capacity [TLC] of 96% of predicted, interquartile range [85%, 110%]), but a diffusion capacity limitation for carbon monoxide (DLCO) was observed at baseline (median DLCO 59% of predicted, interquartile range [49%, 69%]). The cohort had severe PoPH at baseline, with a median mPAP of 47 mmHg (interquartile range [38, 55]; only one patient with mPAP > 20 and < 25 mmHg according to the extended PH guideline definition), a median PVR of 7.2 Wood Units (interquartile range [5, 9.5]), and a median CI of 2.7 L/min/m2 (interquartile range [2.1, 3.3]). As expected for patients with underlying liver disease, this included low (CI < 2 L/min/m2), medium (CI 2–4 L/min/m2) and high (CI > 4 L/min/m2) CI hemodynamic patterns, without major differences in Kaplan–Meier survival curves between these hemodynamic groups (Figure S2). The majority of the cohort (86%) was ultimately treated with targeted PAH therapy. Further demographic and clinical characteristics of the cohort, stratified by hemodynamic disease severity and treatment characteristics, are listed in Table S1.
3.2 Survival Outcomes and Impact of PAH-Targeting Therapy in PoPH
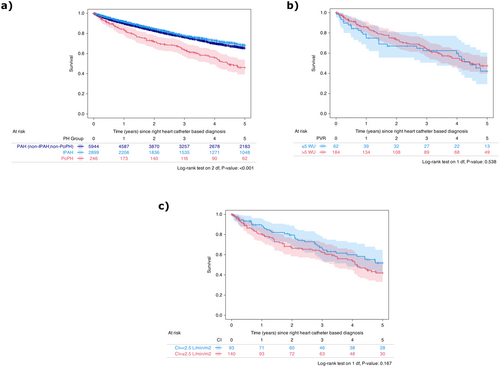
When compared to both patients with IPAH and those with other subtypes of PAH (not classified as PoPH or IPAH), those with PoPH had significantly lower 5-year survival rates (46% vs. 68% vs. 65%, log-rank p < 0.001, Figure 2a), though the median PVR of the PoPH group was, on the average, lower than the median PVR of the entire PAH group excluding PoPH (7.2 WU [5.0, 9.6] vs. 8.4 WU [4.9, 12.9]). Amongst the PoPH patients, however, there was no significant difference in 5-year survival when dichotomized by disease severity, either by a PVR of 5 Wood Units (42% vs. 47%, log-rank p = 0.538, Figure 2b) or a CI of 2.5 L/min/m2 (52% vs. 42%, log-rank p = 0.167, Figure 2c).
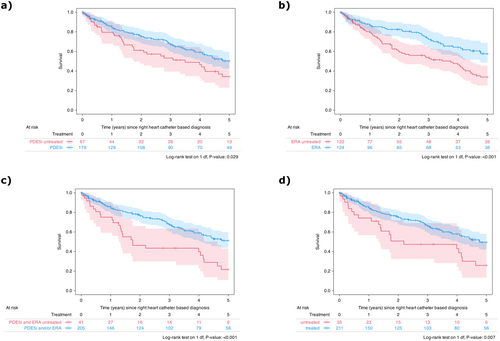
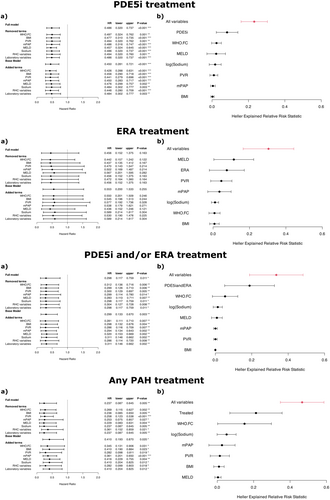
In the PoPH cohort, treatment with PAH-targeted therapies was associated with significantly higher 5-year survival rates compared to those not receiving such treatments, as shown by Kaplan-Meier analysis. This survival benefit was observed for PDE5i (50% vs. 34%, log-rank p = 0.029), ERA (58% vs. 34%, log-rank p < 0.001), and the combination of PDE5i and/or ERA (51% vs. 22%, log-rank p < 0.001), as well as any PAH-targeting treatment (50% vs. 26%, log-rank p = 0.007, Figure 3). To account for potential confounders, multivariable Cox regression analyses were conducted to calculate adjusted hazard ratios (Figure 4). The base model, which adjusted for age, sex, diagnosis decade, and PH expert center, demonstrated a significant association between PAH-targeted therapies (PDE5i, ERA, PDE5i and/or ERA, or any PAH-targeting treatment) and reduced hazard ratios (Figure 4).
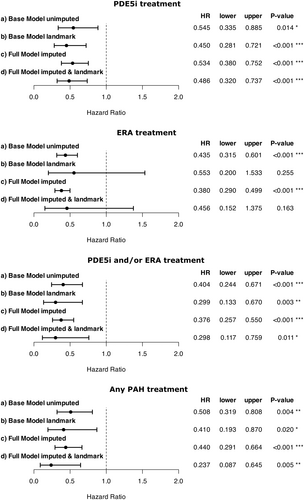
After addressing potential immortal time bias through landmark analysis, the reduction in hazard ratios was confirmed. However, statistical significance was reached only for PDE5i, PDE5i and/or ERA, and any PAH-targeting treatment, but not for ERA monotherapy (Figure 4). This robust association between PAH-targeted therapies and improved survival was further corroborated in the full-model landmark analysis, further adjusting for pulmonary hemodynamics, MELD score, and sodium.
We next examined whether the significant reduction in hazard ratios associated with PAH-targeting therapies persisted in patients with severe underlying liver disease, defined as a high MELD score (≥ 13 points). The full model, accounting for key confounders, demonstrated hazard ratio reductions of 0.496 [0.349, 0.704] (p < 0.001) for PDE5i, 0.231 [0.117, 0.457] (p < 0.001) for ERA, 0.536 [0.289, 0.995] (p = 0.048) for PDE5i and/or ERA, and 0.440 [0.291, 0.664] (p < 0.001) for any PAH-targeting therapy.
3.3 Sensitivity Analyses
Sensitivity analyses using the full imputed model and landmark approach, which assessed the impact of adding or removing individual variables from the full proportional hazards model, confirmed the robustness of a protective effect of PAH-targeted therapies (PDE5i therapy, combined PDE5i and ERA therapy) on survival in PoPH patients (Figure 5). Additionally, the benefit of PAH-targeted therapies was supported by Heller's explanation of relative risk scores.
4 Discussion
In this international cohort study of PH patients, we validated the distinct risk profile of PoPH and demonstrated a strong association between PAH-targeted therapies and improved survival. Our findings indicate that: (i) PoPH patients exhibit significantly lower 5-year survival rates compared to the general PAH population, (ii) commonly used PoPH severity thresholds (i.e., PVR > 5 WU; CI < 2.5 L/min/m2) do not effectively identify patients at increased mortality risk in this highly treated cohort, as shown by Kaplan–Meier analyses, (iii) PAH-targeted therapies provide a robust protective effect on survival in PoPH, and (iv) these therapies are associated with improved hazard ratios even in patients with severe liver disease, as reflected by high MELD scores.
In our cohort, PoPH patients exhibited significantly worse long-term survival compared to IPAH patients or the average across PAH groups. However, high-risk features such as PVR > 5 WU or CI < 2.5 L/min/m2 were not associated with worse survival in PoPH patients, which differs from most other PAH groups. Survival reported in the PVRI GoDeep meta-registry aligns with findings from other PoPH registries in the United States, France, and the United Kingdom, which collectively report a median 5-year survival of 35-50% in PoPH [6-8]. Some of these registry studies identified an association between diminished CI and/or elevated PVR and increased mortality in PoPH [7, 19], but others failed to validate this relationship [8-10]. CI was strongly associated with PoPH survival in previous analyses of the United States Veterans Affairs right heart catheterization database [19] and the French National Center for PAH [7]. However, these cohorts were predominantly treatment-naïve, with PAH-targeted therapies used in only 14.5% and 29.2% of patients, respectively. In contrast, in cohorts more comparable to ours—where the majority of PoPH patients received PAH-targeted therapy—CI and other cardiopulmonary hemodynamics were not associated with mortality, and no consistent relationships between hemodynamics and survival were observed [8-10]. We thus hypothesize that this discrepancy reflects the protective effect of PAH-targeted therapy in PoPH. In treatment-naive PoPH population, cardiopulmonary hemodynamics likely play a significant role in predicting survival and outcomes alongside hepatic dysfunction. However, in the presence of targeted PAH therapy, this association is mitigated, and the primary determinant of outcomes in PoPH patients with treated and well-controlled pulmonary vascular disease shifts to the severity of the underlying liver disease. This dichotomy, along with our findings, underscores the importance of the current approach to PoPH management: prioritizing a reduction in clinical risk and controlling pulmonary vascular disease severity, while also considering liver transplantation in well-controlled PoPH patients [1, 18].
While the beneficial effects of targeted PAH therapy on cardiopulmonary hemodynamics, functional capacity, and exercise tolerance in PoPH patients have long been recognized [21, 22], our findings are the first to provide crucial evidence for a survival benefit of PAH-targeted therapy in PoPH. Notably, we observed a strong protective effect of PAH-targeted therapies on PoPH survival, which persisted after multivariable adjustment, including adjustments for pulmonary hemodynamics and liver disease severity, and was further confirmed through in-depth sensitivity analyses. This is particularly valuable given the conflicting results of prior retrospective studies [6-10]. Reassuringly, the observed survival benefit was also independent of the specific therapeutic agent used, which is notable given the limited evidence from randomized controlled studies supporting PAH therapy in PoPH [11, 12] and the restricted geographic availability of certain PAH therapeutics [23]. Our results also align with current guidelines recommending targeted PAH therapy for all PoPH patients [1, 24], and together suggest that such therapies may be an effective means to improve survival in this high-risk group.
Despite being one of the largest retrospective studies of PoPH patients and benefiting from the international, multicenter scope of the PVRI GoDeep meta-registry, our study has several limitations. First, while PoPH was defined by each participating specialty center, direct portal pressure measurements were not captured. This introduces the possibility that some PoPH patients may have had a different etiology of PAH, potentially influencing our findings [1]. Second, although the PVRI GoDeep meta-registry includes an international cohort, data from non-US and non-European centers were limited, restricting the generalizability of our findings to other regions. A further limitation of the study is that only 14% of the study population was untreated, resulting in a relatively small comparison group. Additionally, the potential for treatment selection bias cannot be fully excluded, and unmeasured patient characteristics may have influenced both treatment decisions and outcomes. Lastly, missing data may have introduced bias into our analyses.
In conclusion, this analysis from an international PH meta-registry supports the classification of PoPH as a high-risk PAH subtype, with one of the poorest long-term survival rates among all PAH groups. Our findings demonstrate a robust survival benefit from PAH-targeted therapy in this population, while showing no prognostic value for high-risk hemodynamic thresholds (i.e., PVR, CI) in well-treated PoPH patients. These results align with current PAH guidelines, which advocate for the use of targeted PAH therapy in all PoPH patients and consideration of liver transplantation in those with well-controlled disease. Moreover, our findings identify prognostic and management factors unique to PoPH compared to other PAH subtypes, supporting the use of targeted PAH therapies as a strategy to improve survival in this high-risk and historically underserved population while underscoring the need for further investigation to refine screening, diagnosis, and treatment strategies.
Author Contributions
Study conceptualization: Athiththan Yogeswaran and Werner Seeger. Study design and data collection: All authors. Data analysis: Athiththan Yogeswaran, Meike Fünderich, and Jochen Wilhelm. Drafting of the manuscript: Arun Jose, Athiththan Yogeswaran, Khodr Tello, Jean Elwing, and Werner Seeger. Critical revision and approval of the manuscript for submission: All authors. Werner Seeger serves as the corresponding author of this study and, as such, takes responsibility for the integrity and accuracy of the data analysis. He has full access to all of the data in the study and has approved the final version of the manuscript.
Acknowledgments
The PVRI-GoDeep Consortium further include the following: Imad Al Ghouleh (University of Pittsburgh, Pittsburgh, Pennsylvania, USA); Jeffrey S. Annis (Vanderbilt University Medical Center, Nashville, Tennessee, USA); Anastasia Anthi (Evangelismos Hospital Athens, Athens, Greece); Tobiah Antoine (Department of Internal Medicine, Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany; Institute for Lung Health (ILH), Cardio-Pulmonary Institute (CPI), Giessen, Germany); Evan Brittain (Vanderbilt University Medical Center, Nashville, Tennessee, USA); John Cannon (Royal Papworth Hospital Cambridge, Cambridge, UK); Stephen Y. Chan (University of Pittsburgh, Pittsburgh, Pennsylvania, USA); Victoria Damonte (University of Cordoba, Cordoba, Argentina); Efrosyui Dima (Evangelismos Hospital Athens, Athens, Greece); Diego Echazarreta (Universidad Nacional de La Plata, La Plata, Argentina); Kai Förster (Ludwig-Maximillians-University Munich, Munich, Germany; Faculty of Medicine, Nursing and Health Sciences, Central Clinical School, Monash University, Melbourne, VIC, Australia); Marlize Frauendorf (Johannesburg, South Africa Milpark Hospital); Anne Hilgendorff (Ludwig-Maximillians-University, Munich, Munich, Germany; Faculty of Medicine, Nursing and Health Sciences, Central Clinical School, Monash University, Melbourne, VIC, Australia); Ernesto Junaeda (University of Cordoba, Cordoba, Argentina); Ingrid King (Murdoch Children's Research Institute, University of Melbourne, Melbourne, VIC, Australia); Ziad Konswa (Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, School of Medicine, Baltimore, USA); Philipp Krieb (Department of Internal Medicine, Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany; Institute for Lung Health (ILH), Cardio-Pulmonary Institute (CPI), Giessen Germany); Kurt Marquardt (Department of Internal Medicine, Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany); Hiromi Matsubara (Okayama Medical Center, Okayama, Japan); Karen Osborn (Pulmonary Vascular Research Institute (PVRI), Canterbury, UK); Joanna Pepke-Zaba (Royal Papworth Hospital Cambridge, Cambridge, UK); Stephan Rosenkranz (Clinic III for Internal Medicine, Department of Cardiology, Heart Center at the University Hospital Cologne, Cologne, Germany; Cologne Cardiovascular Research Center (CCRC), Hospital Cologne and Medical Faculty, Heart Center at the University, University of Cologne, Cologne, Germany); Thenappan Thenappan (University of Minnesota, Minneapolis, Minnesota, USA); Ioan Tilea (George Emil Palade University of Medicine, Targu Mures, Romani); Andrea Varga (George Emil Palade University of Medicine, Targu Mures, Romania); Hellen M. Whitford (Faculty of Medicine, Nursing and Health Sciences, Central Clinical School, Monash University, Melbourne, VIC, Australia; The Royal Children's Hospital, Melbourne, Australia; Department of Respiratory Medicine, The Alfred Hospital, Melbourne, Victoria, Australia; The Royal Children's Hospital, Melbourne, Australia; Department of Respiratory Medicine, The Alfred Hospital, Melbourne, Victoria, Australia); Paul G. Williams (Johannesburg, South Africa Milpark Hospital); Zhenguo Zhei (Department of Respiratory and Critical Care Medicine, China–Japan Friendship Hospital, Beijing, China). This study is funded by the Pulmonary Vascular Research Institute (PVRI) and the Cardiovascular Medical Research and Education Fund (CMREF), NIH.
Ethics Statement
The University of Giessen/University Hospital Ethics Committee and the responsible local ethics committees have approved the PVRI-GoDeep central data repository (ClinicalTrials.gov; NCT05329714).
Conflicts of Interest
Arun Jose reports grants from United Therapeutics and National Institute of Health K23 Career Development, payment from the Law firm of Huff, Powell, Bailey in Atlanta, Georgia, and participation on the advisory board of Merck and Janssen. Athiththan Yogeswaran reports personal fees from MSD and support for attending meetings from AOP Germany. Meike Fünderich has nothing to disclose. David Kiely reports grants from Janssen Pharmaceuticals, National Institute of Health Research Sheffield Biomedical Research Centre, and Ferrer, consulting fees from Janssen Pharmaceuticals, Ferrer, Altavant, MSD, and United Therapeutics, support for attending meetings from Janssen, Ferrer, MSD, and United Therapeutics, and participation on the advisory boards of Janssen and MSD. Andy J. Sweatt has nothing to disclose.
Roham T. Zamanian has nothing to disclose. In addition, Dr. Zamanian has a patent FK506 for the treatment of PAH issued to Stanford University. Paul M Hassoun reports personal fees from Merck Co. Antoine Mouawad has nothing to disclose. Aparna Balasubramanian has nothing to disclose. Martin Wilkins reports personal fees from MorphogenIX, Janssen, Chiesi, Aerami, MSD, Benevolent AI, and Tiakis Biotech, and grants from British Heart Foundation and NIHR, outside the submitted work. In addition, Dr. Wilkins has a patent Zip12 as a drug target issued. Allan Lawrie has nothing to disclose. Luke Howard reports personal fees and nonfinancial support from Janssen, personal fees from MSD, Gossamer, and Altavant. Sandeep Sahay reports personal fees from GossamerBio, Merck, Keros, Janssen, United Therapeutics, Liquidia. Horst Olschewski has nothing to disclose. Gabor Kovacs reports grants from Janssen and Boehringer-Ingelheim, consulting fees from MSD, Boehringer-Ingelheim, AOP Orphan, Chiesi, Ferrer, Bayer, Janssen, GSK, Liquidia, AstraZeneca, United Therapeutics, honoraria from MSD, Boehringer-Ingelheim, AOP Orphan, Chiesi, Ferrer, Bayer, Janssen, GSK, Liquidia, AstraZeneca, support for attending meetings from MSD, Janssen, Boehringer-Ingelheim, and AOP Orphan, and participation on advisory boards from MSD, Boehringer-Ingelheim, Ferrer, and Liquidia. Khaled Saleh has nothing to disclose. Hani Sabbour has nothing to disclose. Christina Eichstaedt reports consulting fees from MSD, honoraria from MSD and OMT, and support for attending meetings from OMT. Ekkehard Grünig reports grants from Acceleron, Actelion, Aerovate, Bayer, Ferrer, Gossamer, Insmed, Janssen, Keros, Liquidia, Merck, MSD, Novartis, OMT, United Therapeutics, consulting fees from Actelion, Ferrer, Janssen, Merck, MSD, honoraria from Actelion, AOP, Bayer, Ferrer, GEBRO, GSK, GWT, Janssen, MSD, OMT, phev, and participation on advisory boards from Actelion, Ferrer, and MSD. George Giannakoulas reports speaker fees from ELPEN Pharmaceuticals, Ferrer/Galenica, GossamerBio, Janssen Pharmaceutical Companies of Johnson & Johnson, MSD, travel fees from ELPEN Pharmaceuticals, Ferrer/Galenica, GossamerBio, Janssen Pharmaceutical Companies of Johnson & Johnson, MSD, and advisory board fees from GossamerBio, Janssen Pharmaceutical Companies of Johnson & Johnson, MSD. Alexandra Arvanitaki has nothing to disclose. Yuriy Sirenko has nothing to disclose. Olena Torbas has nothing to disclose. Hector Cajigas has nothing to disclose. Robert Frantz has nothing to disclose. Laura Scelsi has nothing to disclose. Stefano Ghio has nothing to disclose. Raphael W. Majeed has nothing to disclose. Jochen Wilhelm has nothing to disclose. Hossein Ardeschir Ghofrani reports personal fees from Bayer, Actelion, Pfizer, Merck, GSK, Takeda, and Novartis, and grants from German Research Foundation, Excellence Cluster Cardiopulmonary Research, German Ministry of Education and Research, and other entities outside the submitted work. Friedrich Grimminger has nothing to disclose. Khodr Tello reports personal fees from Bayer, AstraZeneca, and Gossamer during the conduct of the study. Jean Elwing has nothing to disclose. Werner Seeger reports personal fees from United Therapeutics, Tiakis Biotech AG, Liquidia, Pieris Pharmaceuticals, Abivax, Pfizer, and Medspray BV outside the submitted work.




