Practical Considerations for Managing Patients on Tyvaso DPI (Treprostinil Inhalation Powder)
Brittany N. J. Davis was affiliated with United Therapeutics Corporation at the time of manuscript submission.
ABSTRACT
Pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD) require a multifaceted, guideline-directed management approach. This includes active patient participation in partnership with their healthcare team to minimize disease impact and improve survival. Inhaled treprostinil, a prostacyclin analog, has been approved for both PAH and PH-ILD in two formulations: Tyvaso (treprostinil nebulizer) and the more recent Tyvaso DPI (treprostinil dry powder inhaler) [United Therapeutics]. Both formulations deliver therapy directly to the lung vasculature, minimizing the risk of ventilation-perfusion mismatch, reducing systemic exposure, and decreasing the incidence of adverse events commonly associated with parenteral and oral prostacyclin formulations. While practical recommendations for the treprostinil nebulizer have been previously published, Tyvaso DPI provides a well-tolerated, convenient administration option for patients requiring prostacyclin therapy. This review provides an overview of inhaled prostacyclin therapy with a focus on practical considerations for managing PAH and PH-ILD patients treated with Tyvaso DPI. Recommendations from a panel of pulmonary hypertension advanced practice providers include patient selection, education, communication, onboarding and monitoring, transition and titration, side effect mitigation, and the availability of clinician- and patient-facing resources.
1 Introduction
Pulmonary hypertension (PH) is a pathophysiological condition characterized by an elevation in mean pulmonary arterial pressure (mPAP) of > 20 mmHg [1, 2]. PH is classified into five groups, based on characteristic pathophysiology, etiologies, and hemodynamic characteristics, each with specific therapeutic management approaches. Among these are two forms of precapillary PH, Group 1, pulmonary arterial hypertension (PAH), and pulmonary hypertension associated with underlying interstitial lung disease (PH-ILD), a subgroup of Group 3 PH.
PAH is considered rare, affecting 15–50 individuals per million [3, 4]. It is a progressive, life-threatening condition associated with poor quality of life and decreased survival [4, 5]. Of the foundational PAH treatments, prostacyclin therapy plays a crucial role in improving hemodynamics, exercise tolerance, and survival [1, 6, 7]. Conversely, PH-ILD is one of the most common forms of PH, developing in 5%–15% of early-stage ILD and up to 86% in later stages requiring transplant [3, 4, 8-11]. It is characterized by inflammation, scarring, fibrosis, and arterial thickening in the lungs [8, 12].
The 2022 ESC/ERS Guidelines and the 7th World Symposium on Pulmonary Hypertension (WSPH) provided recommendations for diagnosing and treating pulmonary hypertension, emphasizing the need for a multifaceted approach to effectively manage both PAH and PH-ILD, including active participation of patients in partnership with their healthcare team to minimize the impact of these diseases and improve survival [1, 13].
Inhaled treprostinil, a prostacyclin analog, has been approved for use in both PAH and PH-ILD in the United States. It is available in two formulations: Tyvaso, treprostinil nebulizer, and Tyvaso DPI, treprostinil dry powder inhaler [United Therapeutics Corporation, Research Triangle Park, NC, USA]. These formulations facilitate drug deposition in the distal airways, reducing the risk of ventilation–perfusion mismatch, systemic exposure, and the occurrence of adverse events (AEs) typically associated with parenteral and oral prostacyclin formulations [14].
Tyvaso DPI was approved based on the results of the BREEZE study [7]. BREEZE was an open-label safety and tolerability study in which 51 PAH patients receiving Tyvaso nebulizer treatment initiated equivalent DPI dosing. After 51 weeks, 6 out of 43 patients achieved doses ≥ 96 μg (corresponding to 18 breaths of nebulized Tyvaso) and 1 patient reached 176 μg (corresponding to 33 breaths of nebulized Tyvaso) [7]. Following 3 weeks of DPI treatment, the mean 6MWD significantly improved by 11.5 m, with improvements sustained through the optional extension phase of the study. In the BREEZE study, 98% of patients expressed satisfaction with Tyvaso DPI, highlighting specific preferred attributes over nebulizer therapy, including its handheld size, portability, as well as ease of set up, use, and cleaning [7].
The recent 7th WSPH task force provided guidance for additional therapy, recommending that PAH patients on combination oral therapy who are at intermediate-low risk on a four-tiered risk scale should be considered for add-on inhaled prostacyclin therapy [15]. As with the introduction of any new agent or delivery method, the PH clinician should carefully consider and determine the most appropriate treatment for each patient to optimize outcomes. Over the past decade, several publications provided insightful recommendations for the practical use of inhaled treprostinil and management of PAH and more recently, PH-ILD patients [16-18]. Although this study has provided clinicians with a valuable foundation for the use of inhaled treprostinil treatment, the recent approval of the DPI formulation necessitates an update to practical guidance to better support clinicians and patients.
This review discusses practical considerations in the management of PAH and PH-ILD patients, specific to Tyvaso DPI treatment. Recommendations encompass patient selection, education, communication, onboarding, transitioning from other inhaled therapy, titration methods, monitoring, and mitigation of side effects.
2 Methods
Five expert advanced practice providers (APPs) with a clinical practice scope of 15 (10–27) years [median (range)] treating PAH and PH-ILD patients were convened by the study sponsor (United Therapeutics) to enhance practice recommendations for the use of inhaled treprostinil therapy in PAH and PH-ILD patients. Two virtual interview sessions were conducted by a project organizer supported by the study sponsor to reflect on published literature and for the clinicians to share best practices and real-life experience with Tyvaso DPI or Tyvaso in the management of their patients. A question-and-answer session was included as part of each virtual meeting. Prior to the meeting, a short premeeting survey related to current experience and clinical practice patterns was completed by each attendee. Drawing on the discussions from the interview sessions, a summary of recommendations was drafted, and the clinicians collaboratively refined the recommendations to support clinical practice. Additionally, a PH-ILD patient who transitioned from treprostinil nebulizer to the DPI formulation was interviewed to provide insights into the patient's journey and experience using both inhaled forms of treprostinil. The authors and contributors from the sponsor reviewed the article at each draft and provided editorial suggestions.
2.1 Tyvaso DPI Device Characteristics—Clinical Implications
Tyvaso DPI is a low-flow, high-resistance inhaler that delivers treprostinil powder at a peak inspiratory pressure of 2 kPa for at least 2 s in a single breath. High-resistance devices require a lower inhalation flow rate, and therefore, a lower effort is required [19]. Clinical studies have shown that mean peak inspiratory pressure in both PAH and PH-ILD patients is well above the pressure required to empty the Tyvaso DPI cartridge and deliver the necessary dose, suggesting a wide range of patients with varying lung capacities could be accommodated [20-26]. In addition, high-resistance, low flow devices like Tyvaso DPI have been shown to enhance drug deposition to the targeted sites [27]. Comparatively, low-resistance devices must have the patient create a higher inhalation flow rate to produce the higher inspiratory pressure required to adequately deliver the medication [27]. Other inhaled powder drugs for respiratory diseases typically require much higher inspiratory pressures than Tyvaso DPI, ranging from 20 to 60 kPa [28].
2.2 Setting up for Success—Considerations for Patient Selection for Appropriate Therapy
While treatment with Tyvaso DPI is indicated for the treatment of patients with PAH and PH-ILD, a variety of factors should also be considered to ensure the appropriate therapy is selected and tailored to the patient [29].
Understanding the diverse PAH and PH-ILD patient populations included in Tyvaso clinical studies provides valuable context for these considerations. The TRIUMPH study included patients with WHO/NYHA Functional Class III/IV symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%) [30]. The INCREASE study included PH-ILD patients, including those with idiopathic interstitial pneumonia (IIP; 45%), chronic hypersensitivity pneumonitis (6%), occupational lung disease (2%), combined pulmonary fibrosis and emphysema (CPFE; 25%), and connective tissue disease associated interstitial lung disease (CTD-ILD; 22%), with effect across subgroups being generally similar [31]. Additionally, the BREEZE study, an open-label safety and tolerability trial, included 51 PAH patients that were already receiving stable treatment with the Tyvaso nebulizer and transitioned to Tyvaso DPI [7].
Shapiro and colleagues describe in their 2021 analysis that patients receiving higher doses of inhaled treprostinil had significantly higher rates of drug persistence and survival over 3 years [32]. Patients on higher doses of inhaled treprostinil (> 9 breaths, 4 times daily or 54 μg/session) also had a delayed time to parenteral therapy which may be an indicator of worsening disease. Since Tyvaso DPI formulation may allow for easier and more convenient titration of higher inhaled doses, it could be particularly useful in patients who do not respond to the current maximum dose recommendations without having to switch them to parenteral administration [32]. Additionally, for stable patients who are candidates for de-escalation of parenteral therapy, Tyvaso DPI may be a potential option for transition, however, further research is needed. While these study populations offer a framework for applying the study results to patient care, real-world patient characteristics must also be considered.
Key objective clinical factors in patient selection include PH Group and subtype, a review of current medical therapies such as antifibrotics in PH-ILD patients, supplemental medical therapies including oxygen and assessment of oxygen delivery systems (to monitor for oxygen desaturation during use particularly for patients with high oxygen delivery rates, disease progression or for and any ventilation–perfusion mismatch), functional status, right ventricular metrics, disease stability or progression, and overall current risk status. In addition to clinical factors, patient-specific considerations play a crucial role in determining the appropriateness of Tyvaso DPI. Baseline symptom burden, including the patient's oxygen requirements, baseline cough (noting current mitigation strategies in place), other active medication side effects, motor coordination and capability, compliance history, current work status, available caregiver support, and daily activity level, should be carefully evaluated. While comorbidities should be considered, inhaled treprostinil remains safe and effective even in these patients [33, 34]. A recently published single-center review suggested that inhaled treprostinil was well tolerated by patients with PH, many who had accompanying cardiac or pulmonary comorbidities, and that treatment discontinuation in this group was associated with a higher risk of disease worsening [35]. Patients with physical or cognitive challenges may require additional family or clinical support to manage the administration requirements of Tyvaso DPI. Conversely, the DPI's small size and convenience may make it appealing to patients who are currently engaged in work outside of the home, travel frequently, or lead active lifestyles. Social determinants of health, including the patient's financial situation and insurance coverage, also play a role in available therapy options. Finally, it is essential to evaluate the patient's support systems, including their access to caregivers and healthcare providers, as well as their overall health literacy and emotional readiness for a change in therapy.
Ultimately, a comprehensive and individualized assessment of both clinical and patient-specific factors is crucial for determining whether the Tyvaso nebulizer or DPI is the most suitable treatment option for each patient.
2.3 Educate and Empower—Key Elements of the Patient Discussion
2.3.1 Initial Onboarding
When initiating a PAH or PH-ILD patient on Tyvaso DPI, as initial inhaled treprostinil treatment or transitioning from nebulizer therapy, initial discussions should be comprehensive, deliberate, and delivered in an unrushed manner. The expert panel agreed that the initial APP visit should be scheduled for at least 45 min and include members of the multidisciplinary team, as outlined in existing guidelines [1, 36] (Figure 1).
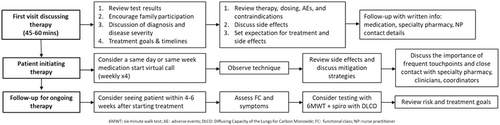
This visit aims to educate the patient about their condition, discuss treatment options, introduce the care team including healthcare providers and clinical coordinators, and explain the role of specialty pharmacy. A significant component of this visit involves reviewing the patient's diagnostic testing results and explaining the routine tests that will be used to monitor their condition and response to treatment (Table 1 and Supporting Information S1: Figure 1). It is important that patients understand the significance of each test and how results guide the determination of disease severity and prognosis and goals for treatment over time.
| Tasks | Provider notes |
|---|---|
| Initial visit patient discussion checklist | |
| Review inhaled treprostinil medication in detail, including mechanism of action, dosing, side effects, and mitigation strategies. | |
| Review medication dose and titration plan. | |
| Review HCP follow-up plan including role of PH care team and specialty pharmacy. Ensure patient knows who to contact for questions or concerns. | |
| Educate on the importance of monitoring pulse oximeter readings at rest, with exertion, and at night. Ensure oxygenation goal is clear and being met with supplemental oxygen requirements as needed. | |
| Discuss patient's self-monitoring of PH symptoms including shortness of breath, fatigue, palpitations, chest pain, presyncope, syncope, and lower extremity edema. Ensure patient knows when they need to contact PH care team. | |
| Conduct risk assessment; share findings with patient (for Group 1 PAH patients). | |
| Plan for follow-up, including any required testing. | |
| Provide encouragement, serve as a supportive advocate, and reinforce the goals of treatment. | |
| Follow-up visit patient discussion checklist | |
| Review patient's clinical status on current PH medication regimen. | |
| Reevaluate risk levels for PAH patients or assess treatment goal achievement for PH-ILD patients. | |
| Evaluate the patient's tolerance to therapy and address any current adverse events. | |
| If treatment escalation is indicated, discuss the rationale and importance of achieving low-risk status or meeting treatment goals. | |
| Review updated dose. | |
| Tailor the titration schedule to best fit the patient's individual needs. | |
| Review medication side effects and discuss mitigation strategies. | |
| Reaffirm treatment goals through shared decision-making. | |
| Plan for follow-up visit, including any required testing. |
Once the patient has a clear understanding of their condition, the clinician should discuss potential treatment options tailored to the patient's clinical and personal circumstances, providing detailed information on the mechanism of action, dosing, route of administration, and potential AEs for each option. After selecting a treatment and mechanism of delivery, it is essential to review the treatment goals, dosing schedule, titration process, and potential side effects. Providing the patient with a list of the anticipated side effects, mitigation strategies, and a booklet or diary for recording frequency, intensity and management practices can be valuable for encouraging medication adherence and success (Supporting Information S2: Supplemental Material 2).
This initial onboarding appointment also serves as an opportunity to establish trust, provide hope, and open lines of communication with the patient and their caregivers. Recognizing that patients may have different learning preferences (e.g., visual, auditory), the use of patient education materials can help reinforce key concepts and provide opportunities for questions. Given the number of care team members involved, it is important that patients are informed about each member's role and how and when to contact them. Additionally, to prevent interruptions in therapy, clinicians should counsel patients to bring their supply of Tyvaso DPI cartridges and inhaler with them when seeking evaluation in the emergency department or if they are admitted to the hospital.
2.4 Side Effect Management and Mitigation—Tools in the Toolbox
Initiation of prostacyclin therapy, especially during dose up-titration, can be accompanied by anticipated adverse effects of the drug [17]. It is highly recommended that patients be informed about these anticipated adverse effects, including their potential severity and duration, and are provided with guidance on how to manage them. Patients should be reassured that these side effects such as cough, as observed in the BREEZE clinical study through the optional extension phase, typically diminish over time [7].
Effective side effect management, along with a clear mitigation plan, is important in ensuring patients can successfully continue and benefit from inhaled prostacyclin therapy. It is also essential for patients and clinicians to differentiate treatment-related side effects and symptoms of worsening disease (Table 2). Clinicians should conduct a comprehensive review of the patient's medical history to account for any factors that could contribute to unanticipated side effects, such as seasonal allergies or other comorbidities, especially in cases where the side effects are persistent or unresponsive to standard supportive measures, such as refractory cough. Special consideration should be given to patients on combination therapies, such as phosphodiesterase type 5 (PDE5) inhibitors, endothelin receptor antagonists (ERAs), or antifibrotics in PH-ILD, as these may increase the frequency or severity of anticipated side effects.
| Treatment-related side effects | Symptoms of worsening pulmonary hypertension |
|---|---|
| Cough | Increased shortness of breath |
| Sore throat | Increased need for oxygen |
| Headache | Decrease in activity tolerance |
| Dizziness | Dizziness |
| Shortness of breath | Edema |
| Blood-tinged sputum | Syncope |
| Fatigue | |
| Hemoptysis |
For patients experiencing persistent side effects, reviewing the DPI device's preparation steps and delivery technique should be the initial mitigation strategy (Figure 2). Correct positioning and optimal breathing technique should be reviewed thoroughly with the patient and their caregiver support team to avoid unwanted side effects. The teach-back method is a beneficial technique in mastering this task and nonshamingly verifying patient understanding [37, 38].
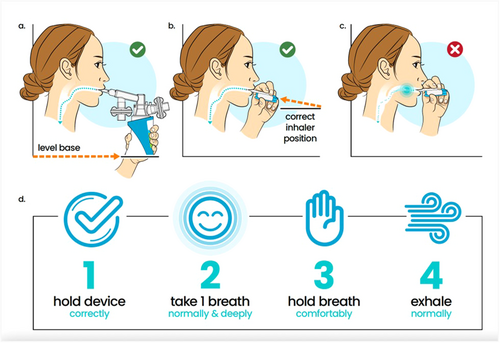
Cough was the most frequently reported side effect in the BREEZE study, occurring in 35% of patients receiving Tyvaso DPI [7]; therefore, addressing cough management should be a key focus during discussions of side effects. Patients should be reminded that DPI cartridges do not require refrigeration, as inhaling cold powder may increase the likelihood of coughing and cause drug accumulation in posterior pharynx. If the cartridges have been refrigerated, they should be brought to room temperature for 10 min before use [29]. Many patients find relief from cough and throat irritation by consuming cold liquids before and after treatments, such as ice-cold water, yogurt, ice cream, or warm liquids such as coffee, or tea with honey. Consuming a small spoonful of honey or peanut butter to coat the throat has also been anecdotally reported as effective. Inhaling the warm, moist air of steam from a humidifier may improve hydration and soothe dry irritated airway passages and lessen cough. Tea, specifically turmeric or ginger, may also help mitigate drug-induced cough and reduce throat inflammation and irritation. Ginger root can be grated and steeped in hot water to make ginger tea or added to other herbal tea blends. Throat pain relievers may act as temporary analgesics and anesthetics. While cough medicine may be used, patients should be cautioned about the risk of aspiration if using cough drops or lozenges. If cough persists or worsens, inhaled steroids, or inhaled short-acting beta-2 agonists, such as albuterol (administered as two inhalations 5 min before DPI treatment), may be considered to relax bronchial smooth muscle and open the airways.
Other common side effects associated with inhaled treprostinil therapy include headache, nausea, and diarrhea, reported in 16%, 6%, and 4% of BREEZE study participants, respectively [7]. Headaches may be managed with anti-inflammatory medications like aspirin or ibuprofen, or analgesics like acetaminophen or tramadol, although headaches may resolve over time or with adjustments to the titration schedule. Persistent nausea may be alleviated with a prescription for ondansetron, while diarrhea can be managed by increasing dietary fiber, which can lessen loose stools, or with loperamide or diphenoxylate/atropine.
It is important for patients and caregivers to understand when to contact their PH care team for support managing adverse effects.
2.4.1 Follow-up Visits
Multiple patient touchpoints may be beneficial, whether through clinic visits, telephone or virtual follow-ups, or communication via the patient portal. It is recommended to schedule an in-person or virtual follow-up with a member of the care team responsible for dosing, titration, and AE management within 4 weeks of initiating treatment, ideally during a time when the patient is expected to administer the treatment. This allows for observation of the patient's breathing technique and the opportunity to provide feedback. Assessing the patient's experience with Tyvaso DPI, including side effects, mitigation strategies, and any necessary treatment adjustments, is a key element of the follow-up appointment [36].
For PAH patients, a risk assessment should be reviewed at every subsequent visit [1, 39]. PH-ILD patients, who are at risk for ventilation–perfusion mismatch with pulmonary vasodilator therapy, should have their oxygen saturation percentage evaluated at rest and with exertion, especially when escalating doses. Additionally, any increased supplemental oxygen needs should be documented through ambulatory oxygen testing and 6-min walk testing (Supporting Information S3: Supplemental Material 3).
At the conclusion of each visit, confirm that the patient and their supporters have had all questions answered. This is also an opportunity to review any changes to the treatment plan and realign care goals as necessary. Encourage patients to adhere to the treatment plan and goals by developing a personalized care plan. It is essential to promote self-efficacy and advocacy in managing symptoms and side effects independently, while emphasizing the importance of promptly reporting significant or worsening symptoms to their healthcare team.
2.5 Treatment Titration
The effectiveness of inhaled treprostinil is a function of dose up-titration, with the goal of achieving the maximum tolerated, clinically appropriate dose for each patient. The INCREASE study demonstrated that higher doses are associated with improved clinical outcomes [40]. Furthermore, a study of PAH patients indicated higher 3-year survival rates for those receiving higher doses of inhaled treprostinil [32, 41]. Notably, clinical benefits have been observed even in cases of disease progression. In an INCREASE post hoc analysis, patients who continued inhaled treprostinil treatment after experiencing a progression event had fewer subsequent progression events [40].
Tyvaso DPI administration requires the patient to inhale a normal, deep breath, lasting 2 s, four times daily. Each treatment involves one or more cartridges, with available dosage strengths of 16, 32, 48, 64, and 80 μg (Figure 3). Cartridges can be combined to achieve higher doses within the same treatment session, if necessary. Doses are typically up-titrated by 16 μg/session at ~1–2-week intervals until the highest tolerated dose is reached. For patients transitioning from nebulizer therapy, the 16 μg titration increment corresponds to approximately three nebulizer breaths (Supporting Information S4: Supplemental Material 4).

It is essential for patients to understand that treatment and dosing are individualized and will progress stepwise based on clinical factors such as tolerability, PH severity, and goals of treatment. During dose up-titration, side effects such as cough, headache, throat irritation, and nausea may intensify. Recommended management strategies for these common side effects are outlined in Table 3.
| Adverse event | Frequency of occurrence in clinical trials | Suggested interventions for mitigation |
|---|---|---|
| Cough | BREEZEa: 35% TRIUMPHb: 54% INCREASEc: 44% |
|
| Headache | BREEZEa: 16% TRIUMPHb: 41% INCREASEc: 28% |
|
| Dyspnea | BREEZEa: 8% INCREASEc: 25% |
|
| Nausea | BREEZEa: 6% TRIUMPHb: 19% INCREASEc: 15% |
|
| Diarrhea | BREEZEa: 4% TRIUMPHb: 10% INCREASEc: 14% |
|
| Sinus issues | — |
|
| Throat irritation | BREEZEa: 4% TRIUMPHb: 14% INCREASEc: 12% |
|
- a BREEZE study period, 3 weeks.
- b TRIUMPH study period, 12 weeks.
- c INCREASE study period, 16 weeks.
Healthcare providers have flexibility in their up-titration approach, depending on the patient's initial response and ability to tolerate the therapy. Some patients may benefit from a “lower and slower” approach or alternative titration plan based on expert opinion. For instance, in cases of worsening cough, the patient can be transitioned to a lower DPI cartridge dose and titrated up more slowly. Alternatively, the patient could be switched to nebulized treprostinil (Tyvaso) to enable smaller dose increments. Anecdotally, using two, lower-dose cartridges instead of one higher-dose cartridge might also be considered. As patients gain experience with inhaled treprostinil, treatment goals and dose should be reassessed and adjusted accordingly.
The overarching goal is to support and encourage the patient throughout their treatment journey, helping them reach a stable, optimal dose.
2.6 Communication and the Care Team
Effective communication and collaboration among the care team are essential in managing patients with PAH and PH-ILD, as emphasized in the most recent ESC/ERS Guidelines and 7th WSPH [1, 13].
The management of these conditions requires a multidisciplinary approach, where patients and their caregivers actively participate in partnership with their healthcare team. Clinical guidelines advocate for patient involvement in decision-making; when patients understand the benefits of their prescribed treatment and management plan, they are more likely to adhere to the recommended approach [1, 36].
Consistency in the guidance provided to patients is crucial for their understanding and compliance. Achieving this consistency requires transparency and communication among the direct clinical care team, specialty pharmacy, and other healthcare professionals. Some centers have implemented multidisciplinary meetings that include representatives from all care team members. This ensures more coordinated and consistent care while reducing discrepancies in messaging and education. Such interdisciplinary models, where all members communicate with each other, help alleviate the burden on patients, minimizing the need for them to relay information between various care team members across different appointments.
Beyond the clinical team and specialty pharmacy, it is vital that patients feel supported throughout the treatment journey. A network of resources, including online communities, in-person support groups, patient volunteer mentors or other patients with treatment experience, often organized by patient advocacy groups or medical associations, can provide valuable support and address questions or concerns patients may have.
2.7 Patient Experience: Catherine's Journey With Tyvaso DPI
No discussion of patient selection, education, treatment titration, and ongoing care of PH-ILD can be complete without considering a real-world perspective on living with PH-ILD. In this section, we offer a perspective from Catherine, who was diagnosed with scleroderma and lupus in 2015. After a series of diagnostic tests in 2018, including a right heart catheterization, she was diagnosed with PH-ILD accompanied by severe lung fibrosis. Reflecting on her active career in IT and past decade as a runner, these diagnoses were far from what she had anticipated.
Initially stable on inhaled treprostinil using the Tyvaso nebulizer, Catherine was invited by her cardiologist to participate in a clinical trial for Tyvaso DPI.
Catherine's experience underscores the critical role of thorough patient education. She expressed deep gratitude for her physician, who provided detailed information about her condition and available treatments with empathy. This foundational knowledge, coupled with her ongoing efforts to expand her understanding, has empowered her to advocate for herself and support others navigating similar challenges.
Catherine emphasized the importance of healthcare providers addressing proper breathing techniques when using Tyvaso DPI. Patients may experience anxiety over whether they are administering the treatment correctly, which can exacerbate their overall stress. She found that step-by-step instructions and ongoing encouragement at each visit were vital in building her confidence. Written materials were also invaluable, allowing her to revisit the information and share it with her husband.
Regarding side effects, Catherine found it helpful to receive a list of potential issues and management strategies. She appreciated guidance that the side effects typically lessen or disappear over time. She advises patients to diligently track their side effects and communicate them to their healthcare team, understanding when to contact them for specific issues. Importantly, Catherine stresses that patients should never discontinue any medication without first consulting their care team.
Catherine also highlights the necessity of adhering to all scheduled appointments and diagnostic tests, including PFTs, echocardiograms, and EKGs. Understanding the significance of these tests and being aware of her numbers has allowed Catherine to take a more proactive role in her healthcare, better anticipating potential adjustments in her therapy. She advises patients to maintain a list of all their medications and supplements to share with their healthcare team.
Finally, Catherine advocates for newly diagnosed patients to engage with support groups and attend educational seminars or webinars. Staying informed and connected to a community provides invaluable tips for disease management and offers opportunities for support and mentorship.
Catherine's journey illustrates the profound impact of comprehensive patient education, effective communication, and multidisciplinary collaboration in managing PAH and PH-ILD. Her experience with Tyvaso DPI highlights the importance of patient-centered care, where understanding, convenience, and continuous support converge to improve outcomes and quality of life.
2.8 Limitations
There are several limitations to these recommendations that the authors acknowledge. There is potential for bias in the selection of experts and the development of surveys. These recommendations were developed by a small cohort of five clinicians and may not represent practice patterns in all geographies where Tyvaso and Tyvaso DPI are available. Further, while drawing on the discussions from the interview sessions with clinicians, a summary of recommendations was drafted, and the clinicians collaboratively refined the recommendations, the recommendations did not follow other systematic consensus approaches such as Delphi methodology with this publication covering several tools, tips, and practical strategies, as also includes a patient author and her perspective. Additionally, the article includes several patient resources. While these resources were reviewed for readability and clinical utility by the clinician authors based on their practice experiences as well as our patient author, subsequent assessments by comprehensive patient panels could further enhance the utility of these materials.
3 Conclusions
In conclusion, Tyvaso DPI provides a practical and portable treatment option for patients with PAH and PH-ILD. The device's low-flow and high-resistance design allows patients to successfully inspire with low effort, dispersing treprostinil with a single, normal, deep breath per cartridge. This formulation enables the delivery of higher doses than those attainable with the nebulized formulation. Further data on dose–response relationships at these higher doses could provide valuable insights into optimizing treatment and improving patient outcomes.
Historically, the absence of standardized screening criteria and the lack of any available treatment options for PH-ILD have led to underdiagnosis and undertreatment of this patient population. The availability of Tyvaso and Tyvaso DPI has begun to address this disparity. Additionally, the recently updated hemodynamic definition of precapillary (mPAP > 20 mmHg, PAWP ≤ 15 mmHg, PVR > 2 Wood Units) PH presents opportunities for earlier diagnosis and intervention. Investigating the outcomes of earlier diagnosis and initiation of treatment with Tyvaso DPI will be important in shaping future treatment strategies.
An individualized and targeted approach, which includes shared decision-making between patients and healthcare professionals, with early intervention, can help ensure that PAH and PH-ILD patients maintain adherence and receive maximum benefit from inhaled treprostinil. The successful management of patients on Tyvaso DPI for PAH and PH-ILD hinges on a strategic, patient-centered approach that begins with careful patient selection and subsequent education as well as ongoing support throughout the treatment journey. The time invested in patient preparedness and a proactive approach at onboarding and titration is key to optimizing therapeutic benefit while significantly improving patient success, reducing the likelihood of treatment discontinuation.
Finally, seamless care team collaboration and fostering a strong patient partnership are essential to achieving optimal outcomes. A multidisciplinary approach, with clear communication, encourages shared decision-making, higher patient satisfaction, and improved health outcomes. Additionally, providing patients with access to a robust support network, including online communities, support groups, manufacturer-provided patient support programs, and patient advocacy resources, ensures they have the necessary support to navigate their condition and treatment.
Incorporating these elements—patient selection, education, dose titration, side effect management, and collaborative care—creates a comprehensive framework for managing PAH and PH-ILD patients on Tyvaso DPI, facilitating a positive treatment experience and ultimately treatment success.
Author Contributions
All authors contributed to the conception and design of the work, critical revision of key intellectual content, and approval of the final version to be published.
Acknowledgments
The authors would like to express our sincere gratitude to our colleagues Kari Roberts, Reem Ismail, Claire Thrasher, Melissa McGruder, Meredith Broderick, and Carolina Lowe for their invaluable contributions. Their insightful review of the manuscript and instrumental role in designing the supplemental resources significantly enhanced the quality and depth of this study. The authors would also like to thank Melanie Nguyen and Priyam Patel for their essential editorial and creative contributions on the supplemental patient resources. Authors also thank April Ingram and Julie Ulloa (Upstart Medical Communications) for their support in medical writing and manuscript preparation. This work was sponsored by United Therapeutics Corporation.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
Jennifer H. Keeley has received honoraria for consulting and speaking services from Johnson & Johnson, Gossamer Bio, and Merck Pharmaceuticals, honoraria for speaking symposiums and consultation from United Therapeutics, and advisory board compensation from Gossamer Bio, Johnson & Johnson, and Merck Pharmaceuticals. Lori Reed has received honoraria for speaking and consultant services for United Therapeutics, Johnson & Johnson, and Merck, and advisory board compensation from United Therapeutics, Johnson & Johnson, and Liquidia. Catherine Falardeau has received honoraria for speaking services for United Therapeutics. Brittany N.J. Davis was an employee of United Therapeutics Corporation at the time of manuscript submission and might have received stock or stock options. Manisit Das is an employee of and may have received stock or stock options from United Therapeutics. Howard Castillo is an employee of and may have received stock or stock options from United Therapeutics. Susanne McDevitt has served as a scientific consultant and sub-investigator for Gossamer Bio, Johnson & Johnson, Liquidia, Merck, and United Therapeutics. Jacqueline Skarre and Loida A. Johnson have nothing to disclose.




