Safety and efficacy of balloon pulmonary angioplasty for technically operable chronic thromboembolic pulmonary hypertension
Jingzhi Wang and Jixiang Liu contributed equally as co-first authors.
Abstract
Balloon pulmonary angioplasty (BPA) has been proven effective for addressing technically inoperable chronic thromboembolic pulmonary hypertension (CTEPH). However, the effectiveness of BPA in technically operable CTEPH patients who, for various reasons, did not undergo the procedure remains an area requiring exploration. This study sought to assess the safety and efficacy of BPA in such cases. We collected and reviewed data from CTEPH patients who underwent BPA in a consecutive manner. Following multidisciplinary team (MDT) decisions, patients were classified into two groups: technically inoperable (group A) and operable (group B). Group B comprised patients deemed technically suitable for pulmonary endarterectomy (PEA) but who did not undergo the procedure for various reasons. All patients underwent a comprehensive diagnostic work-up, including right heart categorization at baseline and the last intervention. This study compared changes in hemodynamic parameters, functional capacity, and quality of life between the two groups. In total, 161 patients underwent 414 procedures at our center, with Group A comprising 112 patients who underwent 282 BPA sessions and group B comprising 49 patients who underwent 132 BPA sessions. Significantly, both groups exhibited improvements in hemodynamics, functional capacity, and quality of life. The occurrence rate of complications, including hemoptysis and lung injury, was similar [12 (63.2%) vs. 7 (36.8%), p = 0.68]. BPA demonstrated favorable outcomes in patients with proximal CTEPH who did not undergo pulmonary endarterectomy. However, the clinical impact of BPA in technically operable CTEPH was found to be less significant than in inoperable cases.
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by the obstruciton of multiple organized occlusive thrombi in the pulmonary artery.1 Patients with CTEPH go through progressive pulmonary hypertension, right heart failure and potentially fatal outcomes if untreated.2, 3 CTEPH could be diagnosed on the basis of the following criteria: (1) the persistence of perfusion defects beyond 3 months of effective antithrombotic therapy for acute pulmonary embolism (PE), with confirmation of chronic thrombus achieved through computed tomography pulmonary angiography (CTPA) or pulmonary angiogram; (2) mean pulmonary artery pressure (mPAP) >20 mmHg confirmed by right heart catheterization (RHC), with pulmonary vascular resistance (PVR) >2 wood units (WU) and pulmonary artery wedge pressure (PAWP) ≤15 mmHg.3, 4
Pulmonary endarterectomy (PEA) is recognized as the standard therapy for operable CTEPH when organized thrombi presented in the main, lobar or segmental arteries.5, 6 However, approximately one-third of CTEPH patients are deemed unsuitable for the operation due to distal lesions, a high risk-benefit ratio, or personal preferences.7, 8 Previous studies have also indicated that BPA may offer promising effects for CTEPH with distal-type lesions.9, 10 Meanwhile, there is limited data supporting the efficacy of BPA for CTEPH with proximal-type lesions.11, 12 In this presentation, we share our experience with BPA treatment for CTEPH patients with proximal-type lesions in China, who were technically operable but did not undergo the procedure.
METHODS
The retrospective study was approved by the institutional review board of the China-Japan Friendship Hospital (No. 2021-136-K94). Written informed consent was waived for this kind of retrospective study in our center.
Patients
We conducted a review of consecutive patients undergoing BPA procedures for CTEPH at China-Japan Friendship Hospital from March 2018 to June 2022. Clinical diagnoses and operational assessments were determined by a multidisciplinary team (MDT), including surgeons, interventional radiologists experienced in pulmonary vascular imaging, and pulmonologists specializing in pulmonary hypertension (PH). Among these patients, 53 were identified with thrombus above the proximal segmental pulmonary arteries (suspected level, Figure 1a,b) and were initially deemed technically operable. However, they opted for BPA due to a high risk-benefit ratio or personal preference. We categorized the patients into two groups: the inoperable group (group A, Figure 1e,f) and the operable group (group B). Group A served as the control group, encompassing patients without PEA indications who ultimately underwent BPA. Group B comprised patients initially eligible for PEA but ultimately undergoing BPA for various reasons. All the patients completed at least one session of BPA.
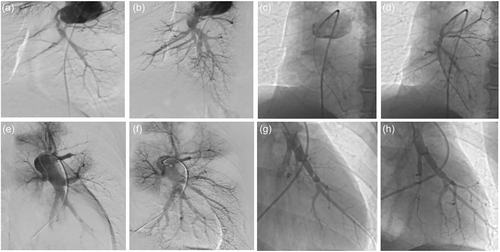
Illustration of pulmonary angiography before and after balloon pulmonary angioplasty. Pulmonary angiography of patients with proximal-type chronic thromboembolic pulmonary hypertension (CTEPH) before (a) and after (b) balloon pulmonary angioplasty. Selective pulmonary angiography showed pulmonary arteries of right lower lobe in the proximal CTEPH before (c) and after (d) balloon pulmonary angioplasty. Pulmonary angiography of patients with distal-type CTEPH before (e) and after (f) balloon pulmonary angioplasty. Selective pulmonary angiography showed pulmonary arteries of right lower lobe in the distal CTEPH before (g) and after (h) balloon pulmonary angioplasty.
Clinical data
All CTEPH patients underwent a standardized diagnosis and assessment work-up before their initial BPA procedure and during the final intervention. Clinical data, encompassing World Health Organization functional class (WHO FC), quality of life questionnaire (emPHasis10), patient health questionnaire-9 (PHQ-9), 6-minute walking distance (6MWD), serum levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP), pulmonary function test results, echocardiography parameters, and hemodynamic data assessed by right heart catheterization (RHC) at rest, were systematically collected and compared.
BPA technique
The standard BPA procedure followed a protocol similar to previous reports.9 BPA was conducted via the femoral vein, utilizing a 6-F guiding catheter inserted into the right or left pulmonary artery through an 80 cm 8 F vascular sheath to achieve a selective approach to the target vessel. Selective pulmonary angiography was performed for patients with both technically inoperable (Figure 1c,d) and operable (Figure 1g,h) CTEPH. Subsequently, a 0.014-inch guidewire was threaded through the target lesions, aided by a balloon catheter or a microcatheter if necessary. The target lesion underwent dilation to an appropriate size through multiple balloon inflations, using semicompliant balloons with a diameter range of 1.5–5.0 mm, depending on the target vessel diameter. In cases where needed, a larger balloon with a diameter range of 8.0–10.0 mm might be employed. Procedures were halted when the accumulated irradiation time reached 1 h or when the contrast medium reached 300 mL. Oxygen was administered at a flow rate of 3–4 L/min to all patients during the procedure.
Complications
Complications related to BPA procedures were categorized into severe and mild complications. Severe complications encompassed situations that necessitated treatment or extended the duration and cost of hospitalization. Mild complications referred to those requiring only observations. In our study, we specifically investigated severe complications, including hemoptysis and lung injury. Mild complications, such as adverse reactions to conscious sedation, were not reported in this study.
Statistical analysis
All continuous variable data are presented as mean ± standard deviation (SD) or as median and interquartile range (IQR) as appropriate. Categorical variables, including gender, medical history, anticoagulant usage, World Health Organization functional class (WHO FC), use of targeted therapy, and the incidence of complications, are expressed as numbers and percentages. The comparison of categorical variables was conducted using the χ2 test for independence or Fisher's exact test. Differences in continuous variables, such as hemodynamic parameters, quality of life, pulmonary function test results, and echocardiography data, were assessed using the independent Student's t test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables. All statistical analyses were performed using R language version 4.0.3 (R Foundation for Statistical Computing). A significance level of p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics and procedures
Data from 165 consecutive patients treated between March 2018 and June 2022 were collected. Excluding four patients who underwent pulmonary endarterectomy (PEA) after the initial intervention, group A comprised 112 patients, and group B comprised 49 patients (including 22 with a high-risk benefit ratio). A total of 414 BPA sessions were conducted for the 161 patients, with 132 BPA sessions in group B and 282 in group A. The study's flowchart is illustrated in Figure 2.
The baseline characteristics of patients with CTEPH before the initial BPA were summarized in Table 1. The mean age of all enrolled patients was 57.8 ± 12.2 years, with 82 (50.9%) of them being female. Table 1 also presented the history of pulmonary embolism (PE), deep vein thrombosis (DVT), chronic obstructive pulmonary disease (COPD), coronary heart disease (CHD), hypertension, and diabetes. All patients received anticoagulant therapy, predominantly with rivaroxaban (64.0%). Furthermore, 101 patients (62.7%) had undergone pulmonary arterial hypertension (PAH) targeted therapy before their initial BPA session.
| All patients (n = 161) | Group A (n = 112) | Group B (n = 49) | |
|---|---|---|---|
| Age, years | 57.8 ± 12.2 | 57.4 ± 12.5 | 57.9 ± 12.5 |
| Female, n (%) | 82 (50.9) | 50 (55.0) | 32 (65.3) |
| BMI, kg/m2 | 24.1 ± 4.5 | 24.5 ± 4.8 | 23.2 ± 5.1 |
| History of PE, n (%) | 106 (65.8) | 74 (66.1) | 32 (65.3) |
| History of DVT, n (%) | 34 (21.1) | 17 (14.0) | 17 (34.7) |
| Comorbidities, n (%) | |||
| COPD | 13 (8.1) | 10 (8.9) | 3 (6.1) |
| Coronary heart disease | 12 (7.5) | 8 (6.6) | 4 (8.2) |
| Hypertension | 42 (26.1) | 26 (23.2) | 16 (32.7) |
| Diabetes | 12 (7.5) | 7 (6.3) | 5 (10.2) |
| Anticoagulants, n (%) | |||
| Warfarin | 45 (28.0) | 33 (29.5) | 12 (24.5) |
| Rivaroxaban | 103 (64.0) | 71 (63.4) | 32 (65.3) |
| LMWH | 13 (8.0) | 8 (7.1) | 5 (10.2) |
| Targeted therapy, n (%) | |||
| PDE5i | 19 (11.8) | 13 (10.7) | 6 (12.2) |
| sGC stimulator | 55 (34.2) | 34 (28.1) | 21 (42.9) |
| ERA | 26 (16.1) | 21 (17.4) | 5 (10.2) |
| Prostacyclin analog | 1 (0.6) | 1 (0.8) | 0 (0.0) |
- Note: Group A: Group of patients with inoperable chronic thromboembolic pulmonary hypertension; Group B: Group of patients with operable chronic thromboembolic pulmonary hypertension.
- Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; ERA, endothelin receptor antagonist; LMWH, low molecular weight heparin; PDE5i, phosphodiesterase 5 inhibitor; PE, pulmonary embolism; sGC, soluble guanylate cyclase.
Treatment response
The effects of BPA, as reflected by changes in hemodynamics, exercise capacity, NT-proBNP levels, and quality of life, are detailed in Table 2. The World Health Organization functional class (WHO FC) showed improvement in 141 (87.6%) patients and remained unchanged in 20 (12.4%) patients. The 6MWD demonstrated an average improvement of 49 meters (13.6% from baseline). Additionally, improvements in cardiac output (CO) and mixed venous oxygen saturation (SVO2) were observed, as outlined in Table 2. The BPA procedure induced improvements in hemodynamics and right ventricular function, along with reductions in forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and diffusing capacity of the lungs for carbon monoxide (DLCO). Simultaneously, the quality of life showed improvement after the BPA procedure, as indicated by emPHasis10 and PHQ-9 scores.
| Group A (n = 112) | Group B (n = 49) | |||||
|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |
| Hemodynamics | ||||||
| Heart rate (bpm) | 70.1 ± 13.2 | 64.3 ± 8.3 | <0.01 | 70.9 ± 14.7 | 65.0 ± 8.9 | 0.04 |
| Mean RAP (mmHg) | 4.6 ± 3.8 | 3.2 ± 2.2 | 0.29 | 4.9 ± 4.5 | 3.4 ± 3.6 | <0.01 |
| Systolic PAP (mmHg) | 70.2 ± 20.3 | 61.1 ± 0.8 | <0.01 | 73.6 ± 17.5 | 63.1 ± 15.8 | <0.01 |
| Diastolic PAP (mmHg) | 23.1 ± 7.0 | 13.9 ± 6.5 | <0.01 | 23.8 ± 6.2 | 17.7 ± 5.5 | <0.01 |
| Mean PAP (mmHg) | 40.4 ± 8.9 | 24.7 ± 10.2 | <0.01 | 39.3 ± 9.7 | 27.9 ± 5.7 | <0.01 |
| Cardiac output (L/min) | 2.9 ± 1.1 | 4.3 ± 1.1 | <0.01 | 3.0 ± 1.0 | 3.7 ± 0.9 | <0.01 |
| PVR (wood unit) | 10.9 ± 6.1 | 5.1 ± 4.6 | <0.01 | 12.2 ± 6.4 | 6.2 ± 2.5 | <0.01 |
| SO2 (%) | 63.9 ± 5.5 | 70.7 ± 7.3 | 0.02 | 64.6 ± 8.3 | 68.2 ± 7.4 | <0.01 |
| NT-proBNP (pg/mL) | 230 (40, 1342) | 205 (10, 569) | <0.01 | 915 (9, 6118) | 215 (12, 2728) | 0.02 |
| 6MWD (m) | 377.1 ± 98.3 | 445.1 ± 80.3 | <0.01 | 361.5 ± 98.8 | 432.0 ± 74.6 | 0.01 |
| WHO-FC (n%) | <0.01 | <0.01 | ||||
| I | 0 (0.0) | 36 (32.1) | 0 (0.0) | 8 (16.3) | ||
| II | 18 (16.1) | 62 (55.4) | 10 (20.4) | 30 (61.2) | ||
| III | 52 (46.4) | 12 (10.7) | 33 (67.3) | 9 (18.4) | ||
| IV | 42 (37.5) | 2 (1.8) | 6 (12.3) | 2 (4.1) | ||
| Pulmonary function | ||||||
| FVC, % pred | 102.9 ± 19.3 | 108.7 ± 19.8 | <0.01 | 101.6 ± 28.1 | 103.4 ± 30.1 | <0.01 |
| FEV1, % pred | 90.0 ± 22.2 | 94.4 ± 22.2 | <0.01 | 81.0 ± 36.2 | 86.3 ± 37.1 | <0.01 |
| DLCO, % predicted | 68.4 ± 15.2 | 70.7 ± 20.5 | 0.11 | 66.4 ± 22.4 | 72.3 ± 22.9 | 0.07 |
| Echocardiography | ||||||
| Pulmonary artery diameter (mm) | 32.6 ± 5.7 | 24.4 ± 8.7 | <0.01 | 33.4 ± 6.5 | 25.3 ± 6.6 | <0.01 |
| RV/LV basal diameter ratio | 1.3 ± 0.4 | 0.9 ± 0.2 | <0.01 | 1.3 ± 0.3 | 1.1 ± 0.3 | <0.01 |
| IVC | 16.6 ± 4.6 | 10.1 ± 3.7 | <0.01 | 14.5 ± 2.1 | 11.8 ± 2.4 | 0.37 |
| Qol | ||||||
| emPHasis10 | 37.0 ± 2.3 | 15.7 ± 1.7 | <0.01 | 39.1 ± 3.1 | 19.0 ± 2.1 | <0.01 |
| PHQ-9 | 10.0 ± 2.1 | 7.0 ± 1.7 | <0.01 | 11.3 ± 2.5 | 6.1 ± 1.6 | <0.01 |
- Note: Values are given as mean ± SD unless otherwise indicate.
- Abbreviations: 6MWD, 6-minute walking distance; BPA, balloon pulmonary angioplasty; DLCO, difusing capacity of carbon monoxide, FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IVC, inferior vena cava; LV, left ventricle; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAP, pulmonary artery pressure; PHQ-9, patient health questionnaire-9; PVR, pulmonary vascular resistance; QoL, quality of life; RAP, right atrial pressure; RV, right ventricle; SVO2, mixed venous oxygen saturation; WHO-FC, World Health Organization functional class.
The mPAP was significantly higher in group B (39.3 ± 9.7 mmHg vs. 27.9 ± 5.7 mmHg, p < 0.01) compared to group A. Similarly, CO was lower in group B (3.0 ± 1.0 L/min vs. 3.6 ± 0.9 L/min, p < 0.05), while PVR was higher (12.2 ± 6.4 WU vs. 6.2 ± 2.5 WU, p < 0.01). Group B also exhibited lower SVO2 (64.6 ± 8.3% vs. 68.2 ± 7.4%, p < 0.05), higher NT-proBNP levels [915 (96,118) pg/mL versus 215 (122,728) pg/mL, p < 0.05], and a shorter 6MWD (380.8 ± 81.8 m vs. 392.6 ± 100.1 m, p < 0.01) compared with group A (Figure 3).
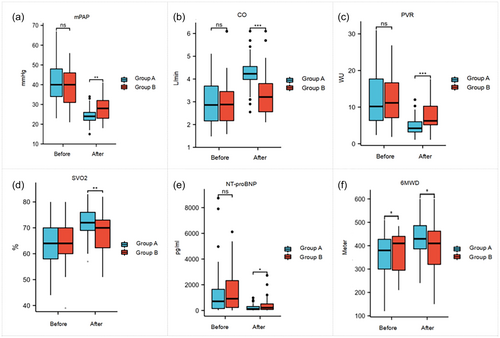
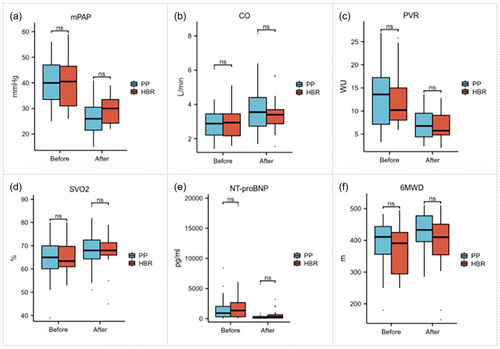
In group B, where 22 patients had a high-risk benefit ratio, significant differences were observed in several parameters: mPAP(40.2 ± 9.2 mmHg vs. 29.5 ± 5.8 mmHg, p < 0.05), CO (2.9 ± 0.9 L/min vs. 3.4 ± 1.0 L/min, P < 0.05), and PVR (12.8 ± 5.9 WU vs. 7.0 ± 3.2 WU, p < 0.05). SVO2 (64.7 ± 9.3% vs. 68.4 ± 8.4%, p < 0.05), NT-proBNP levels [914 (268,386) pg/mL vs. 506 (305,721) pg/mL, p < 0.05], and 6MWD (409.5 ± 64.7 m vs. 431.3 ± 63.0 m, p < 0.05) also showed significant differences. However, when compared with the 27 patients who chose BPA due to personal preference, there was no statistically significant difference between the two groups (Figure 4).
Complications
In a total of 414 BPA sessions, 1971 lesions were treated with BPA (Table 3). Nineteen procedure-related complications occurred during the intervention, including 17 hemoptysis, 2 lung injury. 12 complications occurred in group A and 7 in group B. In the first session of BPA, 10 cases of hemoptysis were observed in the two groups (4 in group A, 6 in group B). In group B, 4 hemoptysis occurred in patient have a high-risk benefit ratio.
| All patients | Group A | Group B | p Value | |
|---|---|---|---|---|
| Sessions, n | 414 | 282 | 132 | |
| Total number of treated lesions, n (%) | 1971 | 1510 | 561 | 0.110 |
| Ring-like stenosis | 490 (25.1) | 330 (23.4) | 160 (29.3) | |
| Web lesions | 864 (44.2) | 670 (47.5) | 194 (35.5) | |
| Subtotal occlusion | 350 (17.9) | 245 (17.4) | 105 (19.2) | |
| Total occlusion | 244 (12.4) | 164 (11.6) | 80 (14.7) | |
| Twisted lesion | 8 (0.4) | 1 (0.1) | 7 (1.3) | |
| Complications, n (%) | 19 (4.6) | 12 (4.3) | 7 (5.3) | 0.682 |
| Hemoptysis | 17 (4.1) | 11 (3.9) | 6 (4.5) | |
| Lung injury | 2 (0.5) | 1 (0.4) | 1 (0.8) | |
| Complications in first session, n (%) | 12 (7.5) | 5 (4.5) | 7 (14.3) | 0.931 |
| Vascular injury of lesion type, n (%) | 10 (6.2) | 4 (3.6) | 6 (12.2) | 0.331 |
| Total occlusion | 6 (3.7) | 2 (1.8) | 4 (8.1) | |
| Subtotal occlusion | 3 (1.9) | 1 (0.9) | 2 (4.1) | |
| Ring-like lesions | 1 (0.6) | 1 (0.9) | 0 (0.0) | |
| Lung injury of lesion type, n (%) | 2 (1.2) | 1 (0.9) | 1 (2.0) | 0.491 |
| Subtotal occlusion | 1 (0.6) | 0 (0.0) | 1 (2.0) | |
| Total occlusion | 1 (0.6) | 1 (0.9) |
The occurrence of hemoptysis in group A included ring-like stenosis (1 case, 0.9%), subtotal occlusion (1 case, 0.9%), and total occlusion (2 cases, 1.8%). In group B, hemoptysis occurred in two cases (4.1%) with subtotal occlusion and four cases (8.1%) with total occlusion after the intervention. Lung injury occurred in both groups, with total occlusion accounting for 0.9% and subtotal occlusion for 2.0%. Overall, no significant difference was observed in the occurrence of complications between groups A and B.
Follow-up
Fifty patients completed 6 months of follow-up, with 16 individuals in group B and 34 in group A (Table 4). All patients remained alive and in good condition during the follow-up period, with no deterioration in symptoms or hemodynamics after 6 months (Figure 5). Regarding the measurement indicators during the follow-up period, there was no statistical significance between the two groups.
| Completed follow-up (n = 50) | Group A (n = 34) | Group B (n = 16) | |
|---|---|---|---|
| Hemodynamics | |||
| Heart rate, bpm | 72.2 ± 10.2 | 73.4 ± 10.4 | 72.1 ± 11.1 |
| Mean RAP, mmHg | 3.6 ± 3.0 | 3.7 ± 2.5 | 3.9 ± 2.2 |
| Systolic PAP, mmHg | 67.1 ± 20.8 | 70.2 ± 20.2 | 60.5 ± 15.6 |
| Diastolic PAP, mmHg | 21.7 ± 7.3 | 23.1 ± 7.4 | 18.7 ± 5.9 |
| Mean PAP, mmHg | 37.9 ± 10.8 | 25.1 ± 9.7 | 32.7 ± 9.3 |
| Cardiac output, L/min | 3.2 ± 1.1 | 3.3 ± 1.0 | 3.0 ± 1.1 |
| PVR, wood units | 9.8 ± 5.0 | 10.1 ± 4.9 | 9.2 ± 5.5 |
| SVO2, % | 64.3 ± 7.6 | 69.2 ± 7.3 | 64.1 ± 6.0 |
| NT-proBNP, pg/mL | 418 (148, 1071) | 423 (183, 1169) | 309 (117, 1010) |
| 6MWD, m | 376.9 ± 83.5 | 376.9 ± 87.2 | 378.7 ± 77.3 |
| WHO-FC, n (%) | |||
| I | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| II | 25 (50.0) | 17 (34.0) | 8 (16.0) |
| III | 20 (40.0) | 14 (28.0) | 6 (12.0) |
| IV | 5 (10.0) | 3 (6.0) | 2 (4.0) |
| Pulmonary function test | |||
| FVC, % pred | 104.3 ± 15.0 | 105.2 ± 15.8 | 102.8 ± 13.8 |
| FEV1, % pred | 88.2 ± 21.3 | 92.3 ± 17.7 | 92.1 ± 10.2 |
| DLCO, % predicted | 73.4 ± 15.9 | 75.4 ± 15.9 | 69.0 ± 15.1 |
| Echocardiography | |||
| Pulmonary artery diameter, mm | 31.3 ± 5.6 | 31.9 ± 6.0 | 29.7 ± 4.1 |
| RV/LV basal diameter ratio | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.0 ± 0.2 |
| IVC, mm | 15.1 ± 3.5 | 15.3 ± 3.6 | 14.6 ± 4.0 |
| Quality of life | |||
| emPHasis10 | 37.0 ± 2.3 | 36.8 ± 1.9 | 36.8 ± 2.0 |
| PHQ-9 | 10.2 ± 2.2 | 10.1 ± 2.0 | 10.3 ± 1.9 |
- Note: Values are given as mean ± SD unless otherwise indicate.
- Abbreviations: 6MWD, 6-minute walking distance; BPA, balloon pulmonary angioplasty; DLCO, difusing capacity of carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; IVC, inferior vena cava; LV, left ventricle; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAP, pulmonary artery pressure; PHQ-9, patient health questionnaire-9; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RV, right ventricle; SVO2, mixed venous oxygen saturation; WHO-FC, World Health Organization functional class.
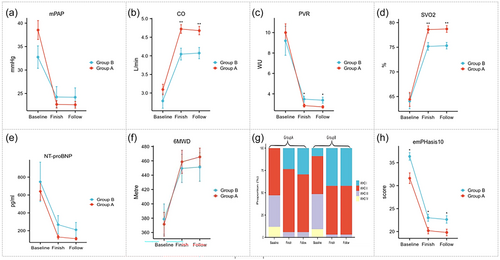
Changes in mPAP, CO, PVR, SVO2, NT-proBNP, 6MWD, WHO-FC, and emPHasis10 during follow-up of 50 patients finished all BPA procedure in groups A and B. Baseline: before the first balloon pulmonary angioplasty; Finish: finish all of procedure; Follow: 6-month follow-up. CO, cardiac output; 6MWD, 6-minute walking distance; mPAP, mean pulmonary artery pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PVR, pulmonary vascular resistance; SVO2, mixed venous oxygen saturation; WHO-FC, World Health Organization functional class. ns, p ≥ 0.05; *, p < 0.05; **, p < 0.01; ***, p < 0.001; for comparisons between groups A and B.
DISCUSSION
In the present study, we elucidated the efficacy and safety of BPA for both technically operable and inoperable CTEPH patients. Our findings indicated BPA produces favorable outcomes in CTEPH patients with proximal lesions who cannot undergo operation. However, there was no discernible difference in safety between inoperable and operable CTEPH patients. Additionally, we underscored the importance of evaluating the evidence of quality of life, providing valuable insights for assessing the effectiveness of BPA during follow-up.
Generally, the enhancement of hemodynamics and exercise capacity has been widely demonstrated through BPA treatment for distal-type lesions.13-15 Cumulative data from multiple centers in Japan have shown positive outcomes in over 308 inoperable CTEPH patients treated with BPA (a total of 1408 procedures). The study reported a notable 44% reduction in mPAP from 43.2 ± 11.0 mmHg to 24.3 ± 6.4 mmHg after undergoing BPA.9 Similar results were observed in our study for patients with inoperable CTEPH. Previously, patients with proximal-type lesions were excluded from BPA due to concerns about its effectiveness and safety.16 However, in 2013, the successful treatment of proximal-type lesions with BPA was reported for the first time. In this report, following treatment, the mPAP in the patients decreased from 41 to 29 mmHg.17 In recent times, there has been a gradual expansion of the indications for BPA.18 In 2019, Arihiro Kiyosue and colleagues reported improvements in hemodynamic and functional parameters achieved through BPA for various types of lesions,19 resembling the effects of PEA. However, it's important to note that this study had a relatively small sample size, enrolling only 33 patients with distal-type lesions and 10 patients with proximal lesions.19 Our study provided relatively more CTEPH cases in the two groups. Compared to group B, group A exhibited advantage in the efficacy of BPA in both aspects of hemodynamics and functional assessment (the decrease of mPAP, 15.8 vs. 8.4 mmHg; the decrease of PVR, 6.2 vs. 4.0WU and the increase of 6MWD, 90.3 vs.54.2 m), the reason may be that the proximal thrombus is not relieved in group B.
Moreover, BPA can improve quality of life and exercise capacity in patients with proximal CTEPH, without increasing the complications. Hemoptysis symptoms and lung injury are common complications of BPA.10 With the modification of BPA technology by Japanese scholars and the development of endovascular technology, the incidence of complications is significantly reduced.20, 21 In 2014, Fukui and colleagues reported no deaths or major complications in 20 CTEPH patients treated with BPA (3.2 ± 0.9 times/case).12 Furthermore, Shun Minatsuki and colleagues reported 13 cases of lung bleeding in 69 BPA sessions for 10 CTEPH patients in the BPA-unsuitable group and 26 cases of lung bleeding in 143 BPA sessions for 33 CTEPH patients in the BPA-unsuitable group in 2019.19 Another study from Japan reported 55 cases of hemoptysis in 475 BPA sessions for 81 patients in group 1 (surgically accessible group) and 173 cases of hemoptysis in 1432 BPA sessions for 263 patients in group 2 (surgically inaccessible group).22 In our study, hemoptysis in 11 patients in group A, 6 in group B. Meanwhile, we examined the complications of different lesion types in both groups. It turned out that there was no significant difference in the occurrence of complications between the two groups. Baseline hemodynamics before the first session were comparable between two groups. Complications were more likely to occur in the operable group, which may be due to injured vessels after the surgery.
Absolutely, addressing symptoms and enhancing the quality of life constitute pivotal aspects of the treatment goals for CTEPH patients. Consequently, the significance of quality of life as a crucial evaluation criterion cannot be overstated. Previous studies have underscored the emPHasis-10 questionnaire as a valid tool for longitudinally measuring the quality of life in patients with CTEPH.23 Paul Hendriks and colleagues further demonstrated a moderate correlation between the emPHasis-10 score and 6MWD at baseline.24 Notably, this correlation persisted both before and after BPA, establishing a comprehensive link between physical capacity and quality of life.25 Moreover, the emPHasis-10 questionnaire boasts the advantage of being easily interpretable by medical staff in clinical practice, rendering it suitable for widespread clinical application.25 It's imperative to recognize that patients with proximal lesions, characterized by a prolonged and intricate disease course, exhibited poorer hemodynamics and cardiac function. This subgroup experienced comparatively less benefit from intervention. This insight emphasizes the multifaceted challenges posed by proximal lesions in CTEPH, shedding light on the complex interplay between disease characteristics and treatment outcomes.
We acknowledge several limitations in our study. First, being a single-center retrospective study, our findings may benefit from validation through future multicenter studies with larger cohorts. Second, the diverse methods used for follow-up information collection, including outpatient consultations, telephone interviews, and interactions with other hospitals, might introduce bias. Third, long-term follow-up studies are essential to strengthen and extend our findings. Lastly, we did not explore the potential effects of combining BPA with PAH-targeting agents, an aspect that warrants investigation in future studies.
CONCLUSIONS
BPA produces favorable outcomes in CTEPH patients with proximal lesions who cannot undergo operation. Compared to the distal lesion, the clinical effect of BPA of proximal lesion was less significant.
AUTHOR CONTRIBUTIONS
Jinzhi Wang and Jixiang Liu collected the clinical information and completed the manuscript. Xincao Tao, Wanmu Xie, Shengfeng Wang, Shuai Zhang, and Zhu Zhang completed data analysis and revised manuscript. Zhihui Fu, Haobo Li, Yunjing Zhang, Yishan Li, Xincheng Li, Yu Zhang, Xincheng Li, and Dong Liu completed data analysis, Qiang Huang, Yunwei Zhao, and Zhenguo Zhai gave the conception and design of the study. All authors approved the final version. Zhenguo Zhai is the guarantor of this research and take responsibility for the integrity of this work.
ACKNOWLEDGMENTS
This study was supported by funds from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-049), National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01-01-02), and China Postdoctoral Science Foundation (2023TQ0383).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All participants provided informed consent, and the Local Ethics Committees approved the study (No. 2021-136-K94).




