On the Statistical Theory of Vacancies in Strongly Anharmonic Crystals
Ordzhonikidze Street 3, 117302 Moscow, USSR.
Abstract
enThe Gibbs free energy of the vacancy formation g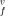 (T, P) in a strongly anharmonic crystal is obtained, using the quasi-chemical approximation for multi-component systems and the improved self-consistent field method for a crystal. The enthalpy h
(T, P) in a strongly anharmonic crystal is obtained, using the quasi-chemical approximation for multi-component systems and the improved self-consistent field method for a crystal. The enthalpy h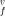 , entropy s
, entropy s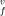 , and volume v
, and volume v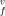 of the vacancy formation are derived versus the temperature and pressure. The vacancy formation parameters, concentration of vacancies, and their effects on the thermodynamic properties of strongly an- harmonic crystals of Ar, Kr, and Xe are calculated along the solid–gas coexistence curves, computed in the preceding paper. The pair potential of Lennard-Jones and those of Barker et al. are used. The three-body interactions are included. All potentials give v
of the vacancy formation are derived versus the temperature and pressure. The vacancy formation parameters, concentration of vacancies, and their effects on the thermodynamic properties of strongly an- harmonic crystals of Ar, Kr, and Xe are calculated along the solid–gas coexistence curves, computed in the preceding paper. The pair potential of Lennard-Jones and those of Barker et al. are used. The three-body interactions are included. All potentials give v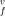 = (1.02 to 1.04) atomic volume over the whole temperature range. The enthalpy and entropy of the vacancy formation decrease with increasing temperature, with the values h
= (1.02 to 1.04) atomic volume over the whole temperature range. The enthalpy and entropy of the vacancy formation decrease with increasing temperature, with the values h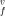 /kT = 9.48 to 9.60 and s
/kT = 9.48 to 9.60 and s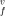 /k = 3.14 to 3.40 at the triple points. The results are compared with experimental data for the thermodynamic properties and also with the available Monte Carlo computations and experimental estimations for the vacancy formation parameters.
/k = 3.14 to 3.40 at the triple points. The results are compared with experimental data for the thermodynamic properties and also with the available Monte Carlo computations and experimental estimations for the vacancy formation parameters.
Abstract
ru[Russian Text Ignored].