Improving the stability of thiol–maleimide bioconjugates via the formation of a thiazine structure
Abstract
Linker stability is critically important for the efficacy and safety of peptide and protein conjugates used for biological applications. One common conjugation strategy, thiol–maleimide coupling, generates a succinimidyl thioether linker with limited stability under physiological conditions. We have shown in previous work that when a peptide with an N-terminal cysteine is conjugated to a maleimide reagent, a thiazine structure is formed via a chemical rearrangement. Our preliminary work indicated that the thiazine linker has favorable stability. Here, we report the evaluation of a thiazine linker as an alternative to the widely used succinimidyl thioether linker for thiol–maleimide bioconjugation. The stability of the thiazine conjugate in comparison to the thioether conjugate was assessed across a broad pH range. Additionally, the propensity for retro-Michael reaction and cross-reactivity with other thiols was evaluated by treating conjugates in the presence of glutathione. The studies indicated that the thiazine linker degrades markedly slower than the thioether conjugate. In addition, the thiazine linker is over 20 times less susceptible to glutathione adduct formation. The NMR study of the thiazine structure confirmed that the formation of the thiazine linker is a stereoselective process that yields a single diastereomer. In summary, we propose the use of the thiazine linker obtained by conjugation of maleimide-containing reagents with peptides or proteins presenting an N-terminal cysteine as a novel approach for bioconjugation. The advantages of this approach are the formation of a linker with a well-defined stereochemical configuration, increased stability at physiological pH, and a strongly reduced propensity for thiol exchange.
Abbreviations
-
- H–Cys–Arg–Tyr–OH
-
- H–CRY–OH
-
- MPA
-
- 3-maleimidopropionic acid
-
- NMR
-
- nuclear magnetic resonance spectroscopy
-
- ROESY
-
- rotating frame Overhauser enhancement spectroscopy
1 INTRODUCTION
The field of protein and peptide conjugates is expanding rapidly with a large number of bioconjugate products entering clinical development.1-3 The list of proteinaceous bioconjugate applications includes the use of fluorescent tags both as in vivo or in vitro diagnostic reagents,4 the use of polyethylene glycol (PEG) or hydrophilic polymers applied to therapeutic agent half-life extension,5 the use of macrocyclic chelators conjugates to generate selective radiochemical therapeutics or imaging agents,6 and the exciting field of antibody–drug conjugates (ADCs), which are mainly used as chemotherapeutic agents in cancer therapy.7 The preparation of homogenous bioconjugates is a prerequisite for the development of safer and more efficacious biological drugs or in vivo diagnostic reagents. To this effect, different functional groups on the side chain of proteins or peptides are utilized to establish a chemoselective and regioselective functionalization of the biopolymers. One widely exploited approach to achieve the required selectivity is to target naturally occurring reduced cysteine thiols in natural proteins or ad hoc introduced cysteine in the sequences.8 The Michael addition of thiols to maleimides is highly chemoselective at pH's ranging between 6.5 and 7.5; generating a thiosuccimide linkage, which, for a long time, was considered extremely stable. This selectivity relies both on the reaction of a soft nucleophile with a soft electrophile (HSAB theory) and on the thiol pKa (approximately pH 8) with conjugation taking place in a timeframe compatible with commercial manufacturing.9 As a consequence of these properties, a large number of readily available maleimide-based conjugation reagents are commercially available.
During product development, besides the biological activity, other factors need to be considered when designing the target bioconjugate structure. Successful commercial manufacturing requires obtaining a bioconjugate with a well-defined chemical structure as well as predictable chemical stability during the different production stages: from the chemical synthesis to the final fill-finish process standpoint. Ideally, the product should also be stable in the biological fluids. In these regards, recent studies have demonstrated the instability of thiosuccinmide linkers.9, 10 In addition, the thiosuccinimide is susceptible to thiol exchange via a retro-Michael addition, which, in biological fluids, can result in the transfer of the maleimide structure to endogenous free thiols such as human serum albumin (HAS) and glutathione (GSH).11 On the other hand, the imido group in the thiosuccinimido structure can undergo spontaneous hydrolysis at neutral pH over time, generating two isomeric succinamic acid thioethers,10 which produces a complex mixture of isomers. The succinamic acid structures formed are not subject to thiol exchange.10 This approach has been proposed and studied for the stabilization of ADCs.12, 13 For this reason, the controlled ring opening of succinimidyl thioethers has been proposed to stabilize ADCs by using maleimide linkers with increased instability toward hydrolysis. The controlled ring opening can be induced by exposing the conjugated product to high pH for a short period of time. Unfortunately, when such an approach is applied to peptide conjugates, a mixture of four isomers can be detected by ultra-high performance chromatography (UHPLC). Such mixtures represent an additional challenge when studying drug stability, performing in vivo biodistribution, and submitting the registration dossier to the regulatory authorities for commercialization approval. Additionally, the conditions required for hydrolyzing the succinimidyl thioether (incubation at high pH and elevated temperatures) can lead to undesired side reactions such as the deamidation of asparagine residues. These alkaline hydrolysis conditions can also lead to the formation of aggregates.14
Additionally, the problem of maleimide instability has been addressed by multiple groups by modifying the original simple aliphatic linker and introducing complex structures.15 Additionally, a new class of linkers based on substituted ethynylphosphonamidates has been recently introduced to overcome some of the maleimide limitations.16 Ethynylphosphonamidate linkers were proven by direct comparison with maleimides to provide increased chemo selectivity for thiols, increase stability to thiol exchange, and increase biostability in biological fluids.16 Taking advantage of the valency of the phosphonamidite structure, this linker allows modulating the hydrophobicity of a cytotoxic payload by the introduction of an additional solubilizing PEG moiety, resulting in additional stability both in serum and in vivo.17 Unfortunately, these complex linkers and payloads are rarely readily commercially available, and the fine tuning and screening of different payloads require an initial step of library generation that hampers the rapid production of lead compounds.
Inspired by early reports of a chemical rearrangement occurring between cysteine and maleimide structures18-20 resulting in a chemical rearrangement to a six-membered thiazinyl structure, we recently reported our study of this rearrangement in the context of short model peptides comprising an N-terminal cysteinyl residue and different maleimides. We proved the general occurrence of this chemical rearrangement as a function of different conditions of pH, temperature, and N-terminal peptide sequences.21 Based on our previous work, the conditions for the formation of the thiazine structure were shown to be mild, rapid, and well-controlled. In addition, preliminary results supported the thiazine structure stability. We have extended this study to include an evaluation of the thiazine structure to both hydrolysis and thiol exchange in comparison to the corresponding thiosuccinimide structure of a peptide with an acetylated N-terminus.
2 MATERIALS AND METHODS
2.1 Materials
3-(2,5-Dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-propylpropanamide was purchased from Aurum Pharmatech LLC (Franklin Park, NJ). Dimethylformamide was acquired from Univar Solutions Inc. (Downers Grove, IL). N,N-Diisopropylethylamine was purchased from Spectrum Laboratory Products Inc. (Gardena, CA). Piperidine was purchased from Tedia Company Inc. (Fairfield, OH). Isopropyl alcohol was purchased from Brenntag Pacific Inc. (Santa Fe Springs, CA). Diisopropyl ether was purchased from Neuchem (Sparks, NV).
Sigma-Aldrich (St. Louis, MO) was the supplier for biotin–maleimide, potassium phosphate monobasic, potassium phosphate dibasic, 1,2-ethanedithiol (EDT), triisopropylsilane (TIS), and N,N′-diisopropylcarbodiimide (DIC).
HPLC-grade trifluoroacetic acid was purchased from Alfa Aesar (Haverhill, MA). HPLC-grade water and acetonitrile were obtained from MilliporeSigma (Burlington, MA). All other amino acid derivatives and reagents were obtained from Bachem AG, Bubendorf, Switzerland. Preparative HPLC purification was performed using a Waters Prep LC system. HPLC and UHPLC analyses were performed on a Thermo Scientific UltiMate 3000 UHPLC system using an Atlantis T3 100 Å, 3 μm, 4.6 mm × 150 mm column and a Waters Acquity HSS T3 100 Å, 1.8 μm, 2.1 × 150 mm column, respectively. A Thermo Scientific Vanquish UHPLC system with a Waters Acquity CSH C18 130 Å, 1.7 μm, 2.1 mm × 150 mm column was used for UHPLC–MS separations. Mass spec analysis was performed in positive ion mode on a Thermo Scientific Q Exactive Focus Hybrid Quadrupole-Orbtitrap™ mass spectrometer. For MS/MS analyses, high-energy C-trap dissociation (HCD) was used for fragmentation.
The apparent pH of the solutions used during the maleimide conjugations was measured using a Mettler Toledo™ SevenCompact™ S220-Basic pH/ion benchtop meter equipped with a Mettler Toledo™ InLab Solids Go-ISM® electrode (pH 1–11; 0–80°C). The electrode was calibrated before each series of measurements using standard buffer solutions purchased from Millipore Sigma (Burlington, MA). All measurements were taken at ambient temperature.
2.2 Manual Fmoc solid-phase peptide synthesis
Peptides H–Cys–Arg–Tyr–OH and Ac–Cys–Arg–Tyr–OH were synthesized manually on a 19.5 mmol scale (with respect to the loading of the first amino acid) using 2-chlorotrityl chloride resin (substitution = 1.56 mmol/g). To load the first amino acid, Fmoc–Tyr(tBu)–OH (1.0 eq, 19.5 mmol) and DIPEA (2.0 eq., 39 mmol) were dissolved in DMF, and the solution was stirred for 30 min. Unreacted sites were quenched by treating the resin with a solution of 17:2:1 DCM/MeOH/DIPEA two times for 15 min each. After loading the first amino acid, the effective resin loading was approximately 0.5 mmol/g. The second amino acid was coupled by reacting Fmoc–Arg(Pbf)–OH (2.0 eq., 39 mmol) with TCTU (1.9 eq., 37 mmol) and DIPEA (2.5 eq, 48.5 mmol) in DMF. SPPS reactions were conducted at room temperature. Fmoc removal was achieved using a solution of 20% (v/v) piperidine/DMF. Fmoc–Cys(Trt)–OH was coupled using DIC (2 eq., 39 mmol) and HOBt hydrate (2 eq., 39 mmol) in DMF. Once the coupling of Fmoc–Cys(Trt)–OH was complete, the resin was split in half; half of the resin would be used to generate the peptide with a free N-terminus, and the other half would be used to generate the peptide with an acetylated N-terminus. N-terminal acetylation was performed using a solution of Ac2O (10 eq, 97.5 mmol) and DIPEA (2 eq, 19 mmol) in DMF for 10 min. All reactions were performed at room temperature. For Fmoc removal, 20% (v/v) piperidine in DMF was used. Post-coupling capping was performed after using a solution of Ac2O (5 eq., 97.5 mmol) and DIPEA (1.0 eq, 19.5 mmol) in DMF for 10 min. The peptidyl resin was cleaved using a cleavage cocktail of TFA/TIS/EDT/water (92.5:2.5:2.5:2.5 v/v). Analysis of crude peptide purity was performed by reversed-phase UHPLC (Waters Acquity HSS T3 100 Å, 1.8 μm, 2.1 × 150 mm column) using 0.1% TFA in water and 0.1% TFA in acetonitrile as mobile phases. Purification of peptides was conducted on a ModCol®, 25 × 5 ID axial compression column with Luna C18 (3) media (10 μm particle size). Peptides were lyophilized using a VirTis freeze dryer to give white powders. Peptide weights were not corrected for water, TFA, or acetate content; therefore, all weights were based on gross peptide weight.
2.3 pH stability study
2.3.1 Ac–CRY–OH–MPA thioether pH stability study
Stock solutions of 0.1 M potassium phosphate in water were prepared at pH 5.5, pH 7.3, and pH 8.0. A stock solution of the Ac–CRY–OH–MPA thioether conjugate was prepared by first dissolving 111.0 mg of the conjugate in water (12 mg/mL). Then, the thioether stock solution was aliquoted into three different centrifuge tubes containing 5.88 mL of either pH 5.5, pH 7.3, or pH 8.0 potassium phosphate solution. Due to a slight decrease in pH due to the acidity of the thioether conjugate, the pH of each solution was adjusted using aqueous 1 N potassium hydroxide. After adjusting the pH, 0.6 mL of the three different peptide conjugate solutions were pipetted into glass HPLC vials. The vials were immediately sealed and either kept at room temperature or placed in an oven set to 37°C. Upon completion of the incubation period, the sample was quenched with 0.4 mL of 2% acetic acid in water, and the sample was immediately stored at −20°C. Samples were collected at 2, 5, 24, 48, 168, and 336 h time points.
2.3.2 H–CRY–OH–MPA thiazine pH stability study
Stock solutions of 0.1 M potassium phosphate in water were prepared at pH 5.5, pH 7.3, and pH 8.0. A stock solution of the H–CRY–OH–MPA thiazine conjugate was prepared by first dissolving 110.9 mg of the conjugate in water (12 mg/mL). Then, the thioether stock solution was aliquoted into three different centrifuge tubes containing 5.88 mL of either pH 5.5, pH 7.3, or pH 8.0 potassium phosphate solution. Due to a slight decrease in pH due to the acidity of the thioether conjugate, the pH of each solution was adjusted using aqueous 1 N potassium hydroxide. After adjusting the pH, 0.6 mL of the three different peptide conjugate solutions were pipetted into glass HPLC vials. The vials were immediately sealed and were either kept at room temperature or placed in an oven set to 37°C. Upon completion of the incubation period, the sample was quenched with 0.4 mL of 2% acetic acid in water, and the sample was immediately stored at −20°C. Samples were collected at 2, 5, 24, 48, 168, and 336 h time points.
2.4 Glutathione stability study
2.4.1 GSH stability study
A 25 mM stock solution of reduced GSH was prepared by dissolving 153.8 mg in 20 mL of 0.25 M potassium phosphate. The pH of the solution was adjusted to pH 7.4 using aqueous 1 N KOH. To 10 mL of the 25 mM GSH solution was added either 4.52 mg of H–CRY–OH–MPA thiazine conjugate (0.45 mg/mL) or 3.93 mg of Ac–CRY–OH–MPA thioether conjugate (0.39 mg/mL). Once the conjugate was dissolved in 25 mM GSH, 0.6 mL of the solution was pipetted into glass HPLC vials. The vials were sealed and were either kept at room temperature or placed in an oven set to 37°C. Upon completion of the incubation period, the sample was quenched with 0.4 mL of 2% acetic acid in water, and the sample was immediately stored at −20°C. Samples were collected at 5, 24, 48, 72, 96, 120, and 192 h time points.
2.4.2 GSSG stability study
A 25 mM stock solution of oxidized GSH (GSSG) was prepared by dissolving 306.37 mg in 20 mL of 0.25 M potassium phosphate. The pH of the solution was adjusted to pH 7.4 using aqueous 1 N KOH. To 10 mL of the 25 mM GSH solution was added either 4.43 mg of H–CRY–OH–MPA thiazine conjugate (0.44 mg/mL) or 3.83 mg of Ac–CRY–OH–MPA thioether conjugate (0.38 mg/mL). Once the conjugate was dissolved in 25 mM GSH, 0.6 mL of the solution was pipetted into glass HPLC vials. The vials were sealed and were either kept at room temperature or placed in an oven set to 37°C. Upon completion of the incubation period, the sample was quenched with 0.4 mL of 2% acetic acid in water, and the sample was immediately stored at −20°C. Samples were collected at 5, 24, 48, 72, 96, and 144 h time points.
2.5 Preparation of base-stressed thiazine sample
A solution of H–CRY–OH–MPA thiazine was prepared in a glass scintillation vial by dissolving 10.0 mg of the conjugate in deionized water. The pH of the solution was adjusted to pH 7.8 using aqueous 1 N KOH. The sample was placed in an oven that was set to 37°C and was incubated for 336 h. Upon completion of the incubation period, the sample was frozen using a dry ice/ethanol bath and lyophilized. The base-stressed thiazine sample was purified by reversed-phase HPLC.
2.6 NMR experiments
All NMR experiments (1H, 13C, and rotating frame Overhauser enhancement spectroscopy [ROESY]) were performed at 600 MHz on a Bruker AVNeo-600 NMR spectrometer. Lyophilized peptide conjugates were dissolved in a solution of 0.1% TFA-D in D2O or H2O/D2O (9:1). All spectra were collected at 298 K using trimethylsilylpropanoic acid (TSP) as an internal standard.
3 RESULTS AND DISCUSSION
3.1 Design and preparation of N-term Cys peptide thioether and thiazine conjugates
The thioether and thiazine conjugate model systems used for the stability studies contained a tripeptide (Ac–Cys–Arg–Tyr–OH or H–Cys–Arg–Tyr–OH, respectively) conjugated to 3-maleimidopropionic acid (MPA) (see Figure 1). The rationale behind the selection of the peptide sequence was to simplify experimental processes and analyses. To this end, the model system was designed to be an N-terminal Cys peptide that could be synthesized in high purity with great water solubility. The model system was also designed to avoid the incorporation of problematic nucleophilic side chains and to be easily detected by UV absorbance. The Arg residue was included to provide an amino acid that would improve solubility without reacting with the succinimidyl thioether or thiazine structures, whereas the Tyr residue was incorporated to provide high molar absorptivity for facile detection of the peptide and related impurities by UV. In regard to the maleimide, MPA was selected for its simple structure and favorable water solubility, which simplified the production of the peptide conjugates as well as NMR analysis.
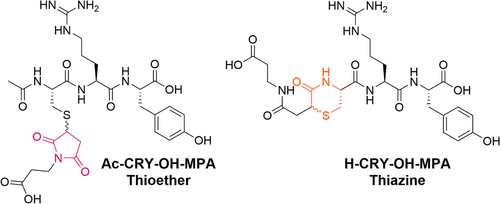
To prepare the thiazine model peptide conjugate, H–CRY–OH was first synthesized with a free N-terminal amine using standard Fmoc-SPPS after which the peptide was conjugated to MPA in unbuffered water. Once the thiol–maleimide conjugation had gone to completion, the pH was raised to pH 7 to initiate thiazine rearrangement. Rearrangement was allowed to proceed to completion for 65 h at room temperature and then the thiazine conjugate was purified by reversed-phase HPLC. This was followed by acetate salt exchange purification using reverse phase HPLC to generate the H–CRY–OH–MPA thiazine conjugate as acetate salt in >97% purity. Conversely, the succinimidyl thioether model peptide conjugate was prepared by synthesizing the N-terminal acetylated peptide Ac–CRY–OH and conjugating the peptide to MPA in water. Subsequent purification and salt exchange yielded the thioether conjugate as the acetate salt in >98% purity. Importantly, the peptide content was not determined for the thioether or thiazine conjugates, so no correction was made for residual acetate or water content.
3.2 Structural characterization of the thiazine isomer
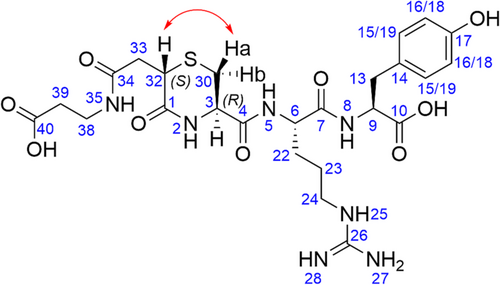
Confirmation of the stereochemical configuration of the H–CRY–OH–MPA thiazine conjugate was accomplished using 1-D and 2-D NMR spectroscopy (see Supporting Information). Peaks were assigned using 1H and 13C NMR (see Figure 2), and the stereochemical configuration was determined using ROESY NMR analysis. Cross-peaks were observed between H(32) and Ha in the ROESY spectrum, whereas no cross-peaks were observed between H(32) and Hb. Taken together, these data revealed that the configuration at C32 was (S) and that the thiazine structure was trans with respect to the substituents at C32 and C3. Interestingly, these results demonstrated that a single stereoisomer was formed during the thiazine rearrangement. The initial R configuration of the C3 carbon of the Cys residues was maintained during the rearrangement. However, further work is required to elucidate the mechanism of the stereoselective rearrangement. Although the precise mechanism of the selectivity is not known, the formation of a single chemical entity after thiazine rearrangement would be advantageous in the development of peptide and protein conjugates, especially at the stage of larger scale API production.
3.3 pH stability study
To determine the stability of the thiazine linker in comparison to the succinimidyl thioether linker, a series of 1:1 pH stability experiments were conducted at room temperature and at 37°C. The decrease in the percentage of thiazine or thioether starting material as determined by integration of UHPLC peak area was measured at various time points in acidic medium (pH 5.5), neutral medium (pH 7.3), and basic medium (pH 8.0). Data of the stability experiments throughout the article are provided as percent of the starting products detected at each time point compared to the starting product peak area at the experiment corresponding time zero. The three different pH solutions were prepared using 0.1 M potassium phosphate buffer in water. All reactions were quenched by adding a dilute solution of aqueous acetic acid to the sample before analysis by uHPLC.
For both the thiazine and thioether conjugates, degradation of the starting material was slow at pH 5.5, which indicates that both structures are stable under acidic conditions. At room temperature, around 95% of the thiazine conjugate remained after 336 h of incubation, whereas roughly 93% of the thioether was present after the same period of time. At 37°C after 336 h, 89% of the thiazine was detected with the main side reactions being hydrolysis and formation of isomeric impurities. On the other hand, 82% of the thioether was observed with hydrolysis being the primary side reaction. This would also suggest that during the manufacturing of maleimide conjugates, operating at a mildly acidic pH has a potential advantage for the stability of the conjugated product (for sample analyses at pH 5.5; see Supporting Information).
Although stabilities were similar for the thiazine and succimidyl thioether under acidic conditions, a large disparity in stability between the two conjugates was observed at physiological pH (pH 7.3). At room temperature after 336 h, 85% of the thiazine conjugate was still intact (see Supporting Information), whereas only 58% of the thioether remained undegraded (see Supporting Information). The stability at pH 7.3 for the thiazine structure for an extended time is extremely important, since it indicates that during the rearrangement step of thiazine conjugate production, the time of rearrangement is unlikely to be critical or to require tight process controls.
When the peptide conjugates were incubated in pH 7.3 buffer at 37°C, an even larger difference in stability was observed between the thiazine and thioether. For the thiazine, around 47% of the starting conjugate remained after 336 h (see Figures 3 and 4) compared to merely 5% of the corresponding thioether after the same period of time (see Figures 5 and 6). For the thiazine, the main impurity was an isomeric impurity which was present in nearly 41% abundance. Conversely, for the thioether conjugate, the hydrolyzed, ring-opened species was the primary impurity observed (92% abundance). From these data, it can be determined that at pH 7.3 and at 37°C, for the thioether, 50% of the product is degraded in approximately 75 h, whereas for the thiazine, 50% degradation is detected after 325 h. The stability of the thiazine bioconjugate at pH 7.3 and at 37°C suggests that it could be advantageous for in vivo therapeutic applications.
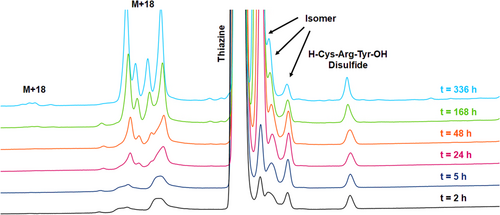
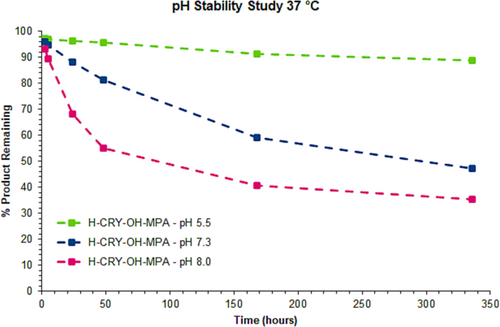
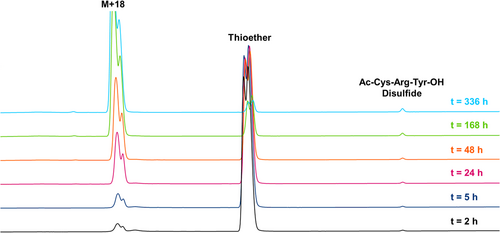
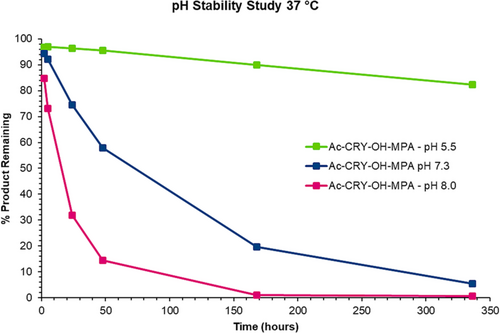
Degradation of the peptide conjugate was accelerated for both the thioether and thiazine conjugates when incubation was performed at pH 8.0. At room temperature, 63% of the remaining thiazine was measured after 336 h; however, only 20% of the thioether conjugate was observed under the same conditions. When incubation in basic buffer was performed at 37°C, about 35% of the thiazine conjugate remained intact after 336 h, and the major impurity was an isomeric impurity that was present in 37% abundance (stability data at pH 8 are provided in the Supporting Information). The approximate 1:1 ratio of the starting thiazine to the isomeric impurity detected under base-stressed conditions over a long period of time suggests that racemization of one of the stereocenters of the thiazine occurred. In the case of the thioether conjugate, nearly all the thioether had completely hydrolyzed after 336 h at 37°C with only 0.6% of the starting conjugate remaining.
3.4 MS/MS analysis of the isomeric impurity from base-stressed thiazine sample
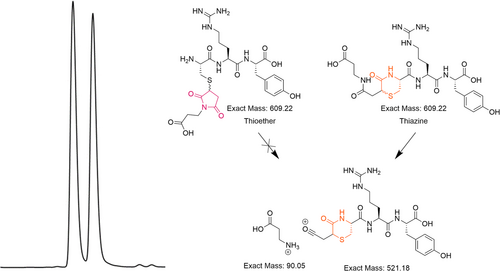
During the pH stability experiments for the thiazine peptide conjugate, an isomeric impurity was formed during incubation under neutral or basic conditions for prolonged periods of time. To determine whether racemization of the starting material had taken place, the chemical structure of the isomeric impurity was elucidated by isolating the supposed racemate and performing LC–MS/MS and NMR analysis.
The base-stressed sample was prepared by dissolving purified H–CRY–OH–MPA thiazine in water and adjusting the pH to pH 8 using potassium hydroxide. The solution was allowed to incubate at 37°C for 2 weeks before purification by reversed-phase HPLC. The purified base-stressed sample contained two peaks that were equal in abundance based on UV absorbance at 220 nm. In our previous studies, we have identified a specific fragmentation pattern that is specific to the thiazine structure.21 The UHPLC–MS analysis revealed that both peaks had the same mass of 609.22 Da corresponding to the expected mass of the H–CRY–OH–MPA conjugate. When the sample was subjected to LC–MS/MS analysis, fragmentation of the earlier eluting peak produced a species with a mass of 521.18 Da, with the second eluting peak providing the same 521.18 Da mass fragment (see Figure 7). These MS/MS results confirmed that both peaks contained the thiazine structure and suggested that the isomeric impurity is not a regioisomer resulting from the rearrangement of the thiazine structure.
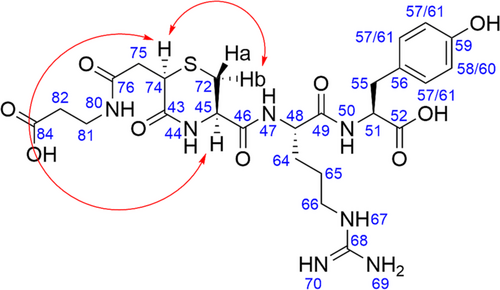
3.5 ROESY NMR characterization of the based-stressed thiazine sample
Further characterization of the isomeric impurity that arose in the base-stressed thiazine sample was performed using ROESY NMR analysis. Peak labeling was determined based on the chemical shifts in the 13C NMR spectrum. The ROESY spectrum of the based-stressed sample which contained a 1:1 mixture of starting conjugate and the isomeric impurity was compared to the ROESY spectrum of the purified H–CRY–OH–MPA thiazine conjugate before basic stress. Any new peaks that did not appear in the spectrum of the pure thiazine conjugate could be assigned to the isomeric impurity. New ROE signals corresponding to the isomeric impurity were observed between H74 and Hb and between H74 and H45 (see Figure 8 and Supporting Information). Altogether, these ROESY cross-peaks confirmed that the isomeric impurity is an epimer of the starting thiazine conjugate (R) configuration at C74. This result was consistent with a base-promoted racemization side reaction. Based on the pH stability studies, the isomeric impurity was the dominant impurity across all tested pH ranges (pH 5.5, pH 7.3, and pH 8.0). The rate of formation of the isomeric impurity rose sharply with increasing pH, whereas the ratio of product to isomeric impurity approached unity with prolonged incubation in basic buffer.
3.6 GSH stability study
One of the most problematic side reactions for succinimidyl thioether conjugates used in biological applications is retro-Michael reaction and loss of the conjugated payload. The loss of payload prevents the conjugate from providing the expected biological activity and may lead to adverse off-target effects. In order to understand the propensity of the thiazine structure for retro-Michael reaction and cross-reactivity with other thiols, a series of 1:1 comparison experiments were performed at 37°C and room temperature in the presence of reduced or oxidized GSH. GSH served as a trapping agent for the free thiol that was generated from the retro-Michael reaction, which allowed the side reaction to being detected by the presence of a GSH adduct. The stability of the thioether and that of thiazine were compared by incubating the conjugates in reduced GSH at physiological pH and analyzing the difference in impurity profiles by UHPLC. Stability experiments were also performed using oxidized GSSG; however, the results were nearly identical to the GSH experiments with respect to the rate of degradation of the peptide conjugates and impurities generated.
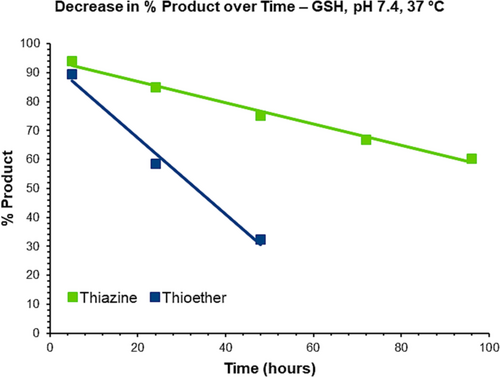
When the H–CRY–OH–MPA thiazine conjugate was treated at room temperature with 25 mM GSH in potassium phosphate buffer at pH 7.4, degradation was fairly slow as 85% of the thiazine was still intact after 192 h (see Figure 9, bottom trace). At 37°C, the degradation of the starting thiazine was faster (see Figure 9, top trace) with 49% of residual thiazine detected after 192 h. The primary impurities that were observed were the already described isomeric impurity, hydrolysis-related impurity, and a GSH adduct. Consistent with earlier experiments where the thiazine conjugate was stressed under basic conditions, the isomeric impurity due to the racemization of C74 was the most abundant impurity that appeared over time. Importantly, the GSH adduct impurity was only present in approximately 2% of abundance even after 192 h, demonstrating that the thiazine structure is resistant to retro-Michael reaction and cross-reactivity with GSH.
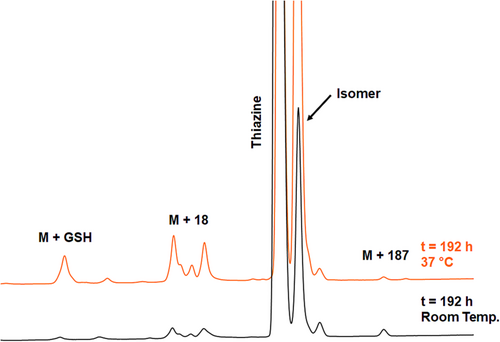
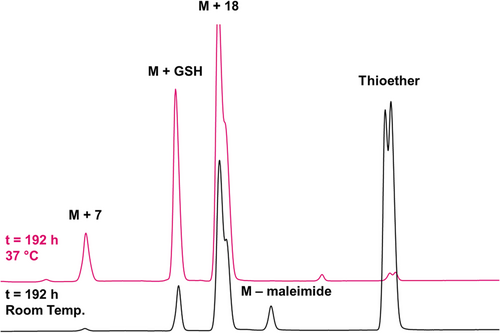
In the case of the thioether conjugate, the degradation in the presence of 25 mM GSH at room temperature and physiological pH is significantly faster compared to the thiazine with about 55% of thioether remaining after 192 h at room temperature (see Figure 10, bottom trace) and only 2% of residual thioether conjugate detected after 192 h (see Figure 10, top trace and Figure 11). The quantification of GSH adduct formation at 37°C revealed that the thioether is considerably more prone to retro-Michael reaction and cross-reactivity with thiols, with approximately 16% GSH adduct detected after treatment with 25 mM GSH at pH 7.4 for 72 h. In the same time range, the amount of GSH adduct for the thiazine was 0.6%.
Conducting the GSH stability studies in the presence of 25 mM GSSG gave almost identical rates of degradation of peptide conjugates compared with using 25 mM GSH (see Supporting Information). However, there was a significant difference in the amounts of GSH adduct formation between GSH and GSSG. Using the 72 h time point as a reference, the amount of GSH adducts detected for the thiazine at room temperature was 0.12%, whereas at 37°C, it was 1.6%. For the thioether at the same time point, the amount of GSH adduct at room temperature was 6.3%, and at 37C was 30%. For both structures, the apparent rate of GSH adduct formation in presence of oxidized GSH seems faster than the rate of GSH adduct formation when reduced GSH was used. This most likely indicates that GSSG was more efficient at trapping the nucleophilic thiol that was generated during retro-Michael reaction (see Table 1).
| Rate of increase in % GSH after 72 h incubation at pH 7.4 (37°C) | ||
|---|---|---|
| Peptide conjugate | 25 mM GSH (%/h) | 25 mM GSSG (%/h) |
| H–CRY–OH–MPA (thiazine) | 0.6% | 1.6% |
| Ac–CRY–OH–MPA (succinimidyl thioether) | 15% | 30% |
Overall, the thiazine conjugate presented a significant reduction of thiol exchange compared with the thioether structure at physiological pH.
3.7 Expanding the scope of the thiazine linker via intra-chain conjugation
To broaden the scope of the thiazine linker bioconjugation strategy and to allow the introduction of a thiazine conjugate at different locations in a peptide primary structure, we sought to demonstrate that thiazine formation could be performed in an intra-chain position of a peptide via the sidechain of an amino acid rather than at the N-terminus. N-terminal thiazine formation may be undesirable for certain peptides or protein conjugates if the N-terminal amino acid residues are involved in binding to its molecular target or in sequences where a free N-terminus is necessary for a particular biological function. Additionally, performing the thiazine formation on the sidechain could allow for modification of the N-terminus with a different chemical moiety using an orthogonal labeling strategy.
A hexamer model peptide 1 was used for demonstrating thiazine conjugation at an intra-chain position (see Figure 12). Standard Fmoc-SPPS was used to generate the protected linear sequence of the resin-bound model peptide: Boc–Gly–Arg(Pbf)–Tyr(tBu)–Lys(ivDde)–Pro–Leu–Tricyclic linker amide resin. To introduce an intra-chain cysteine with a free amino group into 1 for thiol–maleimide conjugation, first, Lys(ivDde) was deprotected using a dilute hydrazine solution, and then, Boc-Cys(Trt)-OH was coupled to the free Ɛ-amino group of Lys. After coupling the Cys to the Lys sidechain, the peptide was cleaved and purified by reversed-phase HPLC.
Two different maleimides were evaluated for intra-chain thiol–maleimide conjugation and subsequent thiazine rearrangement: biotin–maleimide reagent 2 and 3-maleimido-N-propylpropanamide 3 (see Figure 13). The two maleimides were chosen to evaluate the difference in their linker length and chemistry. Previous work demonstrated that using a maleimide reagent with a hexanamide linker that is less electron withdrawing leads to a slower rate of thiazine formation compared to using maleimide reagents with the propylpropanamide linkers. We anticipated that a similar difference in rearrangement rate would be observed in the context of intra-chain conjugation and thiazine rearrangement.
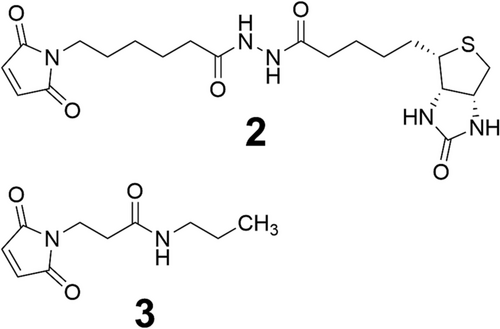
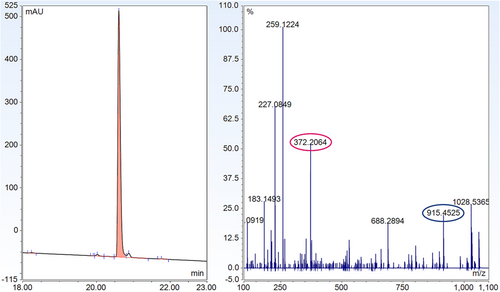
Conjugation of 1 (1 eq.) to maleimide 2 (1.1 eq) to produce the thiazine structure (see Figure 14-4) was carried out in unbuffered water containing 10% (v/v) acetonitrile and 10% (v/v) dimethyl sulfoxide to maintain solubility. UHPLC analysis was used to confirm the completion of the thiol–maleimide reaction. After 6 h, the pH of the solution was adjusted to pH 7.3 using 1 M solutions of potassium phosphate monobasic and potassium phosphate dibasic. The progress of the thiazine rearrangement was monitored at 23, 47, and 64 h by UHPLC. After 23 h, rearrangement proceeded to approximately 70% conversion. When the reaction was monitored after 47 and 64 h, thiazine rearrangement had progressed to 89% and 94%, respectively. Over the course of the thiazine rearrangement, various impurities developed. The main impurity was the incorporation of a second unit of 2, presumably through a reaction with the N-terminal amine of 1. Reversed-phase HPLC purification gave the isolated thiazine conjugate 4 in 97% purity by UHPLC. LC–MS/MS analysis of 4 revealed the presence of a thiazine-specific mass fragment (915.45 Da), confirming the conjugate conversion to the thiazine structure (see Figure 15).
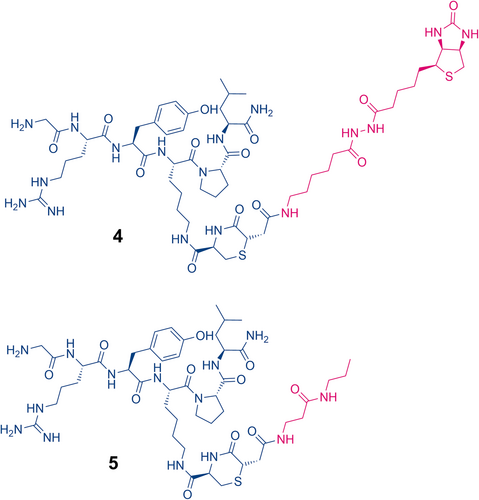
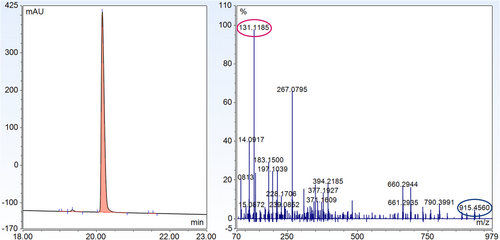
For maleimide 3, conjugation to model peptide 1 was performed in unbuffered water with 10% acetonitrile. UHPLC analysis revealed that the conjugation was complete after 1 h and produced the succinimidyl thioether structure. Subsequently, thiazine formation was initiated by raising the pH of the solution to pH 7.3 using solutions of potassium phosphate in water. After 23 h, conversion of the succinimidyl thioether to the thiazine had reached completion indicating the shorter alkyl linker of 3 leads to faster thiazine formation in the intra-chain position compared to the longer hexanamide linker of 2 (see Figure 14-5). Similar to the thiazine formation with the biotin–maleimide reagent, the largest impurity that was formed during conjugation and thiazine rearrangement was a peptide conjugate with two units of 2. Fortunately, the desired thiazine-containing peptide conjugate 5 could be easily purified and isolated by reversed-phase HPLC to give a product with a purity of 99% by UHPLC. The thiazine structure of conjugate 5 was also verified by LC–MS/MS analysis, which produced the unique thiazine fragment with a mass of 915.46 Da (see Figure 16).
Altogether, we demonstrated that conjugation via a thiazine linker is versatile and flexible. The site of conjugation can be modified in a straightforward manner by appending a cysteine residue onto a lysine sidechain. Similar to thiazine formation using N-terminal cysteine, intra-chain thiazine rearrangement is significantly influenced by linker chemistry. The use of a linker with a more electron-withdrawing substituent at the nitrogen of the maleimide reagent led to a much faster rate of thiazine rearrangement, which is ideal for the preparation of bioconjugates as prolonged incubation in basic buffer can lead to undesired side reactions. Lastly, the intra-chain thiazine conjugate can be rapidly and unambiguously characterized by LC–MS/MS.
4 CONCLUSIONS
In summary, we have evaluated the applicability of the thiazine structure as a linker for bioconjugation by measuring the stability of peptide thiazine conjugates at acidic, neutral, and basic pH and by determining the susceptibility toward retro-Michael reaction and cross-reactivity with other thiols.
A thiazine structure is formed via chemical rearrangement when a peptide or protein presenting a cysteine at the N-terminal position is conjugated with a maleimide-containing linker. The rearrangement requires the N-terminal cysteine to present both a free thiol and a free amine.
In comparison to succinimidyl thioether conjugates, the thiazine degraded slower in various pH conditions and was decidedly more stable at neutral pH. The primary side reaction for the thiazine was racemization at the alpha carbon of thiazine (the carbon between the carbonyl and the sulfur), which occurred when samples were base-stressed for long periods of time at elevated temperatures.
The thiazine structure is also anticipated to have lower sensitivity to oxidation compared to the ring-opened succinimidyl thioether. This is an advantage in chemical biology and proteomics applications where the oxidative stress of proteins is being studied since oxidation of the thiol–maleimide linkage tends to complicate analyses.
ACKNOWLEDGEMENTS
We thank Prof. Dr. Oliver Zerbe and Nadja Bross for the acquisition and interpretation of the NMR data and Sumukh Ray for the LC–MS/MS support.




