Pinpointing the interaction site between semaphorin-3A and its inhibitory peptide
Dedicated in memory of Prof. Dr. Ulf Diederichsen.
Abstract
Semaphorin-3A (Sema-3A) is a chemorepellant protein with various biological functions, including kidney development. It interacts with a protein complex consisting of the receptors neuropilin-1 (NRP-1) and plexin-A1. After acute kidney injury, Sema-3A is overexpressed and secreted, leading to a loss of kidney function. The development of peptide inhibitors is a promising approach to modulate the interaction of Sema-3A with its receptor NRP-1. Few interaction points between these binding partners are known. However, an immunoglobulin-like domain-derived peptide of Sema-3A has shown a positive effect on cell proliferation. To specify these interactions between the peptide inhibitor and the Sema-3A–NRP-1 system, the peptides were modified with the photoactivatable amino acids 4-benzoyl-l-phenylalanine or photo-l-leucine by solid-phase peptide synthesis. Activity was tested by an enzyme-linked immunosorbent-based binding assay, and crosslinking experiments were analyzed by Western blot and mass spectrometry, demonstrating a specific binding site of the peptide at Sema-3A. The observed signals for Sema-3A-peptide interaction were found in a defined area of the Sema domain, which was also demonstrated to be involved in NRP-1 binding. The presented data identified the interaction site for further development of therapeutic peptides to treat acute kidney injury by blocking the Sema-3A–NRP-1 interaction.
Abbreviations
-
- Sema-3A
-
- semaphorin-3A
-
- NRP-1
-
- neuropilin-1
-
- Plxn-A1
-
- plexin-A1
-
- SIP
-
- semaphorin inhibitory peptide
-
- PPI
-
- protein–protein interaction
-
- POD
-
- peroxidase
-
- Bpa
-
- 4-benzoyl-l-phenylalanine
-
- p-Leu
-
- photo-l-leucine
-
- HRP
-
- horseradish peroxidase
-
- Bip
-
- biphenylalanine
-
- 1-Nal
-
- 1-naphthylalanine
-
- 2-Nal
-
- 2-naphthylalanine
-
- TTDS
-
- 1,13-diamino-4,7,10-trioxatridecan-succinamic acid
1 INTRODUCTION
Semaphorins (Sema) are a large class of glycoproteins, which are either secreted or membrane-associated and involved in guiding the axonal growth during neuronal development. They display important roles in addressing the immune system and affecting organogenesis and angiogenesis.1-3 The Sema family comprises around 30 members, which are divided into eight main classes.4, 5 Classes 1 and 2 are only present in invertebrates, and classes 3 to 7 are found in vertebrates. Sema-5c is the only exception, which was observed in vertebrates as well as in invertebrates.6 Sema proteins of class 8 are only present in viruses. Since there are only small structural changes between the eight classes, around 500 amino acid long Sema domain is present in all of these family members (Figure 1).5, 7 The conserved N-terminal domain, consisting as a sevenfold beta-propeller structure, is not only important to form disulfide-bridged homo-dimers but also necessary for binding and signal transduction by its receptors and co-receptors.7, 8
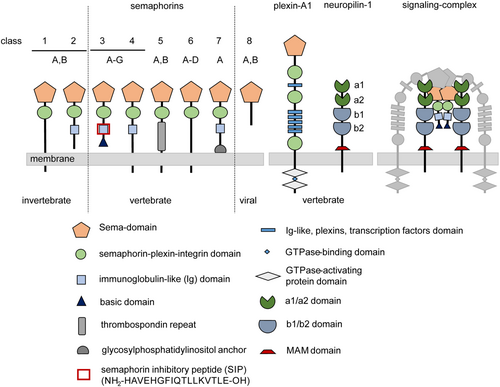
One of the most frequently studied Sema protein is the secreted version of Sema-3A.9, 10 This protein is overexpressed in the podocyte foot processes in injured kidney tissue.11-13 Overexpression leads to degradation of the podocytes and decomposition of renal functions and tissue. It has been shown that the Sema-3A-induced effects are reversible by downregulating the Sema-3A concentration.14 However, to convey the signal, Sema-3A has to bind to its co-receptor neuropilin-1 (NRP-1), and signaling occurs through the receptor plexin-A1 (Plxn-A1), while forming a protein complex.15-20 There are a few reports about the interacting binding domains between Sema-3A and NRP-1. First, the Sema domain is a significant part, as it binds to the a1 and a2 domain of the NRP-1.5 In addition, the immunoglobulin-like domain (Ig-domain) and the basic C-terminal tail (basic domain) are also able to bind the NRP-1 co-receptor through its b1 and b2 domain.21-24 Studies that investigate the Sema-3A and NRP-1 binding would help to identify the therapeutic potential of this system. The investigation of protein–protein interactions (PPIs) is not only necessary for such approaches but also a challenge in chemical biology.25, 26 Recently, Williams et al. described a peptide derived from the immunoglobulin-like domain of Sema-3A (Figure 1, red square), which was found to be responsible for decreasing the effect of collapsing growth cones in a collapse assay—the semaphorin inhibitory peptide (SIP).14 This peptide seems to be a promising starting point for developing possible therapeutic peptides and identifying its mode of action.
First, we identified a peptide, bearing an alanine at position 5 (SIP[H5A]), which shows a strongly improved inhibitory effect compared with the wild-type SIP. Possible inhibitory effects of the peptide variants were quantified by an enzyme-linked immunosorbent assay (ELISA)-based set-up. Next, we modified the peptide at distinct positions and revealed that position 7 can be modified by amino acids with an increased hydrophobicity. Additionally, we investigated possible interaction sites of the inhibitory peptide to the Sema-3A system by photo-crosslinking experiments coupled with mass spectrometry analysis (XL-MS). Thus, we modified distinct positions in the peptide sequence with the photo-activatable amino acids 4-benzoyl-l-phenylalanine (Bpa) and photo-l-leucine (p-Leu). By XL-MS experiments, we have shown the interaction of these peptides in the Sema domain of the secreted Sema-3A. This suggests the binding of the inhibitory peptides to the Sema domain, which may explain the inhibiting effect for binding to NRP-1.
2 MATERIAL AND METHODS
2.1 Materials
Solid phase peptide synthesis was performed by the Fmoc/t-But strategy.27, 28 9-Fluorenylmethyloxycarbonyl (Fmoc)- and tert-butyloxycarbonyl (tBu)-protected amino acids, N,N′-diisopropylcarbodiimide (DIC), and ethyl-2-cyano-2-(hydroxyimino)acetate (Oxyma) were purchased from Iris Biotech (Marktredwitz, Germany) and Sigma-Aldrich (Taufkirchen, Germany). NovaSyn TGR R resin, thioanisole (TA), biotin, N,N-diisopropylethylamine (DIPEA), acetic anhydride, piperidine, 1-hydroxybenzotriazole (HOBt), and 2,2,2-trifluoroacetic acid (TFA) were obtained from Merck (Darmstadt, Germany). The solvents N,N-dimethylformamide (DMF) and dichloromethane (DCM) were purchased from Biosolve (Valkenswaard, Netherlands) and acetonitrile (ACN) from VWR (Darmstadt, Germany). The linker and photoreactive amino acids, N1-(9-fluorenylmethoxycarbonyl)-1,13-diamino-4,7,10-trioxatridecan-succinamic acid (Fmoc-TTDS-OH), Fmoc-4-benzoyl-l-phenylalanine (Fmoc-Bpa-OH), and (S)-2-{[(9H-fluoren-9-yl)methoxy]carbonylamino}-3-(3-methyl-3H-diazirin-3-yl)propanoic acid (Fmoc-l-photo-leucine-OH) were purchased from Iris Biotech (Marktredwitz, Germany). For the ELISA-based assays and the crosslinking experiments, human Fc-tagged Sema-3A, human NRP-1, and anti-Fc Sema-3A antibody were purchased from R&D systems (Minneapolis, USA). Dulbecco's phosphate buffered saline (DPBS) was purchased from Lonza (Basel, Switzerland). Black NuncMaxiSorp™ 96-well plates, Amplex™ Red reagent, and bovine serum albumin (BSA) were purchased from ThermoFisher (Hennigsdorf, Germany). TWEEN-20 was obtained from Carl Roth (Karlsruhe, Germany) and hydrogen peroxide from Merck (Darmstadt, Germany). Low protein binding tubes were purchased from Eppendorf (Hamburg, Germany). Streptavidin-peroxidase (POD) conjugate was purchased from Merck (Darmstadt, Germany). For sample preparation, PNGaseF was purchased from Promega (Walldorf, Germany). The endoprotease Lys-C was purchased from Serva (Heidelberg, Germany), and Asp-N was purchased from ThermoFisher (Hennigsdorf, Germany). NaOH was obtained from Merck (Darmstadt, Germany). Monomeric avidin agarose beads, guanidinium hydrochloride, and monosodium phosphate were purchased from ThermoFisher (Hennigsdorf, Germany). The Corning®-Costar®-Spin-X® tubes were purchased from Merck (Darmstadt, Germany).
2.2 Solid-phase peptide synthesis of Sema-3A inhibitory peptides
Photoactivatable peptides biotin-TTDS-SIP[H5A][Bpa7], biotin-TTDS-SIP[H5A][Bpa11], and biotin-TTDS-SIP[H5A][p-Leu11] were synthesized by a combination of automated and manual solid-phase peptide synthesis (SPPS) and protected from light.
Automated peptide synthesis was performed using the Fmoc/tBu protection group strategy at a tentagel Rink amide resin with a loading capacity of 0.1–0.2 mmol/g. Automated synthesis was implemented on a SYRO I synthesis robot (MultiSyn Tech, Bochum, Germany) using a scale of 15 μmol per peptide. Reaction tubes (2 ml) were equipped with a disposable Teflon frit and the appropriate amount of resin and placed into the reaction compartment of the robot. Amino acids were dissolved in 0.1 M HOBT in DMF to a final concentration of 0.3 M. Separately, DIC was dissolved in DMF to a final concentration of 1.32 M. Oxyma was dissolved in DMF to a final concentration of 1.2 M. A 40% (v/v) piperidine solution in DMF was prepared. All prepared solutions were placed into the amino acid and reagent compartments of the synthesis robot. DMF served as system solvent. Reaction cycles were programmed as followed: Resins were swollen in 800 μl DMF for 10 min. Subsequently, Fmoc was cleaved by applying 600 μl of a 40% piperidine solution for 3 min followed by 600 μl of a 20% piperidine solution for 10 min. For the latter, 300 μl of 40% piperidine in DMF and 300 μl pure DMF were pipetted into each reactor. After four cycles of washing with 600 μl DMF, 400 μl of amino acid solution and 100 μl of Oxyma solution were pipetted to the resin and left for 2 min before another 100 μl of DIC solution were added. The amounts equal 8 eq of amino acid, Oxyma, and DIC. The overall coupling time was 42 min. After one wash cycle with 600 μl DMF, coupling was repeated once followed by another Fmoc cleavage as described above. Synthesis was performed until desired sequence was completed. If not indicated otherwise, Fmoc cleavage was performed as the last step.
Manual peptide synthesis was performed at room temperature (RT) using 3 eq of photo-activatable amino acid, DIC, and HOBt dissolved in 500 μl per syringe. The coupling was carried out twice for 2 h and 16 h. Fmoc-cleavage and coupling was implemented as described for automated synthesis. Coupling of the 1,13-diamino-4,7,10-trioxatridecan-succinamic acid (TTDS) linker was performed manually. Fmoc-cleavage was performed using two times 500 μl of a 20% solution of piperidine in DMF for 15 min per syringe. After the first cleavage step, the resin was washed with DMF, and after the second cleavage step, the resin was washed with DMF, DCM, and DMF. Biotin coupling was performed using 5 eq of biotin dissolved in 500 μl of N-methyl-2-pyrrolidone (NMP) at 60°C for 10–15 min. After the solution cooled down, HOBt was added by transferring the biotin solution to HOBt. The reaction was started by adding 5 eq of DIC for at least 16 h. Acetylation of the N-terminus of SIP[H5A] and analogues was performed by 35 eq of acetic anhydride and 19 eq of DIPEA in DCM for 15 min at RT while shaking. Peptides were purified by reverse-phase high performance liquid chromatography (RP-HPLC) with a Kinetex 5 μm XB-C18 100 Å column (Phenomenex, Torrence, USA). Purity of the peptides was determined by RP-HPLC on a Jupiter 4 μm Proteo 90 Å C12 (Phenomenex) Aeris 3.6 μm 100 Å XB-C18 (Phenomenex) column. All RP-HPLC was carried out by a linear gradient of eluent B (0.08% TFA in ACN) in eluent A (0.1% TFA in H2O). The identity of the peptides was confirmed by MALDI-TOF MS on an Ultraflex II (Bruker Daltonics, Billerica, USA).
2.3 Competitive binding assay
The competitive binding assay as a modified enzyme-linked immunosorbent assay (ELISA) was performed in a black Nunc MaxiSorp 96-well plate. Wells were pre-coated with 100 μl of a 2 μg/ml human NRP-1 solution overnight at 4°C while gently shaking. After coating, solution was aspirated, and wells were washed 3 times by 200 μl of Dulbecco's phosphate buffered saline (DPBS) containing 0.05% (w/v) of TWEEN-20 (washing buffer). Blocking of remaining surface parts was implemented by incubating the wells with 200 μl of a 4% (w/v) bovine serum albumin (BSA) solution in DPBS for 1.5 h at RT. In parallel, the peptides were diluted and pre-incubated with human Fc-Sema-3A. Vacuum dried peptide (30 nmol) was dissolved in 150 μl DPBS, and an equal volume of doubly distilled water to a final concentration of 100 μM in low protein binding vessels and peptide dilution series was prepared in DPBS. Subsequently, 30 nM human Fc-Sema-3A solution in DPBS was prepared and mixed with an equal volume of each peptide concentration to get a final constant human Fc-Sema-3A concentration of 15 nM. The Sema-3A-peptide solution was incubated for 1 h at RT. The BSA solution was aspirated and wells washed three times with washing buffer. A 100 μl of each Sema-3A-peptide dilution was added in duplicates and incubated for 1 h at RT. After another wash, 100 μl of Fc-Sema-3A antibody solution (1:60,000 diluted in DPBS) was incubated for 1 h at RT. Antibody solution was aspirated, and wells were washed three times with washing buffer and incubated with 100 μl of the detection solution. The detection solution contained 10 μM Amplex™ Red reagent and 2.9 mM H2O2 in DPBS. After 15 min without light exposure, detection appeared by excitation at 535 nm, and emission was measured at 590 nm. Data analysis was prepared using GraphPad Prism 5.0.
2.4 Crosslinking experiments
Crosslinking experiments were performed in low protein binding vessels. Photo-activatable peptides were dissolved in equal volumes of bidest water and DPBS to a final concentration of 50 μM. Unlabeled SIP[H5A] was dissolved with the same solvent to a concentration of 300 μM. The peptide stock-solutions were treated with ultra-sonication for 15 min at 35 kHz. A molar ratio of 1:5:100 (human Fc-Sema-3A or NRP1/photo-activatable peptide/unlabeled peptide) was selected for the experiments. The photo-activatable peptide stock-solutions were diluted in DPBS to the above-described ratio starting with 0.26 μM of Fc-Sema-3A or NRP-1. The peptides were pre-incubated for 15 min at RT, before each protein was added to the peptide mixture. The peptide-protein mixture was incubated for 1 h at RT. Subsequently, reaction vessels were transferred to ice with open lids and irradiated by UV-light at 366 nm for 90 min. The crosslinked protein–peptide complexes were analyzed using sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent Western blotting (WB). Samples were prepared by mixing with 2× reducing Laemmli buffer (1:1) after the irradiation by UV-light. Analysis of the WB and the crosslinked complexes was carried out by incubating the WB with streptavidin-POD (1:5000) and anti-Fc-Sema-3A antibody (1:5000).
2.5 Deglycosylation and proteolytic digest
Deglycosylation was performed using PNGaseF. After irradiation by UV-light, the proteins were denatured by adding dithiothreitol (DTT) to a final concentration of 10 nM and incubated at 80°C for 20 min. After cooling down to RT, samples were treated with 10× deglycosylation buffer I and 10% NMP-40 according to the manufacturers protocol. Of the glycosidase, 1 μl was added, and samples were incubated for 3 h at 37°C. The enzymes were heat-inactivated by incubation at 80°C for 20 min. Next, cysteines of the samples were alkylated by iodoacetamide with a final concentration of 15 mM. After 30 min incubation at RT, the pH values of the samples were controlled in order to start the proteolytic digest. Possible adjustments were performed using 1 M NaOH. Proteolytic digestion was performed by adding Lys-C in a ratio of 1:20 (enzyme/protein) and incubated for 18 h at 37°C. The enzyme was heat-inactivated by 80°C for 20 min. The pH value was controlled and adjusted if required. The second digestion was performed adding Lys-C to the protein–peptide mixture in a ratio of 1:20 (enzyme/protein) and incubated for 18 h at 37°C. The enzyme was heat-inactivated by 80°C for 20 min.
2.6 Affinity chromatography and sample preparation
According to the protocol of the manufacturer, monomeric avidin agarose beads were transferred into a spin-X-tube. The beads were washed for six times using DPBS buffer and centrifugation for 1 min at 12,000g at 4°C per centrifugation step. Next, the beads were transferred into a 2 ml low protein binding vessel, and the protein fragments were added to the beads after the proteolytic digest. To load the columns, the mixture was incubated overnight at 4°C on a rotator. Next, the beads and the solution were transferred into spin-X-tubes, and the flow-through was discarded after the centrifugation. The samples were washed 20 times using DPBS buffer by the centrifugation protocol. To elute the fragments from the avidin beads, an elution buffer, containing 6 M guanidinium hydrochloride at pH 6.0 and 100 mM sodium dihydrogenphosphate, was added and incubated for 10 min at 80°C. Next, the elution buffer was centrifuged as described with changing the temperature to RT. The flow through was collected. The elution step was repeated three times, and the samples combined. Samples were prepared for matrix-assisted light desorption ionization–time of flight analysis (MALDI-TOF) analysis by C18 ZIPTIP (Bruker, Daltonics) pipette tips. Afterwards, 1 μl of the desalted sample was targeted with 0.5 μl of a mixture out of 2,5-dihydroxybenzoic acid and 2-hydroxy-5-methoxybenzoic acid (super-DHB, Bruker Daltonics). Measured spectrums were analyzed. In order to identify the mass signals, mass lists were prepared by in silico digestion using Expasy PeptideMass (https://web.expasy.org/peptide_mass/). The sequence was fragmented by selecting Lys-C and Asp-N as proteases and cysteines in a reduced and alkylated form. The sequence of the photo-activatable peptides was also digested in silico. Next, the masses of the crosslinked peptide-protein fragments were calculated with respect to the crosslinking mechanism of Bpa and p-Leu. Calculated theoretical m/z were compared with the experimental determined m/z and analyzed with an average error of 100 ppm.
3 RESULTS AND DISCUSSION
3.1 Generation of inhibitory peptides and evaluation of activity profiles
Recently, finerenone (Kerendia®) was approved as a therapy for the treatment of chronic kidney disease (https://www.ema.europa.eu/en/medicines/human/EPAR/kerendia, 20 July 2022). However, additional approaches are investigated to address the Sema-3A-induced interactions by antibodies and peptides,24, 29, 30 because peptide therapeutics show lower toxicity compared with antibodies or small drugs.31, 32 Thus, peptides derived from the Sema-3A, which inhibit the Sema-3A-induced interactions (semaphorin inhibitor peptides, SIP), seem to be a promising start for the development of therapeutic peptides addressing acute kidney injury.
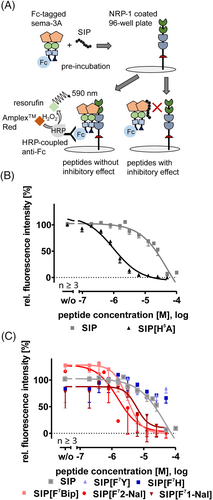
Suitable SIPs and analogues were synthesized by solid phase peptide synthesis (SPPS) using the orthogonal fluorenylmethyloxycarbonyl and tert-butyl (Fmoc/tBu) strategy. The purity of all peptides (≥95%) was tested by reversed-phase high-performance liquid chromatography (RP-HPLC), and identity was validated by matrix-assisted laser desorption/ionization mass spectrometry with time-of-flight detection (MALDI-TOF). To quantify the inhibitory effect of the synthesized compounds, we used an ELISA-based competitive binding assay. Here, we coated a 96-well plate with human NRP-1 and added Fc-tagged Sema-3A protein and the SIP or analogues in different concentrations. Quantification was performed by horseradish-peroxidase (HRP) coupled anti-Fc-Sema-3A antibody, catalyzing the formation of a fluorescence signal from Amplex™ Red to resorufin. Peptides, which disturb the Sema-3A–NRP-1 interaction, lead to a sigmoidal displacement curve with increasing logarithmic peptide concentrations (Figure 2A and Table 1).
| Peptide | Sequence | IC50 [μM] | pIC50 ± SEM | |
|---|---|---|---|---|
1  |
SIP (wild type) | Ac-HAVEHGFIQTLLKVTLE-NH2 | > 10 | n.d. |
2  |
SIP[H5A] | Ac-HAVEAGFIQTLLKVTLE-NH2 | 0.9 | 6.0 ± 0.07 |
3  |
SIP[F7Y] | Ac-HAVEHGYIQTLLKVTLE-NH2 | > 10 | n.d. |
4  |
SIP[F7H] | Ac-HAVEHGHIQTLLKVTLE-NH2 | > 10 | n.d. |
5  |
SIP[F7Bip] | Ac-HAVEHG-Bip-IQTLLKVTLE-NH2 | 2.4 | 5.6 ± 0.02 |
6  |
SIP[F72-Nal] | Ac-HAVEHG-(2-Nal)-IQTLLKVTLE-NH2 | 6.0 | 5.2 ± 0.07 |
7  |
SIP[F71-Nal] | Ac-HAVEHG-(1-Nal)-IQTLLKVTLE-NH2 | 5.0 | 5.3 ± 0.12 |
8  |
[biotin-TTDS] SIP[H5A][F7Bpa] |
biotin-TTDS-HAVEAG-Bpa-IQTLLKVTLE-NH2 | 1.1 | 5.9 ± 0.21 |
9  |
[biotin-TTDS] SIP[H5A][F11Bpa] |
biotin-TTDS-HAVEAGFIQT-Bpa-LKVTLE-NH2 | 1.4 | 5.9 ± 0.19 |
10  |
[biotin-TTDS] SIP[H5A][F11p-Leu] |
biotin-TTDS-HAVEAGFIQT-(p-Leu)-LKVTLE-NH2 | 1.0 | 6.0 ± 0.14 |
- Note: The table shows the peptides with the different exchanged positions, the sequence in one letter amino acid code, the IC50 values, and the pIC50 with the standard error of mean (SEM). IC50 values are displayed with n ≥ 3.
The wild-type SIP (peptide 1) and six analogues (peptides 2 to 7) were synthesized and tested in the ELISA-based assay (Table 1 and Figure 2). The peptide, previously reported SIP, was used as a starting point, and SIP[H5A] was used because of the significant decrease of the IC50 value of 0.9 μM in the ELISA assay compared with the wild-type SIP (peptide 1, IC50 not detectable in our set-up) (Figures 2B and S1).
Next, we searched for a suitable position to introduce the photo-crosslinking amino acid. Phenylalanine at position 7 of the wild-type SIP was exchanged by the amino acids tyrosine or histidine and showed a significant loss of the inhibitory effect, but large aromatic side chains such as in 1-naphthylalanine, 2-naphthylalanine, and biphenylalanine are well tolerated (Figure 2C and Table 1). The increased hydrophobicity seems to be a key factor in the peptide–Sema-3A interaction, indicated by the stronger effect of the biphenylalanine (IC50 2.4 μM).33, 34 This position was identified to be suitable for the exchange to the photo-crosslinker benzoylphenylalanine (Bpa). This amino acid has a hydrophobic side chain and can be activated by irradiation with UV-light.35
3.2 Synthesis of photo-activatable SIP variants
In order to investigate the peptide interaction in more detail to either Sema-3A or NRP-1 by crosslinking and subsequent mass spectrometry analysis, we designed and synthesized photo-activatable SIPs. Instead of the acetylated peptide N-terminus, we introduced a trioxatridecan-succinamic acid (TTDS)-linker and a biotin at the very N-terminus for easy identification of crosslinked protein fragments (Figure 3A). The biotin moiety was introduced to immobilize crosslinked peptide–protein fragments at monomeric avidin agarose beads after the proteolytic digest. The interaction between biotin and the modified avidin agarose beads is similar compared to the normal avidin agarose, which should limit the unspecific bound protein fragments to the bead material.36, 37 The TTDS linker was selected in order to ensure the biotin–avidin interaction, and we used TTDS because it was developed to decrease the hydrophobicity of compounds and peptides in comparison to the standard linker 6-amino-hexanoic acid. This compensates the additional increased hydrophobicity of the hydrophobic peptides and prevents a possible aggregation effect.38 The linker structure, the biotin motif, and the SIP sequence is displayed in Figure 3A.
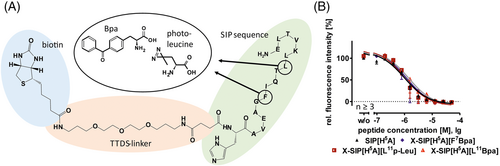
Bpa was introduced at position 7 of SIP[H5A]. Figure 3B and Table 1 demonstrate that the photo-building block and the biotin-TTDS motif at the N-terminus do not show an influence on the inhibitory effect of the crosslinking peptide. For further investigations, leucine at positon 11 was exchanged by the non-proteogenic amino acids Bpa or photo-l-leucine (p-Leu). The biotin-TTDS motif and the introduction of both photo-activatable amino acids at position 11 are accepted and no further loss of the inhibitory effects observable (Figure 3B and Table 1).
3.3 Crosslinking experiments with Sema-3A and NRP-1
The peptides [biotin-TTDS]SIP[H5A][F7Bpa] (peptide 8), [biotin-TTDS]SIP[H5A] [L11Bpa] (peptide 9), and [biotin-TTDS]SIP[H5A][L11p-Leu] (peptide 10) were used to further characterize the binding site of these peptides at Sema-3A and NRP-1 by crosslinking experiments. The proteins were incubated with peptide 8, 9, or 10. To identify unspecific binding sites of the peptides, crosslinking experiments were also conducted in presence of an excess of unlabeled SIP[H5A] (peptide 2). Experiments were performed with a molar ratio of 1:5 (protein/peptide 8, 9, or 10) and 1:5:100 (protein/peptide 8, 9, or 10/unlabeled peptide 2), respectively. Specific binding and crosslinking has been proven by Western Blot (WB) analysis using streptavidin-peroxidase (streptavidin-POD) (Figure 4A,B) and anti-Fc-Sema-3A antibodies (Figure 4C). Successful photo-crosslinking of peptide 8 resulted in bands corresponding to 130 kDa and 100 kDa for Fc-Sema-3A (130 kDa) and Sema-3A lacking the Fc-tag (100 kDa) under reducing conditions (Figure 4A).
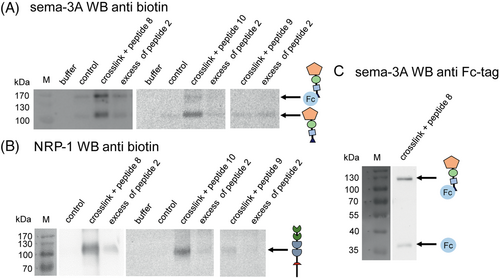
The band intensity decreases when using an excess of peptide 2 in the crosslinking experiments, which indicates a specific binding of peptide 8 (Figure 4A). The WB of Sema-3A under reducing conditions, which was incubated by a horseradish peroxidase (HRP) coupled to anti-Fc-Sema-3A antibody (Figure 4C), shows that the crosslinking band at around 130 kDa is one of the Sema-3A monomers with the Fc-tag. The other band of the crosslinking experiment at around 100 kDa (Figure 4A) indicates that there is another Sema-3A monomer without the Fc-tag, as Sema-3A is a disulfide-linked homodimer.
This suggests that crosslinking occurs at the Sema-3A protein and not at the Fc-tag and shows a specific binding to the protein. Further experiments with both peptides 9 and 10 display a crosslinking band at around 100 kDa and a band at around 130 kDa for the peptide 10 incubated with Sema-3A, revealing also a specific crosslinking to the Sema-3A-protein. The WB shows a much lower intensity for the experiments with peptide 9; thus, no specific crosslinking band is observable (Figure 4A). This indicates that Bpa at position 11 is not able to get close enough (3–8 Å) to the Sema-3A binding site.39, 40
The inhibitory mechanism of the peptides for the Sema3A-NRP-1 system is not known. Thus, we performed additional crosslinking experiments for the NRP-1 in the same way. Here, we expect one band at around 100 kDa under reducing conditions. The WB analysis with streptavidin-POD shows also a crosslinking band, decreasing in intensity with an excess of peptide 2 (Figure 4B). This indicates that the peptides do not only bind to Sema-3A but also to NRP-1 (Figure 4B).
3.4 Sample preparation and mass spectrometry
In order to investigate the binding mechanism of the photo-activatable peptides, we analyzed the crosslinked protein–peptide complexes by MALDI-TOF, as described previously.41, 42
Both Sema-3A and NRP-1 were purchased as glycosylated proteins. Before the linked protein–peptide complexes were digested for mass analysis, N-glycosylations were removed by PNGaseF treatment after denaturation with dithiothreitol. This is crucial, because glycosylations normally protect proteins from proteolytic degradation.43, 44 The reduced cysteine side chains were alkylated after the deglycosylation using iodoacetamide. The samples were digested by adding Lys-C and Asp-N proteases for 18 h each. The enzymatic degradation was analyzed by sodium dodecylsulfate polyacrylamide gel electrophoresis (data not shown). Consequently, the mixture was purified by affinity purification with monomeric avidin agarose. To exclude signals from unspecific binding and crosslinking, the experiments were additionally performed with Sema-3A and NRP-1 in presence of an excess of unlabeled peptide 2. Accordingly, the signals identified in these samples were not included into the binding site analysis.
Mass spectra have been obtained from m/z = 2000 to m/z = 5000. Signals have been identified, and the monoisotopic m/z has been compared with theoretical m/z resulting from in silico crosslinking and digestion of the protein. Since the amino acids asparagine and glutamine may be deamidated due to amidase activity at a pH value between 8.5 and 9.0,45-47 we considered deamidation, alkylation of cysteines, and oxidation of methionines as potential modification for the digested protein and peptide sequences.
All specifically assigned crosslinking signals of the experiment with Sema-3A and peptide 8 or peptide 10 are shown in Table 2. For the analysis of the mass spectra [M+H]+, signals are listed. Unexpectedly, signals referring to potential crosslinking products showed very poor signal intensity. For this reason, we were unable to perform MS/MS experiments to further identify the crosslinking site. Overall, we have identified two signals for the crosslinking experiment with peptide 8, in two out of three independent experiments. The [M+H]+-signals with a m/z of 3429.17 and 2936.70 were identified with an average error of 100 ppm. The signals showing crosslinked peptide-protein fragments, which are in a similar area of the Sema-3A sequence (Table 2).
| Protein fragment | Mcalc protein [Da] | Peptide fragment | Mcalc peptide [Da] | Calculated crosslinked mass | [M+H]+ | Signals in total |
|---|---|---|---|---|---|---|
| Crosslinking signals of peptide 8 with Sema-3A | ||||||
| 260–271 | 1368.69 | X-HAVEAG-XL-IQTLLK-OH | 2058.09 | 3426.78 + 1.05 (deamidated) | 3429.17 | 1 |
| 243–249 | 877.44 | X-HAVEAG-XL-IQTLLK-OH | 2058.09 | 2935.50 (unspecific Glu-N cleavage) | 2936.7 | 1 |
| Crosslinking signals of peptide 10 with Sema-3A | ||||||
| 306–309 | 503.22 | X-HAVEAGFIQT-XL-LKVTLE-NH2 | 2407.29 | 2882.50 | 2883.57 | 1 |
| 385–389 | 527.24 | X-HAVEAGFIQT-XL-LKVTLE-NH2 | 2407.29 | 2906.52 | 2907.62 | 2 |
- Note: The signals of the crosslinking with peptide 8 shows signal distribution between position 240 and 270 in the Sema-3A sequence, indicated by two signals in this range. The experiments with peptide 10 shows two times a [M+H]+-signal at a m/z of 2907.62, which is located in another area of the Sema-3A protein. Comparison of signals for each crosslinking peptide from n = 3 experiments.
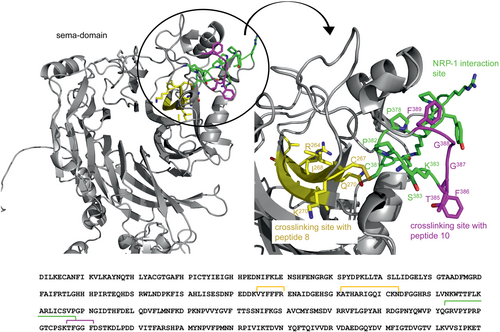
In case of the crosslinking studies with peptide 10, we have identified three signals. The [M+H]+-signals of this experiment were identified with an average error of 50 ppm (Table 2, Table S1). First, it seems that the crosslinking experiment with peptide 10 shows a completely different Sema-3A crosslinking site compared to the experiments with peptide 8. Also, the higher signal distribution might be a side effect due to the faster photolabeling of diazirene moieties compared with the slower benzophenone side chains.48 Interestingly, two of these signals have been found in two out of three independent experiments, which corresponds to a [M+H]+-signal at a m/z of 2907.62. In order to visualize the identified crosslinked Sema-3A sequences, a 3D-model of the human Sema-3A from Alphafold was used (Q14563).49, 50
The signals of the experiments using peptide 8 are located in a defined range of the beta-propeller structure of the Sema-3A protein. This region corresponds to the Sema domain, which is important for the binding to NRP-1 (Figure 5).23 The range around Sema-3A positions 243 to 249 and 260 to 271 seems to be an interesting interaction site for the N-terminal part of the inhibitory peptide. We observed that peptide 2 shows a beta-sheet structure in circular dichroism spectroscopy (data not shown). With respect to beta-sheet inducing amino acids isoleucine, glycine, and valine in the inhibitory peptide sequence, we assumed a sheet–sheet interaction.51-53 However, the [M+H]+-signal at a m/z of 2709.62, which was found in two distinct experiments, covers the loop area at position 385 to 389 of the Sema domain (marked in magenta, Figure 5). Interestingly, this area is in close proximity to the yellow marked crosslinking sites between Sema-3A and peptide 8. Due to the potential of the diazirene moiety to react rather with O–H groups as with C–H groups, we suggest a crosslink interaction with tyrosine 385 (Figure 5).54 If both experimentally assigned signals were compared, the data indicate a binding of the inhibitory peptides in the beta-propeller structure of the Sema domain, where the binding site to the NRP-1 co-receptor is located. Additionally, we suggest that the N-terminal part of the peptide binds in the propeller structure, whereas the C-terminal part interacts with the loop of the Sema domain. Investigations of the Sema-3A–NRP-1–Plxn-A1 complex revealed binding sites of the Sema domain at position 354 to 368 and 478 to 480 to NRP-1.23 Our experiments suggest that the peptides show a higher affinity to the binding site of the Sema domain at position 354 to 368 by blocking the PPI. The magenta-colored crosslinking site also overlaps with the NRP-1 binding module at a tyrosine and a phenylalanine (Figure 5). This site may be the key interaction between the peptide and the loop of the Sema domain.
The investigation between the photo-activatable peptides and the co-receptor NRP-1 was performed as a control, since the WB analysis also showed a crosslinking band. After analyzing the crosslinking experiments with peptides 8 and 10, two signals have been identified as specific crosslinking sites, which are not in close proximity to NRP-1 according to its structure (Table S2 and Figures S6, S7, S8, and S9). This suggests an unspecific binding between the peptides to the NRP-1 co-receptor. Williams et al. suggested a binding of the SIPs to b1 and b2 domain of the NRP-1.14 Our data suggest that the peptide does not bind specifically to the NRP-1 co-receptor. The peptide seems to bind at the Sema-3A protein and therefore is blocking the receptor interactions, as revealed by our crosslinking studies. However, the abundance of signals, which have been assigned was low but show a precise area within the Sema domain. This is also located at the interacting domain to the NRP-1 co-receptor.21, 23 Further studies and methods should be used to confirm the data presented herein.
Our approach shows that there seems to be a specific range of interaction sites of the peptides to the Sema domain of the human Sema-3A protein, which are in close proximity. This demonstrates that the improved inhibitory peptides may interrupt the protein–protein interactions between NRP-1 and Sema-3A due to blocking of the binding site between the secreted protein and the co-receptor.
4 CONCLUSION
In this study, the inhibitory effects and the potential binding site of Sema-3A derived peptides to Sema-3A or its co-receptor NRP-1 were investigated. The peptides were screened by using amino acid exchange and afterwards modified by photoactivatable amino acids, in order to perform photo-crosslinking MS studies. After synthesis, the inhibitory effect of the different peptide variants has been successfully tested by an ELISA-based competition assay. The incorporation of the photoactive amino acids Bpa and p-Leu showed no significant loss compared to peptide 2. The crosslinking studies and the detection by WB analysis showed, that these peptides bind to both Sema-3A and NRP-1. Mass analysis of the crosslinked complexes showed specific binding sites between the inhibitory peptide and Sema-3A. This crosslinking site is in line with the common hypothesis of the Sema-3A binding site to the NRP-1 co-receptor. Our data demonstrate that our improved peptides might be able to block the interaction between Sema-3A and NRP-1, which explains the inhibitory potential of these peptides.
These studies may help to develop potential peptide-based therapeutic approaches to treat chronic kidney disease. The investigated binding sites will help to modify and optimize peptides for selective and specific targeting of the Sema-3A and NRP-1 interaction and therefore reduce the effects of chronic kidney disease.
ACKNOWLEDGMENTS
The authors kindly acknowledge Ronny Müller, Regina Reppich-Sacher, Kristin Löbner, and Janet Schwesinger (Leipzig University) for their excellent technical support. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare the following competing financial interest(s): Part of the work was financially supported by Bayer AG. This work was further supported by the European Union, the federal state of Saxony, and the “European Regional Development Fund.”




