Charge inversion at position 68 of the glucagon and glucagon-like peptide-1 receptors supports selectivity in hormone action
Abstract
Glucagon and glucagon-like peptide-1 (GLP-1)are two structurally related hormones that acutely regulate glucose control in opposite directions through homologous receptors. The molecular basis for selectivity between these two hormones and their receptors is of physiological and medicinal importance. The application of co-agonists to enhance body weight reduction and correct multiple abnormalities associated with the metabolic syndrome has recently been reported. Substitution of amino acids 16, 18, and 20 in glucagon with those found in GLP-1 and exendin-4 were identified as partial contributors to balanced, high potency receptor action. The amidation of the C-terminus was an additional glucagon-based structural change observed to be of seminal importance to discriminate recognition by both receptors. In this work, the molecular basis for receptor selectivity associated with differences in C-terminal peptide sequence has been determined. A single charge inversion in glucagon and GLP-1 receptor sequence at position 68* was determined to significantly alter hormone action. Changing E68* in GLP-1R to the corresponding Lys of GCGR reduced receptor activity for natural GLP-1 hormones by eightfold. The enhanced C-terminal positive charges in GLP-1 peptides favor the native receptor's negative charge at position 68*, while the unfavorable interaction with the C-terminal acid of native glucagon is minimized by amidation. The extension of these observations to other glucagon-related hormones such as oxyntomodulin and exendin, as well as other related receptors such as GIPR, should assist in the assembly of additional hormones with broadened pharmacology. Copyright © 2010 European Peptide Society and John Wiley & Sons, Ltd.
Introduction
The glucagon and glucagon-like peptide-1 (GLP-1) receptors are members of the family of B1 G-protein-coupled receptors (B1 GPCR) that share approximately 50% sequence identity and a conserved mechanism of ligand recognition and receptor activation1. Structurally, each receptor is composed of a C-terminal intracellular domain, seven transmembrane helices (core domain), and an N-terminal extracellular domain (NTD). The NTD binds the C-terminal half of the ligand while the core domain binds the N-terminus region, leading to receptor activation2. There is no reported crystal structure for a full length family B1 GPCR, therefore structure–function relationships are established in part by biochemical analysis with structurally altered ligands and receptors. Glucagon and GLP-1 have been the focus of several studies directed at the determination of the molecular basis for family B1 GPCR receptor action and selectivity2-7.
Glucagon and GLP-1, like their receptors, share a high level of sequence homology (Figure 1(A)). These hormones serve significant but opposing roles in regulating glucose homeostasis and are of sizeable clinical importance to the management of diabetes. Glucagon acts primarily at hepatic glucagon receptors (GCGR) to increase plasma glucose, while GLP-1 functions during nutrient ingestion at pancreatic β-cell GLP-1 receptors to enhance insulin synthesis and secretion8. GLP-1 effects on blood glucose, β-cell protection, appetite, and body weight have led to the use of multiple GLP-1R agonists for the treatment of type 2 diabetes9-11. In contrast, glucagon is used to treat severe hypoglycemia, while antagonists have been developed for the treatment of type 2 diabetes. More recently, the unique pharmacology of GCGR/GLP-1R co-agonists was reported to promote enhanced weight loss when compared to selective GLP-1R agonists in rodent studies4, 12.
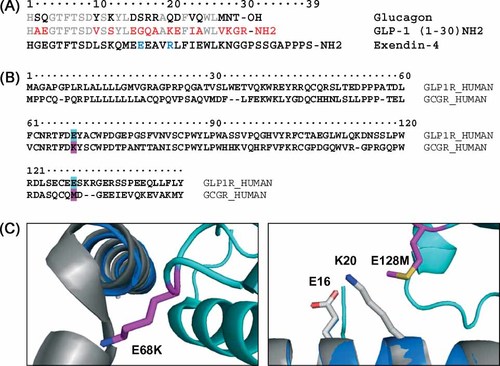
(A) Sequence homology of glucagon and GLP-1. Conserved (gray); glucagon (black); GLP-1 (red). Exendin-4 sequence is shown in black as a reference. The unique E16 and R20 of exendin-4 are highlighted in blue. (B) Glucagon receptor (GCGR) and GLP-1 receptor (GLP-1R) sequence from 1–145 (NTD region). GLP-1R mutation sites at position 68* and 128* are highlighted in cyan and corresponding GCGR amino acids are shown in magenta. (C) Ex-4 (9–39) GLP-1R NTD co-crystal structure (PDB ID: 3C5T). The glucagon crystal structure (1GCN) was overlaid on the co-crystal structure. GLP-1R positions 68* and 128* were mutated to their corresponding GCGR residues in PyMOL (DeLano Scientific LLC). Ex-4 (gray); GLP-1R NTD (cyan); glucagon (blue); receptor mutations (magenta); peptide mutations E16 and K20 (white)
Structure–activity studies of GCGR/GLP-1R co-agonist peptides demonstrated that full potency at GLP-1R could be achieved using a glucagon nucleus with three amino acid substitutions (E16, A18, K20) and amidation of the C-terminal acid4. The exendin-4 (Ex-4) GLP-1R NTD crystal structure13 supports the amino acid substitutions. E16 appears to stabilize the α-helical structure via an intramolecular salt bridge with R20, which further interacts with E128* (* refers to receptor amino acid) of the GLP-1R13. A18 appears proximal to a hydrophobic pocket in the NTD, and this may contribute to the increased receptor potency relative to the native positively charged R18 of glucagon.
The C-terminal acid to amide substitution provided the most substantial decrease in selectivity, primarily through increased potency at GLP-1R. Thus, the importance of the C-terminal acid in glucagon selectivity is quite evident. Biophysical structure analysis through circular dichroism studies showed increased helicity for peptide amides, indicating that the improved potency at GLP-1R may result in part through the enhanced secondary structure4. However, direct C-terminal charge interaction with each receptor is a potential additional contributor to the relative degree of activity. The Ex-4 GLP-1R NTD crystal structure did not provide structural evidence for the specific glucagon C-terminal amide modification. The specific receptor location for preferentially binding ligands that terminate as acids was hypothesized to be one where a charge inversion from negative in GLP-1R to positive in GCGR might exist.
Sequence alignment of GLP-1R and GCGR (Figure 1(B)) revealed divergence of charge at position 68* from a Lys in the latter to a Glu in the former. This receptor site appears proximal to the C-terminus of the ligand in the GLP-1R NTD crystal structure (Figure 1(C)). The purported role of E68* in the exendin-4 structure was hydrogen bonding to S32. Our results demonstrate that the crystal structure is limiting and does not anticipate the interaction with other glucagon-related peptides that terminate prior to the purported site of ligand interaction. Through replacement of GLP-1R position E68* with the corresponding Lys from GCGR our results assign a role for amino acid 68* in supporting selectivity in the recognition of glucagon, GLP-1, and related hormones.
Experimental Procedures
Boc Peptide Synthesis and Cleavage
Peptide syntheses were performed using 0.2 mmol 4-methylbenzhydrylamine (MBHA) resin (Midwest Biotech, Fishers, IN, USA) on a modified Applied Biosystems 430A peptide synthesizer. Solid phase peptide syntheses utilized in situ neutralization for Boc-chemistry as described previously14. Completed peptidyl-resins were treated with HF/p-cresol (10:0.5 v/v) at 0 °C for 1 h. HF was removed in vacuo and the deprotected peptide was precipitated and washed in diethyl ether. The peptide was dissolved in 20% acetonitrile/1% acetic acid and lyophilized. The following side chain protecting groups were used for Boc-amino acids (Midwest Biotech): Arg(Tos), Asp(OcHex), Asn(Xan), Glu(OcHex), His(BOM), Lys(2-Cl-Z), Ser(Bzl), Thr(Bzl), Trp(CHO), and Tyr(Br-Z). Peptide molecular weights were confirmed by electrospray ionization or MALDI-TOF MS and purified as described elsewhere.
Peptide Purification
Following cleavage from the resin, crude peptide extracts were analyzed by analytical reversed-phase HPLC. Analytical separations were conducted in 0.1% TFA with an acetonitrile gradient on a Zorbax C8 column (0.46 × 5 cm). After analytical analysis, the crude extract was purified by semi-preparative chromatography in 0.1% TFA with an acetonitrile gradient on a Vydac C4 or C18 column (2.2 × 25 cm). Preparative fractions were analyzed for purity by analytical reversed-phase HPLC utilizing the conditions listed for analytical separations. Peptide masses and purity were confirmed by ESI-MS or MALDI-TOF MS. Purified peptides were lyophilized and stored at 4 °C.
Cloning of Receptor Mutants
GLP-1R cDNA (Open Biosystems, Catalog No. MHS1768-97 430 513, Huntsville, AL, USA) was subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen). GLP-1R point mutants were produced by a modified megaprimer mutagenesis method using a single flanking reverse primer (5′ GTGGATGTAGTTCCTGGTGC 3′) and mutagenic forward primers (E68K*: 5′ G ACC TTC GAT AAA TAC GCC TGC) (E128M*: 5′ CG GAG TGC GAG ATG TCC AAG CGA G 3′). Plasmid DNA was obtained using the Plasmid Midi Kit (QIAGEN, Valencia, CA, USA). Mutations were confirmed using ABI Big Dye version 3.1 kit and sequencing on ABI3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Cell Culture, Transfection, and Functional Experiments
HEK293 cells were maintained in Dulbecco's modified Eagle Medium (HyClone, Logan, UT, USA) supplemented with 10% bovine growth serum (HyClone, Logan, UT, USA), antibiotics, and 10 mM HEPES. Cells were plated in 10-cm tissue culture dishes and transfected with 6 µg DNA using the FuGENETM 6 transfection reagent (Roche, Indianapolis, IN, USA). Cells were trypsinized 24 h after transfection and 40 µl were plated at 20 × 103 cells/well in 96 half-well plates (Corning, Corning, NY, USA). Serial dilutions of glucagon and GLP-1 peptides were added to wells at a final volume of 50 µl containing cell suspensions and shaken for 30 min at room temperature. Following incubation, 25 µl of diluted cAMP XL-665 (CIS bio international, Bedford, MA, USA) was added to all the wells followed by addition of 25 µl anti-cAMP cryptate conjugate (CIS bio international). Assay plates were shaken for an additional hour and time-resolved fluorescence was read at 665 nm and 590 nm on an Envision plate reader (Perkin-Elmer, Wellesley, MA, USA). EC50 values were calculated using Origin software (OriginLab, Northampton, MA, USA).
Results
E128M* GLP-1R Interaction with Peptide Position 20
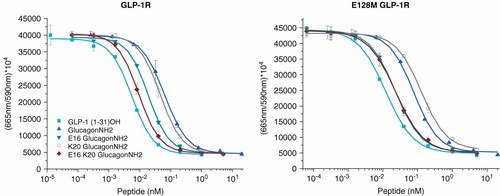
We have previously reported the selective structural changes to native glucagon (peptides 2 and 7) to achieve potency comparable to native GLP-1 (peptides 1 and 9). Two pairs of K20Q glucagon amide analogs (peptides 4, 6 and 5, 7) differing only in the presence of E16 were evaluated at the native GLP-1R and E128M* GLP-1R mutant (Figures 2 and 3 and Table 1). Absolute activity and relative potency (GLP-1R/E128M*) were calculated for each peptide to yield a value describing differential receptor recognition. GLP-1 (1–31)OH (peptide 1) demonstrated an approximately threefold decrease in potency as a result of the one specific receptor change of Glu with methionine. Comparison of peptides 4–7 illustrated the slightly increased potency of K20 analogs at the native receptor that was reversed at the mutated receptor, as captured in the lower percentage ratio (peptides 6, 7 vs 4, 5). The enhanced reduction in the K20 analogs was strikingly similar to that observed for native GLP-1 acid (peptide 1) and supports a specific charge interaction that is eliminated when the native negative charge at position 128* was eliminated through the E/M mutation.

Sequence listing of peptides evaluated in biological assays. Numbers 1–16 correspond to compound numbers in Table 1. Conserved (gray); glucagon (black); GLP-1 (red); non-native to glucagon and GLP-1 (blue)
GLP-1R and E128M* GLP-1R mediated cAMP induction by Q20/K20 glucagon-based analogs in a FRET based competitive immunoassay. This figure is available in colour online at wileyonlinelibrary.com/journal/jpepsci.
| No. | Peptide | GLP-1R EC50 (nM) | GLP-1R % | E128M EC50 (nM) | E128M % | GLP-1R/E128M % |
|---|---|---|---|---|---|---|
| 1 | GLP-1 (1–31)OH | 0.0056 ± 0.0017 | 100 | 0.015 ± 0.005 | 100 | 37 |
| 2 | Glucagon | 0.36 ± 0.08 | 2 | — | — | — |
| 3 | E16 glucagon | 0.16 ± 0.06 | 4 | — | — | — |
| 4 | GlucagonNH2 | 0.061 ± 0.023 | 9 | 0.096 ± 0.058 | 16 | 64 |
| 5 | E16 glucagonNH2 | 0.022 ± 0.007 | 25 | 0.026 ± 0.010 | 58 | 85 |
| 6 | K20 glucagonNH2 | 0.045 ± 0.009 | 12 | 0.14 ± 0.08 | 11 | 32 |
| 7 | E16 K20 glucagonNH2 | 0.0094 ± 0.0019 | 60 | 0.030 ± 0.011 | 50 | 31 |
| 8 | Chimera 3 | 0.035 ± 0.009 | 16 | — | — | — |
| 9 | GLP-1 (1–30)NH2 | 0.0089 ± 0.0022 | 63 | — | — | — |
| 10 | G30 GLP-1 (1–30)NH2 | 0.0049 ± 0.0006 | 114 | — | — | — |
| 11 | GLP-1 (1–29)NH2 | 0.0084 ± 0.0028 | 67 | — | — | — |
| 12 | E16 GLP-1 (1–29)NH2 | 0.0024 ± 0.0010 | 233 | — | — | — |
| 13 | Glucagon Cex | 0.086 ± 0.015 | 7 | — | — | — |
| 14 | G29 glucagon Cex | 0.035 ± 0.006 | 16 | — | — | — |
| 15 | Oxyntomodulin | 0.15 ± 0.03 | 4 | — | — | — |
| 16 | E16 A18 K20 glucagon NH2 | — | 100* | — | — | — |
- EC50 values represent peptide concentrations at which half-maximal activation occurs. Average EC50 values are calculated from a minimum of three independent experiments and standard deviations are shown for all peptides. GLP-1R (%) is calculated using the equation EC50(peptide 1)/EC50(peptide x) × 100. GLP-1R/E128M* (%) is calculated using the equation: GLP-1R EC50/Mutant Receptor EC50 × 100.
- * Peptide 16 relative % is compared to previous values obtained in a different assay system relative to GLP-1 (1–31)-OH (EC50 0.028 nM-unpublished data).
Position 68* Interaction with the C-Terminus
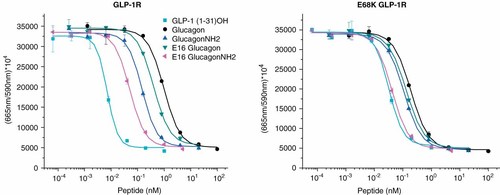
Native GLP-1 (peptide 1) exhibited an eightfold reduction at E68K* GLP-1R, demonstrating the destructive influence of losing a negative charge and/or replacing it with a positive charge (Table 2). A similar comparison of peptides 2–5 showed a difference in potency favoring the peptide amides (Figure 4 and Table 2). C-terminal amides were ∼6 times more potent at native receptors, which is consistent with results we reported previously using a different assay system4 (peptides 2, 3 vs 4, 5). When compared to the previous assay system4, this assay maintains a high degree of consistency for amino acid additions of E16 and K20 in addition to the aforementioned amide modification. Absolute potency values are different but relative changes in potency for each system are consistent. At the E68K* mutated receptor the relative difference in potency between amides and acids narrowed. The C-terminal acid analogs (peptides 2, 3) displayed modestly increased potency at E68K* relative to GLP-1R, whereas the potency of the amide analogs (peptides 4, 5) was reduced approximately twofold. The results suggest that the negatively charged peptide C-terminus forms a more favorable interaction with the positively charged position 68* mutated receptor than the negatively charged native receptor. The fact that the amides maintained greater potency than the acids at the E68K* receptor suggests that the relative change may be more a function of removing a destructive interaction of a negatively charged receptor with a C-terminal peptide acid. However, the influence of the C-terminal amide to enhance secondary helical structure and the influence on receptor activity remain an additional indirect factor for consideration.
GLP-1R and E68K* GLP-1R mediated cAMP induction by C-terminal amide/acid glucagon-based analogs. This figure is available in colour online at wileyonlinelibrary.com/journal/jpepsci.
| No. | Peptide | GLP-1R EC50 (nM) | GLP-1R % | E68K EC50 (nM) | E68K % | GLP-1R/E68K % |
|---|---|---|---|---|---|---|
| 1 | GLP-1 (1–31)OH | 0.0056 ± 0.0017 | 100 | 0.044 ± 0.020 | 100 | 13 |
| 2 | Glucagon | 0.36 ± 0.08 | 2 | 0.23 ± 0.11 | 19 | 157 |
| 3 | E16 glucagon | 0.16 ± 0.06 | 4 | 0.12 ± 0.04 | 37 | 133 |
| 4 | GlucagonNH2 | 0.061 ± 0.023 | 9 | 0.12 ± 0.02 | 37 | 51 |
| 5 | E16 glucagonNH2 | 0.022 ± 0.007 | 25 | 0.039 ± 0.009 | 113 | 56 |
| 6 | K20 glucagonNH2 | 0.045 ± 0.009 | 12 | — | — | — |
| 7 | E16 K20 glucagonNH2 | 0.0094 ± 0.0019 | 60 | — | — | — |
| 8 | Chimera 3 | 0.035 ± 0.009 | 16 | 0.051 ± 0.011 | 86 | 69 |
| 9 | GLP-1 (1–30)NH2 | 0.0089 ± 0.0022 | 63 | 0.075 ± 0.024 | 59 | 12 |
| 10 | G30 GLP-1 (1–30)NH2 | 0.0049 ± 0.0006 | 114 | 0.022 ± 0.004 | 200 | 22 |
| 11 | GLP-1 (1–29)NH2 | 0.0084 ± 0.0028 | 67 | 0.036 ± 0.013 | 122 | 23 |
| 12 | E16 GLP-1 (1–29)NH2 | 0.0024 ± 0.0010 | 233 | 0.011 ± 0.001 | 400 | 22 |
| 13 | Glucagon Cex | 0.086 ± 0.015 | 7 | 0.13 ± 0.01 | 34 | 66 |
| 14 | G29 glucagon Cex | 0.035 ± 0.006 | 16 | 0.30 ± 0.03 | 15 | 12 |
| 15 | Oxyntomodulin | 0.15 ± 0.03 | 4 | 1.6 ± 0.4 | 3 | 9.4 |
| 16 | E16 A18 K20 glucagonNH2 | — | 100* | — | — | — |
- EC50 values represent peptide concentrations at which half-maximal activation occurs. Average EC50 values are calculated from a minimum of three independent experiments and standard deviations are shown for all peptides. GLP-1R (%) is calculated using the equation EC50(peptide 1)/EC50(peptide x) × 100. GLP-1R/E68K* (%) is calculated using the equation: GLP-1R EC50/Mutant Receptor EC50 × 100.
- * Peptide 16 relative % is compared to previous values obtained in a different assay system relative to GLP-1 (1–31)-OH (EC50 0.028 nM-unpublished data).
The N-terminal half of E16, glucagon amide (peptide 5), was replaced with the homologous GLP-1 sequence (peptide 8). There was no apparent difference demonstrated by this chimeric peptide (peptide 8) at GLP-1R and E68K*, indicating that position 68* has no apparent role in interaction with the N-terminal half of GLP-1. Additionally, the sizeable eightfold potency decrease of native GLP-1 at E68K* (peptide 1) shows that position 68* is selectively involved in the recognition of the GLP-1 C-terminus.
GLP-1 Position 30 Interaction with Receptor Position 68*
Peptides 8–12 were studied to determine whether the charge at position 30 is the source of differential interaction with GLP-1 receptor position 68* (Table 2). The native amide form of GLP-1 (peptide 9) was comparably potent at the native receptor and equally reduced in potency at the E68K* receptor mutant compared to the native GLP-1 (peptide 1). The absence of any difference in the native GLP-1 acid and amide peptides stands in contrast with the notable difference in the C-terminal glucagon acid and amide. Two important differences are the increased length of the GLP-1 peptides and the specific nature of the amino acid at position 30. Substitution of R30 with G30 (peptide 10) did not substantially affect potency at GLP-1R but reduced the magnitude of the difference by half (approximately eightfold loss to approximately fourfold loss of potency at E68K*). Removal of position 30 (peptide 11) did not change the relative potency at GLP-1R (peptide 9) and also resulted in a comparable loss in potency relative to the G30 analog at E68K*. The presence of R30 in peptides 1 and 9 resulted in the most significant loss in potency upon receptor mutation with Lys at position 68* (approximately eightfold loss). The fact that substitution of amino acid 30 with Gly or its deletion partially eliminated this effect indicates that R30 is an important element in the reduced ability of such peptides to bind K68* receptors.
Glucagon C-Terminal Extension Interactions with Receptor Position 68*
Given the observed destructive interaction of E68K* receptor with R30 we chose to explore oxyntomodulin (OXY, peptide 15), the biosynthetic precursor to glucagon that differs only by an eight-residue cationic C-terminal extension. OXY activated the receptors half-maximally at concentrations of 0.15 nM and 1.6 nM, with the native receptor being of greater potency (Figure 5 and Table 2). The greater than tenfold decrease suggests that there is an interaction between the receptors and the positively charged tail of OXY. Interestingly, OXY has been reported to be approximately tenfold reduced in its potency at GCGR relative to glucagon15.
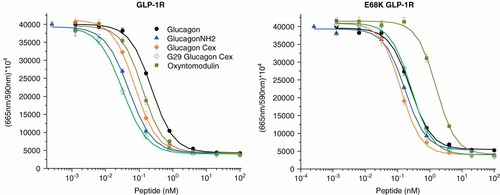
GLP-1R and E68K* GLP-1R mediated cAMP induction by glucagon-based analogs with C-terminal extensions. This figure is available in colour online at wileyonlinelibrary.com/journal/jpepsci.
A second glucagon-related peptide with a sizable C-terminal sequence extension is Ex-4. Addition of Cex appends 10 uncharged amino acids to the C-terminus of glucagon. Glucagon Cex (peptide 13) properties were strikingly similar to glucagon-amide (peptide 4). Structural analysis shows that Cex folds back upon Ex-4 to form a so-called Trp-cage16. The natural C-terminal Thr of glucagon, unlike the comparably positioned Gly of Ex-4, was envisioned to potentially distort the Trp-cage. Substitution to G29 (peptide 14) resulted in slightly enhanced potency at GLP-1R and dramatically decreased potency at E68K*, relative to 13. Once again, these results demonstrate that the specific charge at receptor position 68* can be significant in determining receptor selectivity and in this specific instance unlike OXY it is more difficult to ascribe the effect to a particular charge–charge interaction. The experimental results across the breadth of this set of glucagon analogs demonstrates that the nature of the charge at receptor position 68* exerts a sizable influence on selectivity across a set of glucagon-related peptides and potentially other less homologous ligands like GIP where interest exists in identification of co-agonists.
Discussion
Receptor mutagenesis studies of full length GLP-1R and GCGR have proved to be useful in determining residues important for selectivity and potency2, 3, 17-19. A study by Graziano used mutation of several adjacent GLP-1R residues to corresponding GCGR amino acids at different sites throughout GLP-1R17. Mutation of residues 29–32 resulted in a significant reduction in binding selectivity at GLP-1R relative to the mutant from 1500 to 32. However, when coupled to adenylate cyclase, selectivity did not change significantly upon receptor mutation. Although binding did not appear coupled to activity in this case, their results showed that mutations in the GLP-1R NTD influenced selectivity for the ligands glucagon and GLP-1. In an additional study, Runge and colleagues used domain shuffling of GCGR/GLP-1R and the glucagon/GLP-1 ligands to demonstrate N-terminal peptide recognition by the core domains of the receptor while showing C-terminal recognition by the NTDs of the receptors2, 3. These data coupled with current structural data have provided insights toward understanding the molecular basis for specificity at GCGR and GLP-1R.
Our first objective in this study was to explore the differential interaction at position E128* with Q20/K20 peptides. In order to do this, we chose to mutate position E128*, which has been shown to form a salt bridge with position 20 of Ex-4 (9–39) in the GLP-1R NTD co-crystal structure13. In this set of analogs, peptides 4 and 5 (Q20 analogs) are employed as a reference point to 6 and 7 (K20 analogs). E128M* mutation showed reduction in potency of K20 peptides, but not Q20 peptides relative to the GLP-1 receptor. The differential recognition (GLP-1R/E128M*) of Q20 and K20 peptides by the two different receptors indicates the importance of E128*-K20 for optimal potency.
In a recent study with native GLP-1, E128Q* mutation of GLP-1R showed no change in potency relative to the native receptor19. However, E128A* GLP-1R showed an approximately 2.5-fold decrease relative to the native receptor19. Collectively, the relative decrease in potency from our E128M* mutation with the two other structural changes (E128A*, E128Q*) described elsewhere suggests that a polar side chain at position 128* such as Glu or Gln is more favored for interaction with Lys at position 20.
Selection of Position 68*
Position 68* of GLP-1R, although not conserved, lies in a highly conserved turn between β1 and β2. The highly homologous GIPR structure demonstrated that D66* (analogous to D67* of GLP-1R) hydrogen bonds to the backbone amides of position 67*, 68*, 69*, and indole nitrogen of W71* (analogous to 68*, 69*, 70*, and 72* of GLP-1R) to stabilize this loop for ligand binding20. The high homology shared within this stretch of sequence throughout the glucagon-receptor family serves a global structural role in NTD stabilization and also fixes this loop region proximal to the C-terminal end of the ligand. The fact that position 68* diverges throughout the receptor family suggests that this residue may have a role in selectivity for the highly divergent C-termini of the glucagon-related family of peptides. This provided a basis for further study into the role of position 68* using receptor mutagenesis.
C-Terminal Amide/Acid
Peptides 2–5 examined two C-terminal acids and two C-terminal amides with S16 or E16. E16 did not contribute to differential recognition at the GLP-1R and E68K* receptors. It is expected that the C-terminal acid should pair more favorably with the Lys relative to the C-terminal amide based on charge complementarity. Both C-terminal acids showed increased potency at the E68K* receptor, while both amides had reduced potency relative to the native GLP-1 receptor. This shows a clear difference in recognition of the C-terminal acid and amide by E68* and K68* of the receptors. Although reduced in potency, the peptide amides maintained two- or threefold greater potency compared to the acids at E68K*. Collectively, our CD data4 and the current receptor mutagenesis data show that glucagon amide potency is increased at GLP-1R due to a combined effect of enhanced helicity and direct receptor interaction at position 68*.
Studies on corticotropin-releasing factor (CRF) and PTH have demonstrated the importance of the C-terminal amide for stabilization of the peptide α-helix as well as for direct receptor interaction21, 22. CRF is a 41 amino acid peptide terminating in a C-terminal amide. In the receptor-bound conformation, the C-terminal amide nitrogen hydrogen bonds to the carbonyl of M38, which stabilizes the α-helical conformation. Two additional hydrogen bonds are formed between the C-terminal amide and the backbone of receptor position V97*21. These intra and intermolecular interactions are critical for full potency of CRF at the CRF type 1 receptor (CRFR1). The N-terminal fragment of parathyroid hormone is a 34-amino-acid peptide terminating in a C-terminal amide. Similar to CRF, the PTH (1–34) C-terminal amide forms an intramolecular hydrogen bond with the backbone carbonyl of Val31 and two intermolecular hydrogen bonds to the backbone of receptor position T163*22. These data support the plausibility of our hypothesis that enhanced potency of glucagon amide at GLP-1R results from enhanced helicity as well as direct receptor interaction.
Position 30
Evaluation of a set of GLP-1 analogs demonstrated that the presence of the charge at position 30 enhances differential recognition relative to GLP-1 peptides without position 30 charge. R30 does not enhance potency at the native (E68*) receptor, but does cause greater decrease in potency in the presence of K68* relative to GLP-1 peptides truncated to 29 amino acids or with Gly30. GLP-1 charge at position 30 is shared by OXY, GIP, GLP-2, and PACAP while GRF is charged at position 29. This may suggest that the charge is important for either recognition by the NTD or to provide added selectivity for the native receptor. GLP-1 has two equipotent forms of 30 and 31 amino acids terminating in a C-terminal amide and acid, respectively. Although full potency can be achieved after removal of position 30 as shown by peptide 11 (C-terminal amide), placement of the C-terminal amide and acid is important. Our data shows that a 29-residue glucagon with a C-terminal acid is unfavorable for potency at GLP-1R. Similarly, GLP-1 7–35 acid (analogous to 1–29 in this study) was shown to have less potent insulinotropic action in a perfused rat pancreas relative to the native ligand23.
C-Terminal Extension (Cex)
While the C-terminal extension of Ex-4 is not required for maximum potency at GLP-1R24, Cex may be used to add solubility, additional size, and modified GCGR/GLP-1R selectivity to glucagon-based peptides. Importantly, the Ex-4 GLP-1R NTD structure shows a potential hydrogen bond between Ser32 of Ex-4 (9–39) and position 68*13. We wanted to examine this interaction in the context of glucagon sequence using two glucagon Cex peptides that differed only at position 29. Peptide 13 contained Thr, which is native to glucagon and the other Gly (14), which is present in Ex-4 and GLP-1. The superior potency of 14 at GLP-1R suggests a preference for the more flexible Cex conformation, which may align S32 with E68*. Receptor mutation to K68* caused a significant decrease in potency for 14, which may suggest removal of a S32–E68* hydrogen bond. However, peptide 13 potency only decreased modestly following receptor mutation. Therefore, interactions present between 13 and position 68* of GLP-1R are not altered substantially upon mutation from Glu to Lys. The exact conformation that T29 Cex assumes is not known, but it does not affect recognition by position 68* any differently than the much shorter peptide 4.
OXY
The eight additional amino acids of OXY reduced potency at GCGR more than tenfold15 while enhancing potency at GLP-1R threefold relative to glucagon in the results reported here. In another study, a > 500-fold increase in binding at GLP-1R was observed for OXY relative to glucagon25. This large increase would provide evidence for a highly favorable interaction between the OXY tail and GLP-1 receptor. The molecular basis at the receptor for OXY's increase at GLP-1R and substantial reduction in potency at GCGR was not known previously. A single E68K* mutation resulted in a tenfold decrease in potency for OXY relative to GLP-1R. It is a reasonable explanation that the positively charged tail interacts unfavorably with K68* of GCGR, thus explaining the large decrease in potency. The tenfold decrease observed from a single mutation at GLP-1R parallels the difference seen from multiple changes between GCGR and GLP-1R. Glu/Lys divergence at position 68* considerably impacts OXY's receptor profile at GCGR and GLP-1R.
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) represent another example within family B where a charged C-terminal extension influences selectivity. PACAP exists in two forms of 27 and 38 amino acids. Similar to OXY, PACAP's extension is highly basic with 6 of 11 positively charged residues. PACAP and VIP bind to three different receptors belonging to two subtypes26. Subtype I is the PAC1-R that prefers PACAP relative to VIP with > 1000-fold difference in affinity27. Subtype 2 encompasses the VPAC1-R and VPAC2-R that bind the two ligands with similar affinities27. Structural analysis of the PAC1-R splice variant hPAC1-Rs revealed a high degree of charge complementarity between the C-terminal residues of PACAP and the receptor27. Specifically, receptor residues 116–120 (DEYES) interacted with the basic tail of PACAP. Truncation of PACAP residues 29–32 (KRYK) resulted in a greater than tenfold loss of affinity for hPAC1-Rs, which is consistent with loss of contacts observed in the structure. In VPAC1-R, Y118* is replaced with a Lys, which sets up a potential negative interaction. The order of affinity at VPAC1-R is reversed, favoring the shortened PACAP-27 and VIP relative to PACAP-38. Our data suggests that OXY may use a similar mechanism to differentiate itself from glucagon in selectivity between GCGR and GLP-1R. Although, binding orientation to the NTD is proposed to be different for OXY and PACAP, each uses some degree of charge complementarity or repulsion between tail and receptor to achieve selectivity.
Conclusions
The ability to control selectivity of ligands for family B GPCRs may have utility in the treatment of metabolic diseases such as diabetes and obesity4, 12, 28. Recently, the natural peptide hormone OXY has emerged as a potential anti-obesity agent. Interestingly, OXY's receptor profile indicates a level of co-agonism, albeit a significantly reduced potency at GCGR and GLP-1R relative to the native ligands. The recent design of substantially more potent co-agonists on a glucagon backbone revealed key residues for obtaining maximum potency at GCGR/GLP-1R and introduced questions related to the structural mechanism of interaction. The data reported here have addressed such questions and indicate that a single GLP-1R point mutant E68K* influences potency of multiple ligands. The data indicate more modest changes in potency for the C-terminal amide/acid substitution at E68K* and Q20/K20 at E128M*, while there was a significant shift in potency for OXY at E68K*. This work provides additional insight into the molecular basis for specificity of co-agonist peptides and OXY at GCGR/GLP-1R that could not be captured entirely by previous structural studies.




