A Multimarker Model for Prostate Cancer Risk Assessment: Improving Diagnostic Accuracy Beyond PSA
ABSTRACT
Objective
This study aimed to evaluate the association between biochemical markers and prostate cancer (PCa) risk by analyzing patients with benign prostatic hyperplasia (BPH) and PCa. Additionally, the study sought to assess the diagnostic accuracy of a multimarker model compared to prostate-specific antigen (PSA) alone.
Methods
A cross-sectional study was conducted with data from 2931 patients (1374 with BPH and 1557 with PCa) from the Prostate Cancer Data Set of the National Population Health Data Center. Biochemical markers, including PSA, apolipoproteins, lipid profiles, and metabolic markers (calcium and phosphate), were analyzed. Univariate and multivariate logistic regression analyses were performed to assess the associations with PCa risk. The diagnostic performance of the multimarker model was evaluated using receiver operating characteristic (ROC) curve analysis.
Results
Total PSA levels were significantly higher in PCa patients, and the free/total PSA ratio was lower (p < 0.001). Apolipoprotein A1, LDL cholesterol, calcium, and phosphate were also significantly associated with PCa risk (p < 0.001). The multivariate logistic regression model, incorporating multiple markers, showed improved diagnostic accuracy (AUC 0.731, 95% CI: 0.713–0.749), with sensitivity of 68.4% and specificity of 65.8%.
Conclusions
Combining multiple biochemical markers with PSA enhances the diagnostic accuracy for PCa, offering additional predictive value. This multimarker approach has the potential to improve PCa screening and reduce unnecessary biopsies.
1 Introduction
Prostate cancer (PCa) is one of the most common malignancies in men, with its incidence increasing significantly with age. According to global cancer statistics, PCa ranks as the second most prevalent cancer in men and the fifth leading cause of cancer-related death worldwide [1, 2]. Early detection and accurate diagnosis are essential to improving survival rates and reducing the disease burden. Currently, prostate-specific antigen (PSA) testing is the primary method for PCa screening, having been widely used since its introduction in the late 1980s. While PSA screening has facilitated earlier detection, it remains controversial due to its limited specificity and the risk of overdiagnosis [3, 4].
PSA alone is insufficient to distinguish between benign prostatic hyperplasia (BPH) and PCa, as elevated PSA levels can occur in both conditions. As a result, many men undergo unnecessary biopsies or overtreatment, leading to potential side effects and heightened anxiety. This has driven research into identifying additional biomarkers that could improve the diagnostic accuracy of PCa screening. Several studies have explored the potential role of other biochemical markers, including lipid profiles and metabolic indicators, in PCa risk assessment [5, 6]. Emerging evidence suggests that dyslipidemia, along with alterations in metabolic markers such as calcium and phosphate, may be linked to the development of PCa.
In light of the limitations of PSA testing and the growing interest in multimarker approaches, the aim of this study is to investigate the association between various biochemical markers and the risk of PCa. By analyzing a comprehensive panel of biomarkers—including PSA, lipid profiles, and metabolic markers—this study seeks to develop a more accurate predictive model for PCa diagnosis. Additionally, it aims to assess whether combining multiple markers can enhance the sensitivity and specificity of PCa screening compared to PSA alone.
2 Patients and Methods
2.1 Study Design and Data Source
This study presents a cross-sectional analysis using data from the 2022 Prostate Cancer Data Sets, obtained from the National Population Health Data Center, China (https://www.ncmi.cn//phda/dataDetails.do?id=CSTR:A0006.11.A0005.201905.000531). The data sets include both biochemical and clinical information from patients diagnosed with BPH and PCa. The database was carefully structured to capture relevant clinical and biochemical data related to prostate diseases. It is organized into multiple relational tables, encompassing patient demographics, diagnostic data, biochemical markers, and treatment histories. Each table is indexed by a unique patient identifier (ID), ensuring consistent data linkage across clinical and laboratory records. Data integrity is maintained through the application of referential integrity constraints within the database.
2.2 Data Generation and Quality Control (QC)
Data generation followed standardized procedures for laboratory testing and clinical assessments across participating centers. Biochemical data, including PSA levels, lipid profiles, and metabolic markers, were measured using validated assays and protocols to ensure consistency across laboratories. Clinical information, including patient demographics and diagnoses, was verified through the integration of electronic health records (EHR). To ensure data quality, both automated and manual QC checks were performed. Data entries with missing values exceeding 30% in key variables were excluded, and variables with less than 70% data completeness were removed from the analysis. Standardized coding schemes were applied to categorical variables, and all data points were validated against predefined reference ranges.
2.3 Inclusion Criteria
Patients were eligible for inclusion if they had a confirmed pathological diagnosis of either BPH or PCa. Only cases with complete diagnostic records and relevant biochemical data were considered for analysis.
2.4 Exclusion Criteria
Patients without a definitive pathological diagnosis were excluded. Additionally, cases with missing data in more than 30% of key variables were excluded to ensure the integrity of the analysis.
2.5 Biochemical and Clinical Variables
The primary biochemical variables analyzed included total PSA, the free/total PSA ratio, globulin, apolipoproteins (A1 and B), lipid profiles (LDL cholesterol, triglycerides), calcium, phosphate, and other relevant markers. Demographic factors, including age and body mass index (BMI), were also included in the analysis.
2.6 Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics of the BPH and PCa groups. Continuous variables were presented as medians with interquartile ranges (Q1, Q3) or means with standard deviations, depending on their distribution. Group comparisons for continuous variables were conducted using the Mann−Whitney U test or the independent t-test. Univariate logistic regression analysis assessed the association between each biochemical marker and PCa risk. Significant markers were included in a multivariate logistic regression model to identify independent predictors of PCa. Odds ratios (OR) with 95% confidence intervals (CI) were reported. Diagnostic performance of biomarkers and the multivariate model was evaluated using receiver operating characteristic (ROC) curve analysis. All analyses were performed using SPSS (version 27), with a significance level of p < 0.05.
2.7 Ethical Considerations
The data set used in this study was anonymized and obtained with prior permission from the National Population Health Data Center. As the study involved secondary data analysis with no direct patient interaction, additional informed consent was not required.
3 Results
3.1 Baseline Characteristics Analysis
The baseline characteristics of the patients with BPH and PCa are summarized in Table 1. The study included a total of 2931 patients, of whom 1374 were diagnosed with BPH and 1557 with PCa.
| Characteristic | BPH group [n = 1374] | PCa group [n = 1557] | z value | p value |
|---|---|---|---|---|
| Age [years, M (Q1, Q3)] | 68.00 (62.02, 74.03) | 70.00 (62.04, 73.20) | −2.774 | 0.006 |
| BMI [kg/m², M (Q1, Q3)] | 24.51 (22.41, 26.64) | 24.80 (22.94, 26.71) | −2.672 | 0.008 |
The median age of the BPH group was 68.00 years (Q1, Q3: 62.02–74.03), while the median age in the PCa group was 70.00 years (Q1, Q3: 62.04–73.20), indicating a statistically significant difference (z = −2.774, p = 0.006). Similarly, the median BMI was significantly different between the two groups, with the BPH group having a BMI of 24.51 kg/m² (Q1, Q3: 22.41–26.64) compared to 24.80 kg/m² (Q1, Q3: 22.94–26.71) in the PCa group (z = −2.672, p = 0.008).
Although the differences in age and BMI were statistically significant, the clinical implications remain limited due to their relatively small magnitude. The observed trends align with established risk factors for PCa, such as advanced age and increased BMI.
3.2 Comparison of Biochemical Markers Between Groups
Biochemical markers of patients in the BPH and PCa groups were compared, as shown in Table 2. These markers included PSA, lipid profiles, and metabolic indicators, which were analyzed to identify associations with PCa.
| Biochemical marker | BPH group (n = 1374) | PCa group (n = 1557) | t/z value | p value |
|---|---|---|---|---|
| Total PSA [μg/L, M (Q1, Q3)] | 7.31 (2.81–8.83) | 8.55 (2.21–30.53) | −6.733 | < 0.001 |
| Free/total PSA ratio [M (Q1, Q3)] | 0.15 (0.11–0.23) | 0.10 (0.07–0.18) | −16.409 | < 0.001 |
| Globulin [g/L, M (Q1, Q3)] | 40.50 (38.41–42.60) | 41.35 (39.41–43.32) | −7.205 | < 0.001 |
| LDL cholesterol (mmol/L, mean ± SD) | 2.64 ± 0.69 | 2.85 ± 0.74 | −8.145 | < 0.001 |
| Triglycerides [mmol/L, M (Q1, Q3)] | 1.13 (0.81–1.42) | 1.23 (0.91–1.61) | −4.928 | < 0.001 |
| Creatine kinase [U/L, M (Q1, Q3)] | 79.30 (57.6, 103.5) | 84.30 (62.0, 108.8) | −3.803 | < 0.001 |
| Apolipoprotein A1 (g/L, mean ± SD) | 1.24 ± 0.23 | 1.29 ± 0.24 | −6.177 | < 0.001 |
| Apolipoprotein B (g/L, mean ± SD) | 0.86 ± 0.19 | 0.91 ± 0.20 | −7.303 | < 0.001 |
| Calcium (mmol/L, mean ± SD) | 2.22 ± 0.10 | 2.25 ± 0.10 | −7.549 | < 0.001 |
| Inorganic phosphate (mmol/L, mean ± SD) | 1.08 ± 0.16 | 1.15 ± 0.18 | −10.933 | < 0.001 |
| Chloride [mmol/L, M (Q1, Q3)] | 104.80 (102.5, 106.7) | 104.40 (102.4, 106.4) | −3.389 | 0.001 |
| Free calcium (mmol/L, mean ± SD) | 1.14 ± 0.05 | 1.15 ± 0.05 | −5.846 | < 0.001 |
| Creatine kinase isoenzyme [U/L, M (Q1, Q3)] | 14.20 (10.91, 15.23) | 14.80 (11.81, 16.42) | −7.313 | < 0.001 |
| Free PSA [µg/L, M (Q1, Q3)] | 1.33 (0.50, 1.41) | 0.92 (0.31, 2.93) | −1.538 | 0.124 |
| HDL cholesterol [mmol/L, mean ± SD] | 1.17 ± 0.30 | 1.20 ± 0.31 | −2.462 | 0.014 |
| Sodium [mmol/L, M (Q1, Q3)] | 142.60 (141.11, 144.12) | 142.70 (141.12, 144.13) | −0.328 | 0.743 |
| Serum uric acid [mmol/L, M (Q1, Q3)] | 329.25 (279.61, 385.41) | 328.80 (279.12, 379.43) | −1.040 | 0.298 |
| Potassium [mmol/L, mean ± SD] | 4.04 ± 0.37 | 4.02 ± 0.33 | 1.197 | 0.232 |
| Lactate dehydrogenase [U/L, M (Q1, Q3)] | 151.50 (135.30, 166.40) | 152.80 (136.01, 170.43) | −1.924 | 0.054 |
| Alkaline phosphatase [U/L, M (Q1, Q3)] | 64.80 (54.71, 74.32) | 63.90 (53.92, 77.32) | −0.540 | 0.589 |
| Creatinine [µmol/L, M (Q1, Q3)] | 80.10 (71.72, 90.61) | 79.70 (71.62, 89.32) | −1.366 | 0.172 |
The median total PSA levels were significantly higher in the PCa group (8.55 μg/L, IQR: 2.21–30.53) than in the BPH group (7.31 μg/L, IQR: 2.81–8.83) (z = −6.733, p < 0.001). Additionally, the free/total PSA ratio was significantly lower in the PCa group (0.10, IQR: 0.07–0.18) compared to the BPH group (0.15, IQR: 0.11–0.23) (z = −16.409, p < 0.001).
Other significant differences were observed in serum globulin, with higher levels in the PCa group (41.35 g/L, IQR: 39.41–43.32) compared to the BPH group (40.50 g/L, IQR: 38.41–42.60) (z = −7.205, p < 0.001). Apolipoprotein A1 (1.29 ± 0.24 g/L vs. 1.24 ± 0.23 g/L, p < 0.001) and apolipoprotein B (0.91 ± 0.20 g/L vs. 0.86 ± 0.19 g/L, p < 0.001) levels were also elevated in the PCa group.
Lipid markers showed significant differences, with LDL cholesterol (2.85 ± 0.74 mmol/L vs. 2.64 ± 0.69 mmol/L, z = −8.145, p < 0.001) and triglycerides (1.23 mmol/L, IQR: 0.91–1.61 vs. 1.13 mmol/L, IQR: 0.81–1.42, z = −4.928, p < 0.001) being higher in the PCa group.
Additional markers, such as creatine kinase, calcium, phosphate, and chloride, also demonstrated statistically significant differences (p < 0.05). These findings suggest that PSA levels, lipid profiles, and metabolic indicators are closely associated with the likelihood of developing PCa.
3.3 Univariate Logistic Regression Analysis
Univariate logistic regression analysis was conducted to evaluate the relationship between biochemical markers and PCa risk. Table 3 provides the OR, 95% CI, and p values for each marker.
| Biochemical marker | Coefficient (β) | z value | OR (95% CI) | p value |
|---|---|---|---|---|
| Total PSA (μg/L) | 0.029 | 9.688 | 1.030 (1.024–1.036) | < 0.001 |
| Free/total PSA ratio | −1.839 | −5.528 | 0.159 (0.083–0.305) | < 0.001 |
| Globulin (g/L) | 0.080 | 6.937 | 1.083 (1.059–1.108) | < 0.001 |
| Apo A1 (g/L) | 0.980 | 6.058 | 2.665 (1.941–3.660) | < 0.001 |
| Apo B (g/L) | 1.379 | 7.138 | 3.969 (2.718–5.796) | < 0.001 |
| LDL-C (mmol/L) | 0.420 | 7.922 | 1.522 (1.372–1.689) | < 0.001 |
| Triglycerides (mmol/L) | 0.233 | 4.604 | 1.263 (1.143–1.394) | < 0.001 |
| Creatine kinase isoenzyme (U/L) | 0.016 | 2.981 | 1.016 (1.006–1.027) | 0.003 |
| Calcium (mmol/L) | 4.280 | 5.762 | 72.253 (16.848–309.859) | < 0.001 |
| Phosphate (mmol/L) | 2.375 | 10.395 | 10.754 (6.872–16.829) | < 0.001 |
| Chloride (mmol/L) | −0.038 | −3.247 | 0.962 (0.940–0.985) | 0.001 |
| HDL-C (mmol/L) | 0.301 | 2.456 | 1.352 (1.063–1.720) | 0.014 |
Total PSA was strongly associated with increased PCa risk (OR: 1.030, 95% CI: 1.024–1.036, p < 0.001). Conversely, the free/total PSA ratio demonstrated a robust negative association with PCa risk (OR: 0.159, 95% CI: 0.083–0.305, p < 0.001), suggesting that a lower ratio strongly predicts PCa.
Several markers, including globulin (OR: 1.083, 95% CI: 1.059–1.108, p < 0.001), apolipoprotein A1 (OR: 2.665, 95% CI: 1.941–3.660, p < 0.001), apolipoprotein B (OR: 3.969, 95% CI: 2.718–5.796, p < 0.001), and LDL cholesterol (OR: 1.522, 95% CI: 1.372–1.689, p < 0.001), were significantly associated with increased PCa risk.
Metabolic markers such as calcium (OR: 15.251, 95% CI: 7.402–31.423, p < 0.001) and inorganic phosphate (OR: 10.754, 95% CI: 6.872–16.829, p < 0.001) were also positively associated with PCa. In contrast, HDL cholesterol showed a negative association (OR: 0.568, 95% CI: 0.362–0.892, p = 0.014).
These results emphasize that biochemical markers, including PSA, globulin, lipid profiles, and metabolic indicators, are significantly associated with PCa and may serve as potential diagnostic indicators.
3.4 Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis was performed to identify independent predictors of PCa, incorporating variables significant in univariate analysis. The results are summarized in Table 4.
| Biochemical marker | Coefficient (β) | z value | OR (95% CI) | p value |
|---|---|---|---|---|
| Total PSA (μg/L) | 0.035 | 10.399 | 1.036 (1.029–1.043) | < 0.001 |
| Globulin (g/L) | 0.055 | 4.112 | 1.056 (1.029–1.084) | < 0.001 |
| Apo A1 (g/L) | 1.412 | 4.574 | 4.102 (2.240–7.511) | < 0.001 |
| LDL-C (mmol/L) | 0.311 | 5.366 | 1.364 (1.218–1.528) | < 0.001 |
| Calcium (mmol/L) | 2.489 | 2.986 | 12.051 (2.352–61.749) | 0.003 |
| Phosphate (mmol/L) | 2.468 | 10.078 | 11.799 (7.301–19.068) | < 0.001 |
| HDL-C (mmol/L) | −0.565 | −2.457 | 0.568 (0.362–0.892) | 0.014 |
Total PSA (OR: 1.036, 95% CI: 1.029–1.043, p < 0.001) remained a strong independent predictor of PCa. Similarly, globulin (OR: 1.056, 95% CI: 1.029–1.084, p < 0.001), apolipoprotein A1 (OR: 4.102, 95% CI: 2.240–7.511, p < 0.001), and LDL cholesterol (OR: 1.364, 95% CI: 1.218–1.528, p < 0.001) were independently associated with increased PCa risk.
Calcium (OR: 12.051, 95% CI: 2.352–61.749, p = 0.003) and phosphate (OR: 11.799, 95% CI: 7.301–19.068, p < 0.001) remained significant predictors in the multivariate model, while HDL cholesterol (OR: 0.568, 95% CI: 0.362–0.892, p = 0.014) showed a protective effect.
3.5 ROC Curve Analysis
ROC curve analysis was conducted to evaluate the diagnostic performance of individual markers and the multivariate logistic model (Table 5, Figure 1).
| Marker | AUC | 95% CI | p value |
|---|---|---|---|
| Multivariate logistic model | 0.731 | 0.713–0.749 | < 0.001 |
| Total PSA (μg/L) | 0.572 | 0.551–0.593 | < 0.001 |
| Globulin (g/L) | 0.577 | 0.556–0.598 | < 0.001 |
| Apolipoprotein A1 (g/L) | 0.572 | 0.551–0.593 | < 0.001 |
| LDL cholesterol (mmol/L) | 0.588 | 0.567–0.608 | < 0.001 |
| Calcium (mmol/L) | 0.562 | 0.541–0.583 | < 0.001 |
| Inorganic phosphate (mmol/L) | 0.615 | 0.595–0.635 | < 0.001 |
| HDL cholesterol (mmol/L) | 0.525 | 0.504–0.545 | 0.022 |
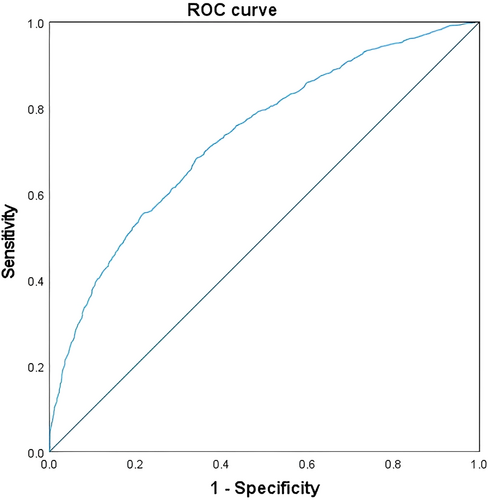
The multivariate model achieved an area under the curve (AUC) of 0.731 (95% CI: 0.713–0.749, p < 0.001), indicating good discriminatory ability. Among individual markers, phosphate exhibited the highest AUC (0.615, 95% CI: 0.595–0.635, p < 0.001), followed by LDL-C (AUC: 0.588, 95% CI: 0.567–0.608, p < 0.001) and globulin (AUC: 0.577, 95% CI: 0.556–0.598, p < 0.001).
Markers such as total PSA (AUC: 0.572, 95% CI: 0.551–0.593, p < 0.001) and calcium (AUC: 0.562, 95% CI: 0.541–0.583, p < 0.001) demonstrated moderate diagnostic utility. HDL-C had the lowest AUC (0.525, 95% CI: 0.504–0.545, p = 0.022).
4 Discussion
This study explored the association between multiple biochemical markers and PCa risk, using data from a large cohort of patients diagnosed with either BPH or PCa. The key findings highlight the significant role of PSA in PCa detection, while also identifying additional markers—such as free/total PSA ratio, apolipoprotein A1, globulin, and lipid profiles—that could improve diagnostic accuracy.
4.1 PSA and Free/Total PSA Ratio
Our results confirm the well-established association between elevated total PSA levels and increased PCa risk, aligning with previous studies that underscore PSA's pivotal role in screening for PCa [7]. The free/total PSA ratio, which was notably lower in PCa patients compared to BPH patients, further corroborates its diagnostic value in distinguishing between benign and malignant prostatic conditions. This finding supports the use of this ratio as an additional tool to refine diagnostic decision-making, particularly in cases with borderline PSA levels.
4.2 Other Biochemical Markers
Beyond PSA, several other biochemical markers, including globulin, apolipoprotein A1, apolipoprotein B, and low-density lipoprotein cholesterol (LDL-C), were significantly associated with PCa risk. The multivariate analysis demonstrated that these markers, particularly apolipoprotein A1 and globulin, remained independently predictive of PCa. Elevated levels of these lipid and protein markers have been implicated in cancer progression, possibly through mechanisms involving inflammation, cellular metabolism, and tissue remodeling [8, 9]. This reinforces the potential role of lipid metabolism in PCa development and suggests that incorporating lipid profiles into routine screening could improve risk assessment.
Additionally, metabolic markers such as calcium and inorganic phosphate were strongly correlated with PCa, supporting the hypothesis that metabolic dysregulation may contribute to PCa progression. Elevated serum calcium levels have been linked to more aggressive forms of PCa, likely due to calcium's role in regulating cell signaling pathways involved in cell growth and differentiation. Similarly, inorganic phosphate has been shown to promote cancer cell proliferation and may play a role in tumor growth [10, 11]. Our findings extend these observations, emphasizing the importance of metabolic markers in understanding the pathophysiology of PCa.
4.3 Multimarker Model and Diagnostic Accuracy
The multivariate logistic regression model developed in this study demonstrated superior diagnostic performance compared to PSA alone, with an AUC of 0.731. This result emphasizes the value of a multimarker approach in improving the diagnostic accuracy of PCa screening. While PSA remains the cornerstone of screening, its diagnostic limitations—particularly in patients with intermediate or borderline PSA levels—are well-documented. Incorporating additional biomarkers, such as apolipoprotein A1, globulin, and lipid profiles, could enhance both the sensitivity and specificity of PCa screening, reducing false positives and negatives and enabling more accurate patient stratification.
Recent studies have further highlighted the role of lipid metabolism in PCa progression [12]. For instance, Suh et al. demonstrated that elevated serum triglyceride levels are robustly associated with increased PCa risk, higher Gleason grade groups (GG2−5), and advanced clinical stages (locally advanced and metastatic disease) [13]. While our model focused on apolipoproteins (A1 and B) and LDL cholesterol, their findings suggest that triglycerides may serve as an independent prognostic marker for tumor aggressiveness. Although our data set did not include Gleason grades or clinical staging, the observed associations between lipid markers (e.g., LDL-C) and PCa risk align with the broader hypothesis that dysregulated lipid metabolism contributes to prostate carcinogenesis. Future studies integrating triglyceride levels and other lipid subcomponents into multimarker models could further refine risk stratification and address tumor aggressiveness [14-16].
4.4 Strengths and Limitations
Our study's strengths include its large sample size (2931 patients), which enhances the generalizability of the findings. Large-scale studies are essential for validating biomarkers and ensuring their clinical relevance. Another strength is the inclusion of a broad range of biochemical markers, not limited to PSA, but also encompassing lipid and metabolic markers [17]. This comprehensive analysis provides a more nuanced understanding of the biochemical factors influencing PCa risk [18]. Furthermore, the study employed robust statistical methods, including univariate and multivariate logistic regression, to control for confounding variables. The use of ROC curve analysis further strengthened the validity of the results.
However, the study has some limitations. First, its cross-sectional design precludes the establishment of causal relationships between biochemical markers and PCa development. While the associations observed in this study are statistically significant, longitudinal studies are necessary to confirm whether these markers predict future PCa risk [19, 20]. Additionally, the sample was drawn from a single clinical center, which may introduce selection bias and limit the generalizability of the results. Future studies should replicate these findings across multicenter cohorts with more diverse demographic profiles to assess the broader applicability of the identified biomarkers.
Moreover, unmeasured confounding factors—such as lifestyle influences on biochemical markers—were not fully accounted for in this study. Future research should incorporate lifestyle factors (e.g., diet, physical activity, and medication use) to provide a more comprehensive understanding of the relationship between biomarkers and PCa risk.
4.5 Future Directions
While the multivariate model improved diagnostic accuracy, the AUC of 0.731 suggests that additional refinement is needed. Incorporating genetic or molecular markers may further enhance the predictive power of the model. Longitudinal studies examining the causal links between these biomarkers and PCa, along with validation in larger and more diverse populations, are essential for advancing the clinical utility of these findings [21, 22]. Additionally, as highlighted by Suh et al., the inclusion of clinical staging and Gleason grade data in future cohorts will be critical to evaluate whether our multimarker model can predict not only cancer presence but also disease severity [13].
4.6 Conclusion
In conclusion, this study demonstrates that a multimarker approach—incorporating PSA, lipid profiles, and metabolic markers—can significantly enhance the diagnostic accuracy of PCa screening compared to PSA alone [23, 24]. The findings suggest that incorporating these markers into routine clinical practice could improve risk assessment and patient stratification. However, further validation in larger, more diverse cohorts, along with longitudinal studies and the integration of AI technologies, is necessary to fully realize the clinical potential of these biomarkers.
Author Contributions
Bin Yang designed the study. Penglu Yang and Bin Yang conducted data extraction, interpreted the study results, and wrote the first draft of the manuscript parts. Bin Yang revised the manuscript.
Ethics Statement
The data set used for this study was anonymized and obtained from the National Population Health Data Center with prior permission.
Consent
As this study involved secondary data analysis with no direct interaction with patients, no additional informed consent was required.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Prostate Cancer Date Set at https://www.ncmi.cn//phda/dataDetails.do?id=CSTR:A0006.11.A0005.201905.000531.