Impact of PSA nadir, PSA response and time to PSA nadir on overall survival in real-world setting of metastatic hormone-sensitive prostate cancer patients
Abstract
Background
To evaluate the impact of prostate-specific antigen (PSA) nadir, PSA response and time to PSA nadir (TTN) in metastatic hormone-sensitive prostate cancer (mHSPC) patients on overall survival (OS) in the era of combination therapies.
Methods
Different PSA nadir cut-offs (including ultra-low PSA) were tested for OS analyses. Additionally, PSA response ≥99% was evaluated, as well as TTN categorized as <3 versus 3–6 versus 6–12 versus >12 months. Multivariable Cox regression models predicted the value of PSA nadir cut-offs, PSA response and TTN on OS. Sensitivity analyses were performed in de novo and high volume mHSPC patients.
Results
Of 238 eligible patients, PSA cut-offs of <0.2 versus 0.2–4.0 versus >4.0 ng/mL differed significantly regarding median OS (96 vs. 56 vs. 44 months, p < 0.01), as well as in subgroup analyses of de novo mHSPC patients and multivariable Cox regression models. A more stringent PSA cut-off of <0.02 versus 0.02–0.2 versus >0.2 ng/mL also yielded significant median OS differences (not reached vs. 96 vs. 50 months, p < 0.01), even after additional multivariable adjustment. A PSA response ≥99% was also significantly associated with better OS than counterparty with <99% response, even after multivariable adjustment (both p < 0.02). When TTN groups were compared, patients with longer TTN harbored more extended OS than those with short TTN (<3 vs. 3–6 vs. 6–12 vs. >12 months: 34 vs. 50 vs. 67 vs. 96 months, p < 0.01). Virtually similar results were observed in sensitivity analyses for high volume mHSPC patients.
Conclusions
In times of combination therapies for mHSPC, a PSA nadir of respectively, <0.2 and <0.02 ng/mL are associated with best OS rates. Moreover, a relative PSA response ≥99% and a longer TTN are clinical important proxies for favorable OS estimates.
1 INTRODUCTION
Treatment of metastatic hormone-sensitive (mHSPC) and castration resistant prostate cancer (mCRPC) has extremely changed within the last two decades and is still under current investigation for best treatment options and sequences.1, 2 Within this period, treatment has been intensified from androgen deprivation therapy (ADT) monotherapy for all mHSPC/mCRPC patients towards combination with either new androgen receptor signal inhibitors (ARSI), chemotherapy or both depending on tumor characteristics such as metastatic burden.3-5
In clinical decision making, despite radiographic markers, easily applicable and available estimates for treatment response and survival estimates are crucial for clinicians and patients. In this regard, European Urology Association (EAU) guidelines still refer to prostate-specific antigen (PSA) nadir categories derived from the SWOG 9346 trial published in 2006 in which overall survival (OS) and treatment response was stratified according to PSA nadir of <0.2 ng/mL versus 0.2–4.0 ng/mL versus >4.0 ng/mL.6, 7 However, in this prospective trial all mHSPC patients received ADT monotherapy. Conversely, a real-world study of mHSPC patients treated with intensified combination therapy suggested a cut-off of ≤0.05 ng/mL.8 Current data of the prospective TITAN trial (apalutamide for mHSPC) suggested an even stricter PSA nadir cut-offs (<0.02 ng/mL vs. 0.02–0.2 ng/mL vs. >0.2 ng/mL) for OS estimates.9
Moreover in post hoc analyses of the prospective phase III TITAN, PROSPER (enzalutamide for nonmetastatic CRPC) and SPARTAN (apalutamide for nonmetastatic CRPC) trials, relative PSA decreases/responses have additionally be shown to be an predictor of extended treatment response and OS.9-12
Nowadays real-world data become an even more important tool to assess the translation from phase III trials into clinical scenario. However, only few real-world studies focused on the above outlined estimates and were mainly limited by sample size or focusing solely on mCRPC or mHSPC patients treated with ADT monotherapy.13-16
We addressed this knowledge gap and relied on our institutional metastatic prostate cancer database to investigate the effect of absolute PSA nadir, relative PSA response and time to PSA nadir in a contemporary mHSPC cohort. We hypothesized that intensified treatment with combination therapy according to EAU guidelines will uncover important insights of PSA estimates of real-world mHSPC patients.
2 MATERIALS AND METHODS
2.1 Study population
After approval of the local ethic committee (number: SUG-5-2018) and in accordance with the Declaration of Helsinki, all mHSPC patients at the Department of Urology, University Hospital Frankfurt, Germany, were retrospectively identified (n = 516). Inclusion criteria consisted of mHSPC status and availability of PSA nadir and its date. Exclusion criteria consisted of patients with unknown follow-up status. These selection criteria yielded 238 eligible mHSPC patients.
2.2 PSA nadir, PSA response and time to PSA nadir
PSA nadir was defined as the lowest PSA value measured during administered treatment of each mHSPC patient. Moreover, PSA response was defined as the percentage of PSA decline in relation to initial PSA before treatment initiation for mHSPC. Time to PSA nadir was calculated as the time (in month) from treatment start for mHSPC until the lowest measured PSA value.
2.3 Statistical analysis
Descriptive statistics included frequencies and proportions for categorical used variables. Medians and interquartile ranges (IQR) were reported for all applied continuously coded variables. The χ2 test was used to test for statistical significance in proportions' differences. In addition, the t test and Kruskal-Wallis test examined the statistical significance of distributions' differences.
Kaplan Meier curve-derived estimates, as well as Cox regression models were used for OS analyses and additional univariable and multivariable logistic regression models. Adjustment was performed for absolute PSA value at mHSPC diagnosis, age at mHSPC diagnosis, metastatic burden (low vs. high volume according to CHAARTED criteria17), Eastern Cooperative Oncology Group (ECOG) status, Gleason Score and number of received therapy lines for metastatic prostate cancer. For time to mCRPC analyses, additional adjustment for treatment in mHSPC was performed.
Applied PSA cut-offs were the more historical cut-offs of the SWOG 9346 trial <0.2 ng/mL versus 0.2–4.0 ng/mL versus >4.0 ng/mL and a stricter cut-off (<0.05 ng/mL vs. 0.05–2.0 ng/mL vs. >2 ng/mL), as well as the ultra-low cut-off derived from post hoc analyses of the TITAN trial: <0.02 ng/mL versus 0.02–0.2 ng/mL versus >0.2 ng/mL.
For PSA response analyses, a PSA decline of ≥99% versus <99% was evaluated. Additionally for time to PSA nadir, time intervals of <3 versus 3–6 versus 6–12 versus >12 months were tested. In all applied methodologies, sensitivity analyses were performed for de novo mHSPC patients, as well as high volume mHSPC (according to CHAARTED criteria17) and the endpoint of time to mCRPC.18 Moreover, in time to PSA nadir analyses, additional sensitivity analyses were performed in mHSPC patients with PSA nadir <2 ng/mL and ≥90% PSA response to avoid bias for insufficient treatment responders.
All tests were two sided with a level of significance set at p < 0.05. R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses.
3 RESULTS
3.1 Descriptive baseline characteristics
In total, 238 mHSPC patients qualified for analyses with a median follow up of 22 months (IQR: 5–45 months). Median age was 70 years (IQR: 63–75 years) at diagnosis of metastatic disease (Table 1) with a median PSA of 47 ng/ml (IQR: 12–300 ng/mL).
| Variable | Overall, n = 238 | PSA nadir ≤0.02 ng/mL, n = 43 (18.1%) | PSA nadir 0.02–0.2 ng/mL, n = 55 (23.1%) | PSA nadir >0.2 ng/mL, n = 140 (58.8%) | p Value |
|---|---|---|---|---|---|
| Age at PCa diagnosis, median (IQR) | 66 (60–71) | 65 (59–69) | 66 (58–72) | 66 (62–71) | 0.11 |
| Age at metastatic PCa, median (IQR) | 69 (62–74) | 66 (61–71) | 69 (59–75) | 69 (64–75) | 0.3 |
| PSA at mHSPC, median (IQR) | 54 (14–330) | 10 (3–44) | 36 (11–273) | 94 (29–473) | <0.01 |
| PSA nadir, median (IQR) | 0.35 (0.05–2.0) | 0.001 (0.0001–0.01) | 0.07 (0.04–0.1) | 1.4 (0.5–8.4) | 0.03 |
| PSA decline ≥ 99%, median (IQR) | 119 (50.0) | 37 (86.0) | 40 (72.7) | 42 (30.0) | <0.001 |
| PSA at mCRPC, median (IQR) | 12 (3–60) | 2.4 (0.01–14.4) | 2.4 (0.7–9.5) | 35 (6.8–91) | 0.02 |
| Systemic therapy lines for PCa, median (IQR) | 2 (2–3) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.08 |
| ECOG status ≥ 2 | 8 (3.4) | 0 (0) | 3 (5.5) | 5 (3.6) | 0.037 |
| Gleason Score 8–10 | 171 (71.8) | 32 (74.4) | 37 (67.3) | 102 (72.9) | 0.3 |
| Local therapy with RP/RT | 85 (35.7) | 25 (58.1) | 26 (47.3) | 34 (24.3) | <0.001 |
| High volume mHSPC | 137 (57.6) | 17 (39.5) | 25 (45.5) | 95 (67.9) | <0.001 |
| High risk mHSPC | 152 (63.9) | 20 (46.5) | 34 (61.8) | 98 (70.0) | <0.001 |
| De novo mHSPC | 178 (74.8) | 22 (51.2) | 37 (67.3) | 119 (85.0) | <0.001 |
| Therapy for mHSPC | |||||
| ADT monotherapy | 9 (3.8) | 2 (4.7) | 4 (7.3) | 3 (2.1) | <0.01 |
| ARSI | 127 (53.4) | 31 (72.1) | 26 (47.3) | 70 (50.0) | |
| Docetaxel | 64 (26.9) | 2 (4.7) | 20 (36.4) | 42 (30.0) | |
| Triplet therapy | 21 (8.8) | 3 (7.0) | 2 (3.6) | 16 (11.4) | |
| Other | 14 (5.9) | 4 (9.3) | 2 (3.6) | 8 (5.7) |
- Abbreviations: ARSI, androgen receptor signaling inhibitor; ECOG, Easter Cooperative Oncology Group; IQR, interquartile range; mCRPC, metastatic castration-resistant prostate cancer; PCa, prostate cancer; RP, radical prostatectomy; RT, radiation therapy.
3.2 PSA cut-off: <0.2 versus 0.2–4.0 versus ≥4.0 ng/mL
After stratification according to PSA cut-offs <0.2 versus 0.2–4.0 versus ≥4.0 ng/mL, 41.2% achieved a PSA nadir <0.2 ng/mL versus 39.5% a PSA nadir 0.2–4.0 ng/mL versus 19.3% with a PSA nadir >4.0 ng/mL. In Kaplan Meier curve estimates, significant OS differences between all three groups were observed (Figure 1A, p < 0.01), with a median OS of 96 versus 56 versus 44 months for PSA cut-offs <0.2 versus 0.2–4.0 versus ≥4.0 ng/mL and median 24-months OS rates of 91.3% versus 86.2% versus 76.9%, respectively. After multivariable adjustment, a PSA nadir >4.0 ng/mL was independently associated with worse OS (hazard ratio [HR] = 5.93, p < 0.01), than patients with a PSA nadir <0.2 ng/mL. A PSA nadir 0.2–4.0 ng/mL did not reach significance.

In subgroup analyses of de novo mHSPC patients, 33.5% of patients achieved a PSA nadir <0.2 ng/mL versus 44.1% a PSA nadir 0.2–4.0 ng/mL versus 22.3% with a PSA nadir >4.0 ng/mL. Median OS also differed significantly between the three compared groups (Figure 1B, p < 0.01) with 63 versus 54 versus 36 months and median 24-months OS of 88.2% versus 85.7% versus 72.1%, respectively. After multivariable adjustment, a PSA nadir >4.0 ng/mL was also independently associated with worse OS (HR = 5.20, p < 0.01). A PSA nadir 0.2–4.0 ng/mL did not reach significance.
In additional subgroup analyses of high volume mHSPC patients 32.8% of patients achieved a PSA nadir <0.2 ng/mL versus 45.3% a PSA nadir 0.2–4.0 ng/mL versus 29.7% with a PSA nadir >4.0 ng/mL. Median OS between the three compared groups were 58 versus 49 versus 36 months (Figure 1C) and median 24-months OS rates were 89.0% versus 85.1% versus 74.7%, respectively. After multivariable adjustment, a PSA nadir >4.0 ng/mL was also independently associated with worse OS (HR = 5.53, p < 0.01). A PSA nadir 0.2–4.0 ng/mL did not reach significance.
3.3 PSA cut-off: <0.05 versus 0.05–2.0 versus ≥2.0 ng/mL
Applying a more stringent PSA cut-off of <0.05 versus 0.05–2.0 versus ≥2.0 ng/mL yielded also significant OS differences (Figure 2A, p = 0.02) with median OS of not reached (NR) versus 56 versus 46 months for a PSA cut-off of <0.05 versus 0.05–2.0 versus ≥2.0 ng/mL and 24-months OS of 98.1% versus 84.6% versus 79.0%, respectively. After multivariable adjustment, a PSA nadir ≥2.0 ng/mL was independently associated with worse OS, compared to a PSA nadir of <0.05 ng/mL (HR = 3.59, p = 0.02). A PSA nadir 0.05–2.0 ng/mL did not reach significance.
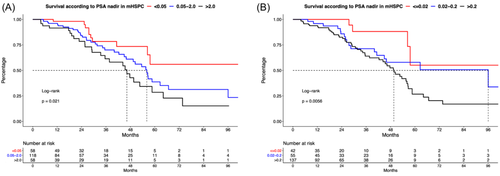
3.4 PSA cut-off: ≤0.02 versus 0.02–0.2 versus >0.2 ng/mL
Applying the ultra-low PSA cut-off of ≤0.02 versus 0.02–0.2 versus >0.2 ng/mL, 18.1% of patients achieving a PSA nadir of ≤0.02 ng/mL (Table 1). When patient groups of the three cut-offs were compared, patients with a PSA nadir ≤0.02 harbored significantly lower PSA at mHSPC diagnosis (10 vs. 36 vs. 94 ng/mL, p < 0.01) than patients with a PSA nadir of 0.02–0.2 and >0.2 ng/mL. Moreover, patients reaching a PSA nadir ≤0.02 ng/mL harbored lower rates of high volume mHSPC disease, de novo metastatic disease, ECOG status ≥2 and higher rates of local therapy to the prostate (all p ≤ 0.03).
OS was significantly different between these three compared groups in univariable analyses (Figure 2B, p < 0.01). Specifically, median OS was NR versus 96 versus 50 months for PSA cut-offs of ≤0.02 versus 0.02–0.2 versus >0.2 ng/mL. Median 24-months OS rates were respectively 100% versus 85.8% versus 83.1%. After multivariable adjustment, a PSA nadir >0.2 ng/mL was a significantly predictor of worse OS, relative to a PSA nadir ≤0.02 ng/mL (HR = 3.54, p = 0.03). A PSA nadir 0.02–0.2 ng/mL tended to predict worse OS (HR = 3.22, p = 0.060).
3.5 PSA response: ≥99% versus <99%
When OS analyses were stratified according to relative PSA decline, mHSPC patients with a PSA response ≥99 versus <99% showed a median OS of 58 versus 50 months (HR = 0.67, p = 0.1, Figure 3A) and 24-months OS of 88.7 versus 86.3%. However, after multivariable adjustment for differences in patient und tumor characteristics, a PSA response ≥99% was associated with better OS (HR = 0.5, p = 0.036).

In subgroup analyses of de novo mHSPC patients (Figure 3B), median OS was 63 versus 42 months with 24-months OS rates of 86.6% versus 82.8% for patients with a PSA response ≥99 versus <99%, respectively (p = 0.02). In multivariable Cox regression models, a PSA response ≥99% was also associated with better OS (HR = 0.43, p = 0.02).
In additional subgroup analyses of high volume mHSPC patients (Figure 3C), median OS was 58 versus 44 months and 24-months OS rates were 87.8% versus 82.9% for patients with a PSA response ≥99 versus <99%, respectively (p = 0.03). In multivariable Cox regression models, a PSA response ≥99% did not reach significance (HR = 0.50, p = 0.08).
3.6 Time to PSA nadir: <3 versus 3–6 versus 6–12 versus >12 months
When patients were stratified according to time to PSA nadir, patients with a PSA nadir >12 months harbored lower rates of high volume mHSPC disease and lower absolute PSA nadir (Table 2, both p < 0.01). Moreover, these patients achieved more frequently a PSA response ≥99% (p < 0.01).
| Variable | TTN <3 months, n = 41 (18.1%) | TTN 3–6 months, n = 67 (29.5%) | TTN 6–12 months, n = 65 (28.6%) | TTN >12 months, n = 54 (23.8%) | p Value |
|---|---|---|---|---|---|
| Age at PCa diagnosis, median (IQR) | 67 (64–74) | 67 (62–71) | 64 (60–70) | 66 (56–70) | 0.2 |
| Age at metastatic PCa, median (IQR) | 68 (65–76) | 70 (63–74) | 68 (61–74) | 68 (62–73) | 0.5 |
| PSA at mHSPC, median (IQR) | 46 (13–303) | 62 (16–329) | 37 (13–332) | 52 (8.1–95) | 0.9 |
| PSA nadir, median (IQR) | 0.7 (0.3–9.0) | 0.3 (0.1–1.7) | 0.3 (0.04–1.3) | 0.1 (0.02–0.9) | <0.01 |
| PSA decline ≥ 99% | 12 (29.3) | 36 (53.7) | 36 (55.4) | 33 (61.1) | <0.01 |
| PSA at mCRPC, median (IQR) | 15 (1.6–69) | 18 (1.1–42) | 10.2 (2.3–46) | 9.2 (5.4–37) | 0.5 |
| Systemic therapy lines for PCa, median (IQR) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.2 |
| ECOG status ≥ 2 | 0 (0) | 4 (6.0) | 4 (6.2) | 0 (0) | 0.13 |
| Gleason Score 8–10 | 28 (68.3) | 53 (79.1) | 46 (70.8) | 40 (74.1) | 0.8 |
| Local therapy with RP/RT | 11 (26.8) | 22 (32.8) | 22 (33.8) | 26 (48.1) | 0.15 |
| High volume mHSPC | 26 (63.4) | 44 (65.7) | 41 (63.1) | 17 (31.5) | <0.001 |
| High risk mHSPC | 27 (65.9) | 47 (70.1) | 45 (69.2) | 23 (42.6) | <0.01 |
| De novo mHSPC | 33 (80.5) | 52 (77.6) | 46 (70.8) | 39 (72.2) | 0.7 |
| Therapy for mHSPC | |||||
| ADT monotherapy | 2 (4.9) | 0 (0) | 2 (3.1) | 5 (9.3) | 0.12 |
| ARSI | 22 (53.7) | 33 (49.3) | 30 (46.2) | 34 (63.0) | |
| Docetaxel | 8 (19.5) | 22 (32.8) | 23 (35.4) | 9 (16.7) | |
| Tripplet therapy | 6 (14.6) | 8 (11.9) | 4 (6.2) | 3 (5.6) | |
| Other | 3 (7.3) | 4 (6) | 4 (6.2) | 2 (3.7) |
- Abbreviations: ARSI, androgen receptor signaling inhibitor; ECOG, Easter Cooperative Oncology Group; IQR, interquartile range; mCRPC, metastatic castration-resistant prostate cancer; PCa, prostate cancer; RP, radical prostatectomy; RT, radiation therapy.
In Kaplan Meier curve analyses, median OS was 29 versus 54 versus 59 versus 67 months for patients with time to PSA nadir <3 versus 3–6 versus 6–12 versus >12 months (p < 0.001, Figure 4A) with 24-months OS rates of 68.2% versus 80.6% versus 90.1% versus 100%. In multivariable adjusted Cox regression models, time to PSA nadir >12 months was significantly associated with better survival than patients time to nadir <3 months (HR = 0.23, p = 0.017). Virtually same results were observed for subgroup analyses of de novo (Figure 4B, multivariable HR = 0.15, p = 0.016) and high volume mHSPC patients (Figure 4C, time to nadir 6–12 months multivariable HR = 0.26, p = 0.036; time to nadir >12 months multivariable HR = 0.17, p = 0.02).
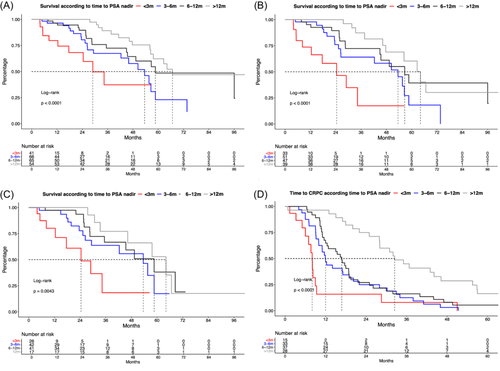
Moreover, time to CRPC differed significantly between these four compared groups: 8 versus 12 versus 17 versus 32 months (Figure 4D, p < 0.001) and time to PSA nadir 3–6 months (HR = 0.18: p < 0.01), 6–12 months (HR = 0.23: p < 0.01) and >12 months (HR = 0.21: p < 0.01) were also significantly associated shorter time to CRPC than patients time to nadir <3 months in multivariable models.
To limit the bias of insufficient PSA response, sensitivity analyses were applied for patients with PSA nadir <2 ng/mL and PSA response ≥90% and yielded virtually same results (not shown).
4 DISCUSSION
We hypothesized that the absolute PSA nadir, relative PSA response, as well as the time to PSA nadir may be used as a substantial predictor for OS estimates in mHSPC patients. Moreover, we hypothesized that intensified treatment with combination therapy for mHSPC may lead to clinically important OS differences than in studies relying on ADT monotherapy. We addressed this knowledge gap with our real world mHSPC database and yielded important findings.
First, when stratification was applied according the EAU guideline suggested cut-offs of <0.2 versus 0.2–4.0 versus ≥4.0 ng/mL derived from the SWOG 9346 trial published in 2006, we found that in our contemporary real world mHSPC cohort, only 19.7% harbored a PSA nadir >4.0 ng/mL compared to 30.8% in the SWOG 9346 trial.6 Moreover, when OS is compared, we observed median OS of 96 versus 56 versus 44 months for PSA nadir cut-offs of <0.2 versus 0.2–4.0 versus ≥4.0 ng/mL in our mHSPC cohort, relative to 75 versus 44 versus 13 months in SWOG 9346 trial.6 These absolute and prolonged OS differences may be explained by the effect of the usage of combination therapies in the setting of mHSPC, as well as subsequent potent treatment drugs for mCRPC, while in the SWOG 9346 trial only ADT monotherapy was administered. However, our findings also suggest that the cut-off derived from the SWOG 9346 trial is still applicable in contemporary mHSPC cohorts for stratification of absolute PSA response. Additionally, we also validated these findings in the subgroup of de novo and high volume mHSPC patients which have in general worse survival than secondary and low volume mHSPC patients.19 Similar results were found by a recently published study of Gebrael et al., where a PSA nadir >0.2 ng/mL with intensified combination therapy for mHSPC was associated with worse survival of median OS of 39 months.20
Second, we applied more stringent PSA nadir cut-offs to validate current findings from previous studies. For example, when using a PSA cut-off of <0.05 versus 0.05-2.0 versus ≥2.0 ng/ml, we also found substantial OS differences between all three groups. A recently published study by Kafka et al., elaborating real world outcomes of 42 mHSPC patients, also suggested a PSA nadir ≤0.05 ng/mL as a predictor for improved OS.8 In consequence, the findings of our cohort with over >230 mHSPC patients validates the findings of Kafka et al.
An post hoc analyses of the TITAN trial presented at ESMO 2023 suggested an even lower PSA nadir cut-off, called ultra-low PSA.21 Here, patients with a PSA nadir ≤0.02 ng/mL at 3 months of treatment with apalutamide were associated with best OS at an 89% survival rate at 42 months of follow up. To test for these ultra-low PSA nadir cut-offs, we further stratified our cohort into PSA nadir ≤0.02 versus 0.02–0.2 versus >0.2 ng/mL. Specifically, we found that 18.1% of our mHSPC patients achieved a PSA nadir ≤0.02 ng/mL. This rate is significantly lower to the number of patients of the TITAN trial of the apalutamide group (49%) and more comparable to the placebo arm (17%). However, our OS data also suggest that PSA nadir ≤0.02 ng/mL is associated with best prognosis. In consequence, the suggested ultra-low PSA cut-off derived from a patient-selected contemporary phase III trial is also applicable in real world setting of mHSPC patients and may be used for approximation of disease progression and OS. However, the cut-off applies to fewer mHSPC patients in real world setting than expected from study-designed environment.
Third, despite absolute PSA responses, we also tested for relative PSA responses in mHSPC patients. Here, we found that a PSA response ≥99% is associated with better OS prognosis. Moreover, we validated these findings in de novo and high volume mHSPC patients. Relative PSA responses may be interpreted as a more sufficient clinical decision tool, since mHSPC patients may differ significantly with their baseline PSA, depending on time of metastatic disease (de novo vs. secondary mHSPC) or metastatic burden (low vs. high volume), supported by an absolute interquartile range of baseline PSA at metastatic disease of 12–298 ng/mL and a maximum PSA of 12,760 ng/mL in our study cohort. Our main findings are in line with data of post hoc analyses of phase III trials, where a PSA response ≥90% was also associated with better OS.9-12
Finally, we stratified patients according to time to PSA nadir. We found that prolonged time to PSA nadir is associated with better survival. These findings may be counterintuitive since previous phase III trials found that especially a rapid PSA response selects patients with better prognosis.9-12 However, our findings are not contrary to these previous findings. In particular, it suggests that after a deep and rapid PSA response an additional response to an even deeper PSA nadir is associated with more favorable OS. To avoid a bias due to insufficient PSA responders, we validated these findings in mHSPC patients with PSA nadir <4 ng/mL and PSA response ≥90% and similar findings were made. These findings are in an agreement with previously made findings from international studies and meta-analyses.14, 16, 22-25
Nonetheless, our study has limitations and needs to be interpreted in light of its retrospective, single-center study design. Moreover, no data were available for PSA nadir landmark analyses at specific time points after treatment initiation. Finally, some subgroup analyses may be limited due to patient sample size or the heterogeneity of compared cohorts and therefore may not reach significance level.
5 CONCLUSION
Taken together, our study demonstrated that in times of combination therapies for mHSPC, a PSA nadir of respectively <0.2 and <0.02 ng/mL are associated with best OS rates. Moreover, a relative PSA response ≥99% and a longer TTN are clinical important proxies for favorable OS estimates.
ACKNOWLEDGMENTS
This study was part of the EPIC-REAP project (Enhancing Prostate cancer care In Germany Combining Real-world data And AI for Enhanced Analysis and Precision) supported by the Mildred-Scheel Nachwuchszentrum Frankfurt. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.