Structure and heme binding properties of Escherichia coli O157:H7 ChuX
Abstract
For many pathogenic microorganisms, iron acquisition from host heme sources stimulates growth, multiplication, ultimately enabling successful survival and colonization. In gram-negative Escherichia coli O157:H7, Shigella dysenteriae and Yersinia enterocolitica the genes encoded within the heme utilization operon enable the effective uptake and utilization of heme as an iron source. While the complement of proteins responsible for heme internalization has been determined in these organisms, the fate of heme once it has reached the cytoplasm has only recently begun to be resolved. Here we report the first crystal structure of ChuX, a member of the conserved heme utilization operon from pathogenic E. coli O157:H7 determined at 2.05 Å resolution. ChuX forms a dimer which remarkably given low sequence homology, displays a very similar fold to the monomer structure of ChuS and HemS, two other heme utilization proteins. Absorption spectral analysis of heme reconstituted ChuX demonstrates that ChuX binds heme in a 1:1 manner implying that each ChuX homodimer has the potential to coordinate two heme molecules in contrast to ChuS and HemS where only one heme molecule is bound. Resonance Raman spectroscopy indicates that the heme of ferric ChuX is composed of a mixture of coordination states: 5-coordinate and high-spin, 6-coordinate and low-spin, and 6-coordinate and high-spin. In contrast, the reduced ferrous form displays mainly a 5-coordinate and high-spin state with a minor contribution from a 6-coordinate and low-spin state. The νFe-CO and νC-O frequencies of ChuX-bound CO fall on the correlation line expected for histidine-coordinated hemoproteins indicating that the fifth axial ligand of the ferrous heme is the imidazole ring of a histidine residue. Based on sequence and structural comparisons, we designed a number of site-directed mutations in ChuX to probe the heme binding sites and dimer interface. Spectral analysis of ChuX and mutants suggests involvement of H65 and H98 in heme coordination as mutations of both residues were required to abolish the formation of the hexacoordination state of heme-bound ChuX.
Introduction
Iron is the fourth most abundant element in the Earth's crust.1 However, due to the low solubility of iron(III) salts at physiological pH in the presence of oxygen, iron uptake, storage, and transport poses a serious obstacle for bacteria to overcome for survival and pathogenesis. Furthermore, invading microorganisms are confronted with the obstacle that 99.9% of iron is sequestered as protein coordinated iron or as heme in hemoglobin, myoglobin, and various other host heme coordinating proteins.2 Through various mechanisms of heme detection and liberation from host hemoproteins, bacteria can access host heme sources and subsequently internalize heme through translocation machinery. The newly internalized heme may then be used as a protein prosthetic group or degraded providing a valuable pool of iron.
Restriction mapping and sequence analysis of selected regions of E. coli O157:H7 DNA, the serotype responsible for outbreaks of hemorrhagic colitis and hemolytic uremic syndrome, has revealed that the organization of the heme transport locus is strikingly similar to that of other enteric bacteria such as Shigella dysenteriae and Yersinia enterocolitica.3, 4 This similarity has permitted the establishment of a general model for heme internalization. Using a laboratory strain of E. coli [1017 (ent::Tn5)] that was unable to import heme into the cytoplasm, it was established that the outer membrane receptor ChuA (E. coli heme-utilization protein A) was required for heme uptake along with the energy-transducing protein complex TonB-ExbB and ExbD.5 Three other conserved members of the heme uptake operon from Y. enterocolitica were shown to be required to complete heme uptake and transport by E. coli K-12 including: the periplasmic heme-binding protein HemT, the heme permease protein HemU, and the ATP-binding hydrophilic protein HemV.3, 4E. coli O157:H7 homologues are similarly named ChuT, ChuU, and ChuV, respectively.
Once internalized, heme can either be stored, degraded, or used directly in a variety of biological processes, many of which are fundamental to bacterial survival and host colonization.6, 7 Furthermore, due to the ability of heme to disrupt normal redox processes and catalyze the formation of deleterious free radicals, heme is also a potentially toxic molecule.8 A tightly regulated balance between heme uptake and degradation is therefore of critical importance. In E. coli O157:H7 and organisms which utilize analogous heme utilization systems, the regulation of cytoplasmic heme processing and roles of the remaining elements remains unclear but likely involves the remaining factors encoded within the heme utilization operon, namely, ChuS, ChuW, ChuX, and ChuY.
ChuS and its homologues have been more extensively investigated, which have been shown to promote the utilization of heme as an iron source as well as to offer protection against heme toxicity.4, 9 Monomeric ChuS was revealed to be composed of a structural duplication that interact across a central core of two pleated β-sheets.10-12 Structures of heme associated forms of ChuS and HemS illustrated that the bowing β-core contributes part of the heme binding cleft together with a three helix subdomain that houses a conserved histidine responsible for iron coordination.10-12
In S. dysenteriae shuW, shuX, and shuY appear to be cotranscribed with the periplasmic heme transport gene shut, but their expression was not required for heme utilization as an iron source.3, 4 A premature stop codon exists within shuW that is absent in E. coli O157:H7 chuW suggesting that ChuW is expressed in a full length form.9 Twelve nucleotides downstream of shuW is the start codon for shuX, that likely is cotranscribed with shuY.13 In the heme uptake locus from Yersiniae pestis, genes homologous to chuX and chuY (orfX and orfY, respectively) were evaluated to be located immediately downstream of the heme receptor hmuR and were therefore likely coexpressed.14 In Vibrio anguillarum, which can use heme and hemoglobin as a source of iron, using a lacZ reporter system it was shown that a homologue of chuX, huvX, was cotranscribed with the uncharacterized gene huvZ under iron limiting conditions but that huvX was not essential for heme utilization.15, 16 An examination of the −10 and −35 elements upstream of huvX revealed sequence with similarity to sigma70-promoters and the presence of a Fur element, suggesting HuvX expression is up-regulated under iron limiting conditions where heme utilization is also stimulated.15 In Plesiomonas shigelloides a homologue to ChuX, HugX was required for heme iron utilization along with HugW and HugZ, and was speculated to have been involved in prevention against heme toxicity.17
Despite extensive genomic analysis of the heme utilization operon in various gram-negative bacteria, functional characterization of ChuX or its homologues have received little attention. Herein we report the first crystal structure of ChuX at 2.05 Å resolution. Through site-directed mutagenesis of conserved histidine residues, visible and Raman spectral analysis, and structural comparison of ChuX with ChuS and HemS we have identified the putative heme binding sites in ChuX and propose a mechanism of ChuX heme binding and release. Based on structural similarity of ChuX with ChuS, HemS, AGR_C_4470p and Q6d2t7, a structural class of heme binding proteins in gram-negative bacteria is emerging.
Results
ChuX protein fold and structural analysis
Recombinant ChuX fused to an N-terminal His6-tag was purified via a two-step protocol, crystallized and a structural solution obtained and refined as outlined in “Materials and Methods”. The structure of ChuX consists of two dimers in the asymmetric unit, termed AB and CD here for clarification [Fig. 1(A)]. However, molecular weight evaluation of ChuX by both size exclusion chromatography and dynamic light scattering suggests that ChuX and reconstituted ChuX-heme both form homodimers in solution (data not shown). At the intramolecular interface within each dimer are equivalent sets of antiparallel β-sheets which bow outward to form an overall saddle motif. These central sheets are composed of six β-strands contributed by one of the ChuX monomers and three others contributed through domain swapping from the partnering ChuX molecule [Fig. 1(B)]. A cluster of hydrophobic residues (V64A-V64B, V64A-L71B, V69A-L133B, F73A-F73B) together with the reverse complements from B to A contribute to interdomain stabilization at this interface across a mean distance of 4.4 Å. The β-strands on which these residues originate rests between the two strands on which the conserved histidines mutated herein were observed to compromise heme coordination (see next section).
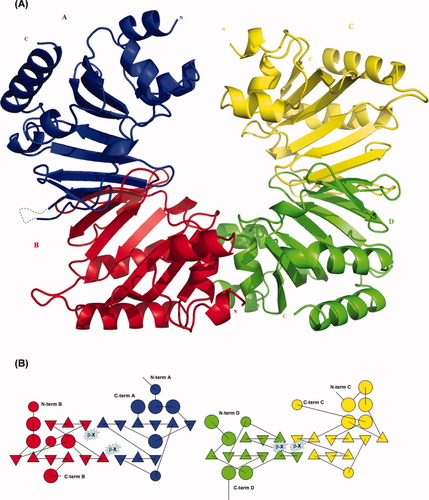
Crystal structure and topographical representation of the four ChuX molecules in the asymmetric unit. (A) Each of the four ChuX monomers that make up two pairs of ChuX homodimers are depicted with different colours. (B) ChuX homodimerization occurs via interactions across the central β-sheet core, involving a domain swap between associated ChuX couples. The β-strand where residues V69, L71, and F73 were mutated in the structure of BetaX are highlighted and interact between equivalent nonpolar residues of red A and blue B, or yellow C and green D monomers respectively. Due to a difference in domain organization between AB and CD couples we propose flexibility in ChuX dimers that may facilitate acceptance of the heme ligand.
Flanking the bowing β-core are twelve α-helices, which differ slightly in their spatial organization between the two dimer couples [Fig. 1(B)] but are structurally conserved between ChuX, ChuS, HemS and AGR_C_4470p. Three of these helices form an α-loop-α-loop-α subdomain that has been shown in ChuS and HemS to delineate part of the conserved heme binding cleft11, 12 and houses the residue demonstrated to be important for axial heme coordination in ChuS (H193) on the second α-helix. In ChuX a conserved glycine residue (G16) is in an equivalent structural position.
Ramachandran analysis of the final ChuX structure shows 488 residues (88.1%) within the most favored region, 55 (9.9%) in additional allowed, 10 (1.8%) in generously allowed, and only a single residue (0.2%) in the disallowed region (K11 from molecule C). Crystallographic statistics for diffraction data and final structural refinement are presented in Table I. Due to an absence of clear electron density in the refined structure of ChuX, a gap exists between residues 90–93 of molecule A.
| Data collection | Native | Se-met |
|---|---|---|
| Space group | P41 | P41 |
| Cell dimensions | ||
| A and B (Å) | 76.56 | 76.83 |
| C (Å) | 140.86 | 141.64 |
| α, β, and γ (°) | 90 | 90 |
| No. of ChuX molecules in asymmetric unit | 4 | 4 |
| Wavelength (Å) | 1.1000 | 0.9795 |
| Resolution range (Å) | 50–2.05 | 50–2.20 |
| Total reflections | 359,110a | 529,767a |
| Unique reflections | 50,833 | 82,255b |
| Completeness (%) | 100.0 (100.0)c | 98.6 (94.7)c |
| Rsym(I) (%) | 4.3 | 4.0 |
| I/σI | 28.3 (1.7)c | 45.3 (6.0)c |
| Refinement statistics | ||
| Resolution range (Å) | 50–2.05 | |
| Rwork, (%) | 21.3 | |
| Rfree, (%) | 25.0 | |
| No. reflections total/Rfree | 47915/3137 | |
| No. atoms, protein/solvent (H2O)/total | 4833/360/5193 | |
| B-factors (Å2) protein/solvent (H2O)/total | 49.3/53.8/49.6 | |
| RMS, bond length (Å)/bond angle(°) | 0.014/1.60 | |
- a Number of reflections following merging.
- b Number of reflections without merging of Bijovet pairs.
- c Values in parentheses are for the outermost shells (Native: 2.12–2.05 Å, SeMet: 2.27-2.20 Å).
ChuX sequence and structure homology
NCBI Blastp sequence analysis suggested that ChuX belongs to a family of conserved putative heme-iron utilization proteins with the conserved domain DUF1008. This analysis demonstrates that ChuX shares sequence similarity with proteins from various human pathogens such as S. dysenteriae (ShuX: 98%), Y. enterocolitica (YE0334: 76%), P. damselae (HutX: 56%), and L. anguillarum (HuvX: 60%). ChuX homologues also appear in photoactive bacteria such as Halorhodospira halophila and Rhodopseudomonas palustris which use either iron or the products of heme degradation in the production of light harvesting photoreceptors.18 Sequence alignment of ChuX and a nonredundant set of homologues revealed many sporadically conserved residues [Supporting Information Fig. 1(A)] especially in the C-terminal half between residues K134 and L145. As histidine residues are frequently responsible for coordination of the central iron atom in heme binding proteins, H65 and H98 were highlighted through this sequence alignment and subsequently targeted for mutagenesis as a result.
Structural comparisons revealed that the ChuX monomer shares structural similarity with AGR_C_4470p from A. tumefaciens (1.3 Å rmsd),19 Q6D2T7_ERWCT from E. carotovora (1.2 Å rmsd) and overall topology with the E. coli metal chelating enzyme glyoxalase I (RCSB PDBID: 1FA8, 3.8 Å rmsd).20 Notably heme binding has not been reported in any of these cases. Furthermore, good secondary structure alignment exists between the ChuX dimer and monomeric structures of two other proteins encoded within the gram-negative heme utilization operon, ChuS and HemS from E. coli O157:H7 and Y. enterocolitica, respectively [Supporting Information Fig. 1(B)]. ChuX aligns with these protein structures to rmsd values between 2.4 and 2.8 Å, depending on which ChuX dimer is used and whether the structure of the apo or holoenzyme of ChuS or HemS is used. This structural similarity is noteworthy given that ChuX shares less than 18% sequence identity with either the N- or C-terminal sequences of ChuS or HemS.
In both ChuS and HemS the histidine side chain responsible for heme binding originates from one of the flanking α-helices. A structurally equivalent histidine residue is absent in ChuX, AGR_C_4470, or Q6D2T7_ERWCT. However, originating from elements of the central β-core on the opposite wall of the cleft are two conserved ChuX histidine residues, H65 and H98 (see Fig. 2) contributed from opposite ChuX monomers suggesting that domain swapping is involved in heme uptake. Comparison of the AB and CD ChuX monomer pairs highlighted that in the AB couple this grouping of helices have shifted slightly away from the central β-sheets by as much as 3.5 Å for the α-carbons, effectively broadening the putative ChuX heme binding cleft [Fig. 2 (B,C)]. In the crystal structures of tandem repeating ChuS-heme and HemS-heme only one of structurally similar clefts had a heme molecule bound. However, an additional conserved histidine residue, H277, resides within the vacant cleft and was initially hypothesized to be responsible for heme binding.10 Despite the structural similarity of ChuX and ChuS, no heme degradation capacity was detected for ChuX using either ascorbate or Cytochrome P450 Reductase-NADPH as electron donors (data not shown).
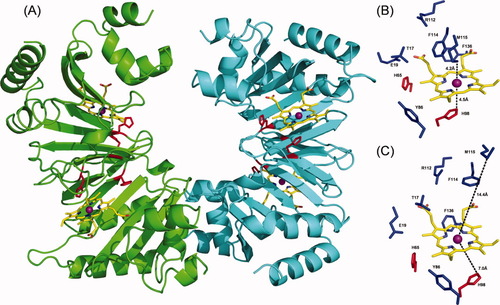
Heme docking to ChuX. Four heme molecules were docked based on ChuX structural similarity with ChuS. Energy minimized heme positions are presented and conserved histidines, H65 and H98 shown to be involved in heme coordination are highlighted red. (A,B) the sequence conserved residues T17, E19, H65, Y86, H98, R112, F114, M115, K134, and F136 are in close proximity to the putative heme binding cleft which could therefore contribute to heme stabilization and/or heme uptake for the (C–D) dimer. (A) heme modeled into one of the proposed closed cleft conformations and (B) into the open cleft conformation.
The first three residues of the conserved segment K134 to L145 reside on the β-sheet responsible for ChuX dimerization. This region of ChuX is also at the base of the conserved heme binding cleft, with K134, a residue originating from the conserved segment of residues at the C-terminal end of ChuX, extending to only 3.8 Å from H65, potentially contributing to the electronegativity of the imidazole group for iron coordination. The other residues of this conserved segment reside on a loop that delineates one edge of the heme binding cleft and may therefore be involved in guiding heme insertion, or stabilizing the heme moiety once it is taken up by ChuX [Fig. 2 (B,C)].
The ChuX structure may represent an open and closed conformation
Structural alignment of the two homodimers in the ChuX asymmetric unit revealed an interesting difference in that one of the four putative heme binding clefts is significantly more open compared to the other three. In order to disrupt hydrophobic interactions in the β-core of each ChuX dimer, V69, L71, and F73 were targeted for mutagenesis to polar glutamate, histidine, and serine respectively. In the three closed cleft conformations, the gap between α-carbons of H84 and M115 at the outermost edge of the cleft are 13.3, 15.3, and 15.5 Å across, whereas the equivalent gap distance of the open form is 23.3 Å. This difference is largely due to a 3.2 Å shift of the α-helix delineated by residues 16–26 that define the lateral edge of the cleft that effectively closes the gap to a final distance of 5.0 Å. In ChuX this helix is structurally equivalent to the helix responsible for axial coordination of heme-iron in ChuS and HemS, that shifts upon heme binding by as much as 3.8 and 4.0 Å, respectively.11, 12 Crystal contact analysis revealed that relatively few contacts are made between ChuX symmetry-related molecules, with the exceptions of contacts made between C-terminal helices with an adjacent symmetry molecule and contacts made between the loop formed by residues F9 to G16 with the turn present at H84 and G85. These interactions occur in a similar fashion for either ChuX homodimer, suggesting that the structural difference observed was not due to crystal packing. Furthermore, notably absent are contacts which would have affected the three helix subdomain of ChuX proximal to the putative heme binding cleft, suggesting that contacts made between dimer couples are more significant. The overall effect of the gap differential is that H65 is effectively buried at the base of the putative heme binding cleft in the closed cleft conformations, while H98 remains oriented into the putative heme binding cleft.
When heme was modeled into the four putative heme binding clefts, positions corresponding to the lowest 10 energy scores converged to an equivalent position in each case [Fig. 2(A)], where the heme iron is axially coordinated between H98 and M115 at a distance of 4.5 and 4.2 Å, respectively in the closed clefts. This distance is comparable to the distance between R100 and H193 of ChuS (8.2 Å) before heme loading.11 In addition to H65, H98, and M115, other conserved residues T17, E19, Y86, R112, F114, K134, and F136 could also contribute to heme uptake and coordination through van der Waals interactions with the pyrrole rings and potentially stabilize the heme propionates by R112, K134, and R139 [Fig. 2(A,B)].
ChuX-heme association characterized by optical and resonance spectroscopies
Heme proteins have characteristic optical and resonance Raman spectra that convey information of ChuX-heme resemble those of other heme proteins in that a Soret maximum is evident at 404 nm [Figs. 3(A) and Fig. 4], and accompanied by a smaller peak for the charge-transfer band at 630 nm, consistent with the formation of a ferric hexacoordinate high-spin complex.21 The resonance Raman spectrum of ferric ChuX obtained in the high-frequency region confirmed this but revealed that two hexacoordinate states are present. The ν3 line at 1479 cm−1 is consistent with the presence of a hexacoordinate high-spin complex while the strong ν3 line at 1506 cm−1 indicates that a hexacoordinate low-spin complex is present as well (see Fig. 5).22 Monitoring the appearance of the 404 nm transition peak during heme reconstitution to ChuX suggests that the association occurs rapidly, reaching its maximum within 5 minutes. Furthermore, differential spectroscopy of heme versus ChuX-heme suggests that the association is 1:1, implying that the ChuX homodimer binds two molecules of heme [Fig. 3(B)]. In addition to the 404 nm Soret transition, a second transition was revealed by the shoulder at ∼375 nm on the left of the main Soret transition (see Fig. 3). This wavelength is consistent with a pentacoordinate heme complex which was confirmed by the detection of a weak ν3 line at 1494 cm−1 in the resonance Raman spectrum of ChuX (see Fig. 5).22, 23 Thus, ChuX forms a majority of hexacoordinate complexes (high-spin and low-spin) with heme with a minor population of pentacoordinate complex, likely via a water molecule.
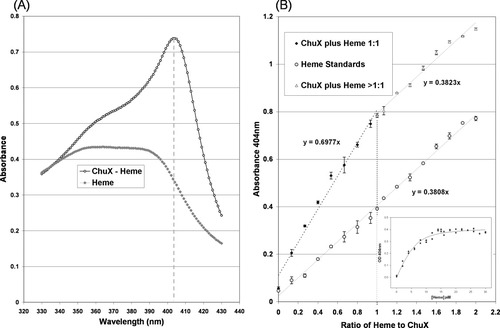
Spectral analysis of ChuX reconstituted with heme. (A) A clear Soret peak is visible at 404 nm for reconstituted ChuX-heme purified by size exclusion chromatography. (B) ChuX binding affinity for heme was determined by the addition of 15 μM ChuX to various amounts of heme (0–30 μM), and absorbance measurements at 404 nm. A transition in the slope of the line of fit for ChuX-heme inflection point is evident for ChuX to heme at a ratio of 1:1. At higher heme concentrations the slope of the line changes to one similar to that observed for free heme, suggesting that ChuX has become saturated. (B inset) is the line of fit of ChuX-heme corresponding to a calculated Kd of 1.99 ± 0.02 μM for ChuX-heme.
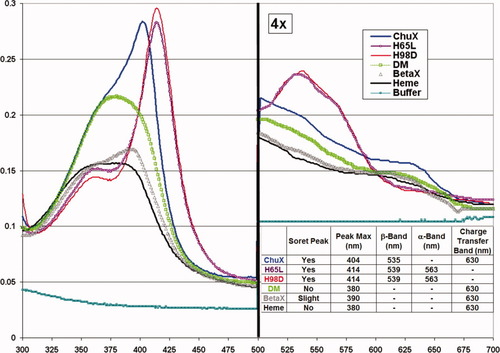
Absorbance spectra of ChuX and ChuX-mutants reconstituted with heme. The region between 500 and 700 nm has been amplified fourfold to highlight peak differences. The ChuX-heme spectrum resembles that of other heme proteins in that a Soret maximum is evident at 404 nm and a charge-transfer band at 630 nm, consistent with the formation of a ferric hexacoordinate complex. Mutations H65L and H98D both affect heme binding, evident in the shift in the wavelength of the Soret peak to 414 nm in their heme reconstituted. Notably, while the H65L and H98D independent mutations did not prevent heme coordination by ChuX, the spectrum of DM-heme reflects a reduction in heme coordination due to the elimination of a clear Soret peak in the 404–414 nm range and reduction in absorption of the α- and β-bands. Mutation of the BetaX form of ChuX resulted in reduces Soret maximum and diminished stability.
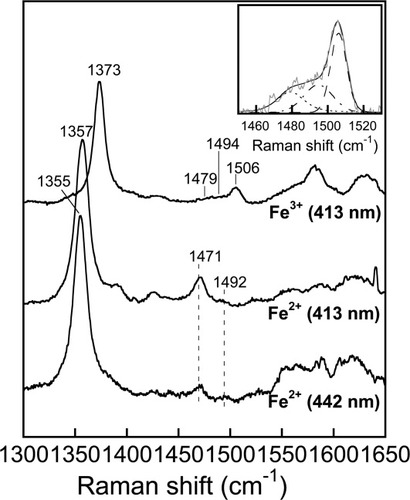
High-frequency region of the resonance Raman spectra of ferrous and ferric ChuX. The spectrum of the ferric form was obtained with an excitation wavelength of 413 nm. Spectral deconvolution of the ν3 region revealed three lines centered at 1479, 1494, and 1506 cm−1, respectively (inset). The widths, Gaussian/Laurentzian ratios and positions of the lines were unconstrained during the regression. The spectra of the ferrous complex were obtained with excitation wavelengths of 413 and 442 nm.
In order to gain insight about the nature of the fifth ligand to the heme of reconstituted ChuX, the resonance Raman spectra of the reduced and reduced-CO complexes were obtained. Heme proteins in which the fifth ligand to the heme is a histidine residue display a Fe-His stretching mode (νFe-His) in the low-frequency region when the heme is pentacoordinate.24 The optical spectrum of ferrous ChuX displays a Soret transition centered at 426 nm and the visible region contains a broad absorbance centered near 556 nm. The absence of strong α- and β-bands together with the relatively high wavelength maximum of the Soret transition indicate that ferrous ChuX is mostly pentacoordinate. This conclusion is corroborated by the resonance Raman spectrum of reduced ChuX, obtained in the high-frequency region, which indicates that the heme was mostly pentacoordinate with a ν4 line at 1355–1357 cm−1 and a strong ν3 line at 1471 cm−1 (see Fig. 5). These resonance Raman lines were detected either with excitation wavelengths at 413 or 442 nm.22, 24 A small population of hexacoordinate heme was detected with a ν3 line at 1492 cm−1. A good quality resonance Raman spectrum was difficult to obtain in the low-frequency region of reduced ChuX and, although the heme is in a pentacoordinate state, we could not detect a strong line in the 210–230 cm−1 range that could be readily identified as νFe-His (Supporting Information Fig. 2). Nevertheless, evidence that the fifth ligand of the heme of reduced ChuX was a histidine residue was provided by the identification of the νFe-CO and νC-O frequencies of the CO complex. In the low-frequency region, isotope substitution was used to identify a major line at 499 cm−1 (489 cm−1 with 12C18O) and a minor one at ∼528 cm−1 (∼515 cm−1 with 12C18O) that correspond to two νFe-CO modes [Fig. 6(A)]. In the high-frequency region, isotope substitution identified a line at 1958 cm−1 that shifted to 1916 cm−1 with 12C18O [Fig. 6(B)]. The amplitude of the isotope shift (42 cm−1) was consistent with this mode corresponding to νC-O (expected shift of 47 cm−1). When plotted on the νFe-CO versus νC-O correlation curve, the frequencies of the main form of the FeIICO complex of ChuX fall on the line of histidine-coordinated hemeproteins [Fig. 6(C)]. The νFe-CO and νC-O frequencies of ChuX, that fall in the lower right portion of the correlation, also indicate that the heme-bound CO is not involved in strong polar or steric interactions with surrounding groups.24 Assuming that the second conformer is also histidine-ligated, the second νC-O line is expected in the 1920–1930 cm−1 range. Presumably, we could not identify the νC-O mode associated with the νFe-CO mode at ∼528 cm−1 due to its low amplitude and the likely overlap with the 12C18O line at 1916 cm−1. This second conformer, which would fall in the upper left region of the νFe-CO versus νC-O correlation line, would represent a fraction of ChuX in which the heme-bound CO is experiencing strong polar interactions with positively charged residues.24
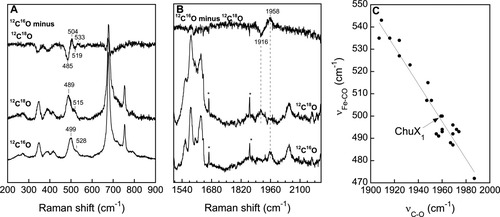
Resonance Raman spectra of the FeIICO complex of ChuX. The spectra were obtained in the low-frequency region (A) and very high-frequency region (B) with 12C16O and 12C18O. The 12C16O minus 12C18O difference spectra are also shown. The excitation wavelength was 413 nm and the power was ∼ 5 mW. The νFe-CO versus νC-O correlation graph is shown in C with the ChuX1 data point (νFe-CO at 499 cm−1 and νC-O at 1958 cm−1) along with many histidine-coordinated hemeproteins. The ChuX1 data point represents the main CO-bound ChuX conformer. A second conformer, with νFe-CO at 528 cm−1 was also identified. The ChuX2 data point should fall in the upper left region of the correlation if it is also histidine-coordinated. The solid line is the best linear fit to the data points.
Spectral analysis of the ChuX-mutants reconstituted with heme indicate that H65L and H98D both affect heme binding as the Soret peak for each has shifted to 414 nm, concomitant with a decrease in the absorbance intensity at 360 nm, while the shoulders in the β-band region intensified and the charge-transfer band disappeared compared to those of ChuX-heme consistent with the formation of a hexacoordinate and low-spin heme complex (see Fig. 4), that may also be complexed with an OH at the sixth coordinate site. Notably, while the independent H65L and H98D mutations did not prevent heme coordination by ChuX, the optical spectrum of DM-heme reflects a reduction in heme coordination due to the elimination of the Soret peak in the 404–414 nm region, as well as a reduction in absorption of the charge-transfer and β-bands. However, a strong optical transition centered near 375 nm was detected indicating the DM-heme was forming a pentacoordinate heme complex. Therefore, in addition to H65 and H98, another residue or water molecule within the heme pocket has the ability to form a coordination bond with the heme.
ChuX heme binding affinity
Based on duplicates of absorbance at 404 nm during ChuX-heme reconstitution [Fig. 3(B inset)], the estimated Kd for ChuX-heme is 1.99 ± 0.02 μM. This estimated Kd for ChuX-heme suggests a slightly weaker heme association than that characterized for other heme binding proteins such as the mammalian hHO-1 (0.84 ± 0.2 μM),25, 26 HasA of 5.0 ± 3.1 nM,27 and ChuS (1.0 ± 0.3 μM),10 but tighter than that reported for HmuO (2.5 ± 1.0 μM),28 IsdG (5.0 ± 1.5 μM) and IsdI (3.5 ± 1.4 μM).29
Discussion
Herein we present the first crystal structure of ChuX, a member of the heme utilization operon from pathogenic E. coli O157:H7, propose that ChuX may help to regulate heme utilization through structure-mediated heme binding and release and demonstrate that ChuX-heme displays mainly a hexacoordinate state (high-spin and low-spin) with a minority proportion of a pentacoordinate and high-spin state. Largely through gene knockouts and subsequent phenotypic characterization, the protein machinery responsible for heme uptake in gram-negative bacteria has been elucidated and can be extended to the human pathogen E. coli O157:H7.3, 4 However, the regulation of heme internalization and the proteins involved in cytoplasmic heme metabolism have only recently received attention. Heme is a hydrophobic molecule capable of freely diffusing into cell membranes where it can alter the bilayer structure, disrupt cell integrity, and promote the light-dependent formation of reactive oxygen species that can cause nonenzymatic redox reactions.8, 30 Organisms must therefore tightly regulate heme acquisition and metabolism through degradation or storage.31 In gram-negative bacteria synchronization of iron requirements with heme uptake has been shown to occur through the transcriptional regulator Fur in E. coli, as well as the chuX homologue huvX in V. anguillarum.15, 32 As heme internalization from host sources may be very efficient, ChuX may therefore be upregulated to serve a cytoprotective role through heme storage or short term sequestering until the organism has returned balance between heme uptake and heme utilization.33 A similar heme storage role has recently been suggested for the gene products ght from Neisseria meningitides34 and hmsT from Y. pestis35, as well as the protein HutZ from Vibrio cholerae36. Due to its ability to bind a molar equivalent of heme and its capacity for heme transfer to other heme utilizing proteins due to its high Kd constant for heme, a similar heme storage role for ChuX may be proposed.37
Structural insights provided from vibrational spectroscopy (EPR and Raman), NMR, and X-ray crystallography have indicated that heme proteins often coordinate the iron center of the heme group between a histidine side chain and a variety of other possible residues including tyrosine,38 methionine,39 cysteine,40 proline,41 histidine,42 arginine,11 and that some heme proteins can even coordinate their heme iron centers via a sole histidine sidechain without additional sidechain assistance.43, 44 Other proximal side chains and polypeptide backbones have been shown to interact with the propionic and vinyl components of the heme group, contributing to the stabilization of the heme moiety within the protein environment which ultimately confers heme protein function. In the structure of dimeric ChuX the conserved residues T17, E19, H65, Y86, H98, R112, F114, M115, K134, and F136 are in close proximity to the putative heme binding cleft which could therefore contribute to heme stabilization and help mediate the structural transition from open to closed conformation or vice versa.
Surprisingly, given the low sequence similarity between ChuX, ChuS and HemS, all three structures have similar topology and structural organization. Furthermore, as the monomeric structures of ChuS and HemS resemble the homodimer formed by ChuX, AGR_C_4470p and Q6D2T7_ERWCT, it could imply an evolutionary relationship whereby chuS initially evolved from a chuX gene duplication event. This notion is further substantiated based on sequence conservation of H98 from dimeric ChuX with the conserved residue H277 in ChuS and homologues. It appears that a new structure family of heme-associated protein from pathogenic bacteria is emerging that may therefore be used to highlight conserved heme binding motifs and suggested the residues responsible for heme stabilization and coordination.
While the structural similarity shared between ChuX and ChuS/HemS suggested a similar mechanism of heme coordination, the axial histidine side chain responsible for heme coordination in ChuS/HemS was notably absent in the structure of ChuX. In ChuX a glycine residue (G16) is in the equivalent structural position and is conserved across the ChuX homologues examined. Spectral analysis of ChuX reconstituted with heme suggested that a histidine residue was involved in ChuX heme coordination and that heme coordination occurred in a 1:1 fashion and that the dissociation constant for ChuX-heme was slightly greater in magnitude larger than for other hemoproteins. When the ChuX sequence was aligned with homologues from various gram-negative bacteria, the conserved residues H65 and H98 were identified as potential heme coordinating residues. Based on the appearance of a Soret peak at 414 nm and strong α/β-bands in the visible region for the H65L and H98D mutants reconstituted with heme, these single point mutations did not abolish heme binding. In fact, they allowed the conversion to a single apparent hexacoordinate low-spin state that was detected in only a fraction of wild type ChuX. However, spectral analysis of the ChuX double H65/H98 mutant demonstrated that an alternate residue or water molecule may be involved in forming a bond with the heme as a pentacoordinate heme was detected.
The capacity for heme coordination by ChuX H65L and H98D single point mutants could therefore categorizes ChuX within a class of proteins associated with heme internalization that utilize a redundant set of residues for heme uptake and coordination. In the extracellular hemophore HasA from Serratia marcescens, two histidine residues are integral for heme binding.27 In the outer-membrane receptor, ShuA, a double histidine mutant abrogated the ability to use hemoglobin as a source of heme.45 Similarly, in the periplasmic heme transport protein ShuT, one tyrosine residue, Y94, has been shown to be the axial heme ligand. A second tyrosine, Y228, has been shown to make significant contributions to the stability of heme-loaded ShuT.46 The involvement of two residues in heme uptake and coordination could imply a conserved mechanism in which one of the residues is initially involved in heme stabilization and acceptance, with the second residue ultimately responsible for axial heme coordination in the holo-form of the protein. Recently, PhuS, the Pseudomonas aeruginosa homologue to ChuS, has been shown to also use alternate histidine ligands in heme coordination.47 Once heme loaded, the nonaxial histidine residue may facilitate recognition of heme-loaded proteins by other proteins involved in heme trafficking that could permit variations in function such as heme sequestering or release as a response to intracellular iron requirements. Furthermore, based on heme docking study, M115 may be act as the second axial residue responsible for heme iron coordination, an idea worth exploring further in subsequent investigations.
Similarities between ChuX and HasA extend beyond a redundant residue set in heme coordination. HasA has been shown to coordinate heme between a residue contributed from a central β-strand, as well as forming a domain swapping dimmer.48 Domain swapping involves a structural exchange with an identical monomer to form an oligomer and has been suggested to regulate protein function.27 Despite that over 60 proteins have been identified as domain swapping dimers, a common role or function mechanism for it remains unclear.49 Domain swapping and structural similarities of the ChuX and HasA dimers, and ChuS and HemS monomers, could therefore exemplify a common mechanism for protein-protein and protein-substrate induced structural changes via a central hinge region.
In the crystallographic asymmetric unit two different ChuX homodimer conformations were observed in which one ChuX dimer assumed a structure with two similarly spaced gaps across the conserved heme binding cleft, while the second dimer had one gap similar in size to those found in the first dimer conformation as well as a noticeably broader one. This gap width differential could suggest a structural mechanism for heme binding and release whereby a central hinge region between the core β-sheets could facilitate heme binding and release. Based on crystal contact analysis, intradimer interactions seem to influence this structural plasticity more significantly, potentially suggesting a mechanism for heme release from ChuX to a structurally similar protein such as ChuS. Furthermore, the decreased stability of the BetaX mutant also suggests that ChuX dimerization is required for proper ChuX function. In the closed conformations the equivalent α-helix shown within ChuS and HemS to be responsible for heme coordination is translated closer to H98. This structural plasticity may reflect an open, heme accepting or donating form and a closed, heme coordinated or sequestering form of ChuX. Similar protein flexibility has been shown to be important for hemoglobin and heme binding HasA to reversibly regulate heme coordination.48, 50 Together, the variation of ChuX juxtapositions in homodimers as well as the docking of heme to ChuX at the two conserved histidine residues H65 and H98 located within the conserved heme binding pocket, suggest a potential structural mechanism for heme binding and sequestering. This plasticity would also help to explain why a double H65/H98 mutant was necessary to abrogate the hexacoordinate state as ChuX may be able to interact with heme via two different histidines within the same heme binding cleft.
In conclusion, due to the structural similarity observed, we have extended the mode of heme coordination of ChuS and HemS to ChuX, and suggest the likely heme binding site in ChuX and highlight the residues responsible for heme uptake, coordination and stabilization. Based on sequence comparisons of ChuX and its homologues, in addition to structural comparisons with ChuS and HemS, we generated a number of site-directed mutants to probe heme binding sites as well as the dimer interface. Furthermore, we have proposed that a hinge region established across the central β-sheet dimer interface may serve as a structural mechanism in regulating heme binding and that ChuX may function as a cytoplasmic heme shuttling protein, thereby regulating heme processing through sequestering or storage. Having demonstrated that homologues to ChuX are present in many gram-negative bacteria, including Enterobacter, Erwinia, Shigella, and Yersinia, we can extend our structural, functional, and mechanistic insights across various organisms with respect to heme utilization and have highlighted an emerging class of heme trafficking proteins.
Abbreviations:
BetaX, ChuX Val69Asp/Leu71His/Phe73Ser triple mutant; DM, ChuX His65Leu/His98Asp mutant; H65L, ChuX His65Leu mutant; H98D, ChuX His98Asp mutant; Heme, ferriprotoporphyrin IX chloride; SAD, single wavelength anomalous dispersion
Materials and Methods
Sequence analysis, protein expression and purification, site-directed mutagenesis
Sequence homologues to E. coli O157:H7 ChuX were initially identified using Blastp (www.ncbi.nlm.nih.gov)51 and subsequently aligned with Multialign (http://bioinfo.genotoul.fr/multalin/multalin.html).52 The sequence accession numbers for homologous sequences used in this alignment include: E. coli O157:H7 (NP_290084), S. dysenteriae (YP_405016), Erwinia carotovora (YP_051098), Y. pestis (NP_991835), Rhodopseudomonas plaustris (YP_569489), Listonella anguillarum (CAD43040), Bradyrhizobium japonicum (NP_773714), Agrobacterium tumefaciens (NP_355414, 2HQV), Photobacterium damselae (CAE46548), Shewanella putrefaciens (ZP_01704968), V. cholerae (EDN11513).
ChuX fused to an N-terminal His6-tag was subcloned into a pET 15b expression vector between NdeI and BamHI restriction cut sites. Based on the apo-ChuX structure and sequence conservation analysis, two single point mutants: H65L and H98D and a double mutant: (H65L/H98D) (termed DM) were created. H65L was initially selected as a single base pair mutation to alter H65 to a nonpolar residue. Subsequently, a H98D mutant was generated as the H65L mutant was evaluated based on spectral analysis to retain heme coordination capacity. A triple residue mutant (BetaX) was also generated which altered three nonpolar residues (V69E/L71H/F73S) shown in the crystal structure to interact across the central β-sheets in ChuX homodimers in an attempt to disrupt ChuX homodimerization. Using the QuickChange® site-directed mutagenesis kit (Stratagene, CA), recombinant ChuX was mutated at these three positions using the following sense primers where base pair changes have been underlined: H65L sense 5′-CGTCACCACGTTAGTACTTACTGCCGATGTAATCC-3′, H98D sense 5′-GCACGGCATGTCCGGGGATATCAAAGCAGAAAACTG-3′, and BetaX sense 5′-GTACATACTGCCGATGAAATCCACGAATCTAGCGGCGAACTGCCTTCGGG-3′. Antisense primers were reverse complements in each case. Polymerase chain reaction conditions were used as recommended by Stratagene followed by digestion of methylated (non-PCR amplified) DNA with Dpn1 for 1 h. Undigested DNA was heat shock transformed into MC1061 cells and confirmed by sequence analysis in each case. Isolated plasmid DNA was transformed into BL21(DE3) cells from which single colonies were picked and grown overnight at 37°C in 5 mL Terrific Broth (Bioshop, Burlington, CAN) supplemented with 100 μg/mL ampicillin. 1 L cultures of BL21(DE3) cells carrying plasmids for the respective recombinant protein were grown at 37°C in Terrific Broth-ampicillin to an OD600 between 0.6–1.0 at which time 0.4 mM isopropyl-1-thio-β-D-galactopyranoside was added to induce protein expression and cultures were grown for an additional 5 h. ChuX protein substituted with seleno-methionine was expressed in the metA- E. coli strain DL41 in LeMaster medium supplemented with 100 μg/mL ampicillin. Potentially reflecting partial copurification with heme, over-expression of ChuX resulted in cells exhibiting a slight yellow colour compared to cultures expressing mutant derivatives. This colouration was removed following purification.
ChuX and mutant derivatives were all purified in the same manner: initially via nickel nitrilotriacetate agarose batch purification in phosphate buffer (pH 8.0), dialyzed overnight in 30 mM Tris (pH 8.0), followed by subsequent purification via anion exchange using a 6 mL Resource Q column on an ATKA Explorer FPLC in 30 mM Tris (pH 8.0), with 30 mM Tris (pH 8.0), 500 mM NaCl, as the elution buffer. Purification was monitored by SDS PAGE and each protein was estimated to be >95% pure before concentration using an Amicon Ultra 15 kDa cutoff centrifugal filtration device (Fisher, Mississauga, CAN). Protein concentrations were determined by BioRad protein assay (Biorad, Mississauga, CAN) using Bovine Globulin G as a protein standard and used immediately for crystallization. ChuX, H65L, H98D, and DM all expressed at high levels (>20 mg/L culture depending on batch) and were soluble in Tris (pH 8.0) or NaH2PO4 (pH 7.0). BetaX expression levels (∼1.0 mg/L culture) were much lower than those for native ChuX or ChuX mutants and precipitated rapidly in both Tris and NaH2PO4 buffer systems. Recombinant ChuX and mutant derivatives used in heme reconstitution experiments were stored in liquid nitrogen and concentrations equalized before subsequent analysis.
Crystallization of ChuX
ChuX crystals were obtained using the hanging drop vapor diffusion method, using freshly prepared protein and reagents. Crystallization was achieved by mixing equal parts 40 mg/mL ChuX in 50 mM Tris (pH 8.0), 50 mM NaCl with reservoir solution consisting of 1.50 M ammonium sulfate, 50 mM Hepes (pH 7.5), and 2% (v/v) polyethylene glycol 400. Large rectangular crystals (3.0 × 1.5 × 0.75 mm) appeared after 2–4 d. For cryo-protection, crystals were looped through Paratone-N, and flash-cooled in N2 cold stream at 100 K. Despite extensive crystallization screening and crystal soaking, a ChuX-heme complex was not obtained.
Data collection and structure determination
ChuX crystals were determined to belong to the space group P41 with four macromolecules in the asymmetric unit. Native and single-wavelength anomalous dispersion (SAD) data sets were collected, respectively, at beamlines X8C and X12C, Brookhaven National Laboratory. SeMet SAD data were collected at a peak wavelength determined by fluorescence scan to be 0.9795 Å, for 360° total with 1.0° oscillations. The native data were collected for 180° in total, using 1.0° oscillations. Both datasets were processed with HKL2000.53 Heavy atom positions were initially determined using HySS that were refined and phases determined with SOLVE.54 Density modification, solvent flattening and automatic chain tracing were performed using RESOLVE.54 The remainder of the model was built by manual fitting using XtalView/Xfit55 and this structure was ultimately refined using native data with the program CNS.56 A structural homology search for the ChuX AB dimer (residues 4–161 from chains A and B) against the SCOP database57 was performed with the program SSM58 and protein structures were superimposed using the CCP4 program LSQKAB (Superpose).58 RCSB PDB IDs for ChuX and its structural homologues include: ChuX, E. coli O157:H7 ( 2OVI); Q6D2T7_ERWCT, E. carotovora (2PH0); AGR_C_4470, A. tumefaciens (2HQV); ChuS, E. coli O157:H7 (2HQ2); and HemS, Y. enterocolitica (2J0R). ChuX topology diagram was depicted using TOPS software.59 Modeling heme coordination by ChuX was done using Rosettadock 3.0 by first approximating the heme position by structural comparison with ChuS-heme followed by rigid body refinement of the heme molecule and evaluation of positions with lowest calculated free energies.60 Crystal contacts were evaluated with CryCo.61
Heme reconstitution
ChuX-heme reconstitution was performed by dissolving heme (ferriprotoporphyrin IX chloride) (Sigma, Oakville, CAN) in 0.5% (v/v) ethanolamine for 30 min that was subsequently dissolved in 50 mM NaH2PO4. This solution was adjusted to pH 7.0 just before incubation with ChuX or mutant derivatives. Small volumes of the heme mixture were then added to protein samples while stirring until a final ratio of 1:1 heme to protein was reached. The mixtures were incubated for 30 min at 21°C, then centrifuged at 4°C for 5 min, 14,000 rpm Absorbance spectra of the supernatant were then measured from 300 to 700 nm for each sample at 1 nm intervals. All absorbance data were collected in duplicate using a microplate spectrophotometer (Bio-Tek Instruments, NY) in 250 μL volumes and mean values were plotted. Similarly, ChuX-heme binding affinity experiments were performed by the addition of increasing amounts of heme as prepared above from 0 to 30 μM to 15 μM ChuX. Following 15 min incubation at room temperature absorbance was measured at 404 nm, the Soret peak or absorbance maximum for the spectra of ChuX-heme.
Samples for resonance Raman spectroscopy
Resonance Raman spectra of the ferric state were obtained from heme-reconstituted ChuX (∼40 μM based on heme content) that had been purified using a Sepharose-G200 column on an ATKA Explorer FPLC in 50 mM NaH2PO4 (pH 7.0). Reduced samples were obtained by equilibrating the ferric protein under an Argon atmosphere followed by the addition of a small amount of anaerobic sodium dithionite solution. FeIICO complexes were obtained by adding to reduced ChuX a known amount of CO gas (either 12C16O (Praxair, Mississauga, Canada) or 12C18O (Icon Isotopes, Summit, NJ)).
Resonance Raman spectroscopy
Resonance Raman spectra were obtained with a previously described equipment.62 Briefly, samples prepared in a custom made cylindrical cuvette were kept spinning while being exposed to the 413 nm light emitted from a Kr laser or the 442 nm light emitted from an Hd/Cd laser. The scattered light was collected at 90°, passed through a notch filter and focused on the entrance slit of a 0.67 spectrometer equipped with 1800 lines/mm grating. The diffracted light was detected with a liquid-nitrogen cooled CCD camera. Cosmic rays were removed by a routine of the Winspec software (Roper Scientific, Princeton, NJ). Several 5 min spectra were collected, averaged, and baseline corrected in the Grams software (ThermoGalactic, Salem, NH).
Coordinates
Coordinates and structure factors for ChuX are deposited in the RCSB Protein Data Bank (www.rcsb.org) under accession code 2OVI.
Acknowledgements
The authors thank Dr. Michael Nesheim for his expertise in designing and interpreting heme binding studies and John Wagner for the generation of the H98D mutant. They thank Drs. Mirek Cygler and Allan Matte for their support. They are also grateful to Brookhaven National Laboratory beam line staff Leonid Flaks and Martin McMillan (X8C) as well as Anand Saxena (X12C), where the SAD and native data for ChuX were collected, respectively.




