CXXC5 is a ubiquitinated protein and is degraded by the ubiquitin-proteasome pathway
Hazal Ayten and Pelin Toker equally contributed first authors.
Review Editor: Jeanine Amacher
Abstract
CXXC5, as a member of the zinc-finger CXXC family proteins, interacts with unmodified CpG dinucleotides to modulate the expression of genes involved in cellular proliferation, differentiation, and death in physiology and pathophysiology. Various signaling pathways, including mitogenic 17β-estradiol (E2), contribute to the expression and synthesis of CXXC5. However, how signaling pathways modulate protein levels of CXXC5 in cells is largely unknown. We previously reported that some key regulators, including retinoblastoma 1 and E74-like ETS transcription factor 1, of the G1 to S phase transitions are involved in the expression of CXXC5 in estrogen-responsive MCF-7 cells, derived from a breast adenocarcinoma. We, therefore, predict that the synthesis of CXXC5 is regulated in a cell cycle-dependent manner. We report here that although E2 in synchronized MCF-7 cells augments both transcription and synthesis of CXXC5 in the G1 phase, CXXC5 protein levels are primarily mediated by ubiquitination independently of cell cycle phases. Utilizing the bioUbiquitination approach, which is based on cellular biotinylation of ubiquitin, in HEK293FT cells derived from immortalized human embryonic kidney cells, followed by sequential immunoprecipitation coupled mass spectrometry analyses, we identified ubiquitinated lysine residues of CXXC5. We show in both MCF-7 and HEK293FT cells that the ubiquitinated lysine residues contribute to the degradation of CXXC5 through the ubiquitin-proteasome pathway.
1 INTRODUCTION
17β-estradiol (E2), the main estrogen hormone in circulation, is a critical signaling molecule that contributes to the physiology and pathology of many organs and tissues, including breast tissue. The effect of E2 in epithelial cells of breast tissues is primarily mediated by the transcription factor (TF), estrogen receptor (ER) α (Ayaz et al., 2019; Yaşar et al., 2017). Upon binding to E2, the activated ERα dimer stably associates with estrogen receptor binding sites on DNA and regulates the expression of primary response genes, some of which encode enzymes for nucleic acid and protein metabolism, TFs, and membrane signaling proteins/receptors (Bourdeau et al., 2007; Hah et al., 2011; Hah & Kraus, 2014; Nott et al., 2009). Primary response gene products, in turn, participate in the expression of secondary response genes involved in the regulation of DNA synthesis, cell cycle, and cell division (Bourdeau et al., 2007; Hah et al., 2011; Hah & Kraus, 2014; Nott et al., 2009).
We (Nott et al., 2009 and Choi et al., 2019) reported that CXXC5 is a primary estrogen-responsive gene. Also known as retinoid-inducible nuclear factor, RINF (Pendino et al., 2009), and WT1-induced Inhibitor of Disheveled, WID (Kim et al., 2010); CXXC5 is a member of the ZF-CXXC family of proteins (Long et al., 2013). The CXXC5 gene is ubiquitously expressed with varying levels in human tissues (Pendino et al., 2009; Xiong et al., 2019), (http://atlasgeneticsoncology.org/gene/52549/). Evidence indicates that morphogenic retinoic acid (Pendino et al., 2009), multifunctional cytokine family member TGF-β (Yan et al., 2018), bone morphogenetic protein BMP4 (Andersson et al., 2009; Kim et al., 2014), the Wnt family of secreted glycolipoprotein Wnt3a (Kim et al., 2015, 2016; Lee et al., 2015), as well as vitamin B2 and vitamin D (Wang et al., 2023) modulate CXXC5 expression in a tissue-specific manner (Aras et al., 2013; Kim et al., 2014; Li et al., 2014; Ma et al., 2017; Ravichandran et al., 2019; Tsuchiya et al., 2016). Alterations in CXXC5 expression result in changes in cellular metabolism, proliferation, and/or differentiation in developmental processes and tissue maintenance (Astori et al., 2022; Joshi et al., 2020; Ma et al., 2024; Marshall et al., 2012; Pendino et al., 2009; Wang, Liao, et al., 2013; Yan et al., 2018; Zhang et al., 2009). In keeping with the functional importance of CXXC5 in physiology, deregulated expression of CXXC5 appears to contribute to various pathologies, including Alzheimer's disease, impaired wound healing in diabetes, acute myeloid leukemia (AML), gastric, prostate, and breast cancer (Benedetti et al., 2017; Bruserud et al., 2015; Fang et al., 2018; Kim et al., 2023; Knappskog et al., 2011; Kühnl et al., 2015; Li et al., 2023; Tan et al., 2018; Yoon et al., 2023).
Despite the importance of CXXC5 in diverse cellular events mediated by distinct signaling pathways in physiology and pathophysiology, the regulation of CXXC5 expression remains largely unknown. We recently explored the structural and functional features of the CXXC5 promoter as the key platform for the assembly of protein complexes to mediate transcription (Pope & Medzhitov, 2018; Stadhouders et al., 2019). We reported that CXXC5 expression is driven by a core CpG island promoter which is associated with various TFs and transcription co-regulatory proteins, as well as proteins involved in histone/chromatin, DNA, and RNA processing in cell models including E2-responsive and ERα-synthesizing MCF-7 cells derived from a breast adenocarcinoma (Yaşar et al., 2021). Of the possible TFs, we verified the association of the retinoblastoma protein (RB1) and E74-like ETS transcription factor 1 (ELF1) with the core CXXC5 promoter and showed the involvement of ELF1 in the expression of CXXC5 (Yaşar et al., 2021). ELF-1 is a member of the ELF subfamily of ETS TFs that regulate many essential cellular processes, including cell cycle and proliferation (Wang et al., 1993). ELF1 acts as an activator or repressor of target gene expressions by binding to a response element on DNA as well as through interactions with RB1 (Larsen et al., 2015), which is a key regulator of the G1/S transition of the cell cycle (Dyson, 2016). Given the involvement of RB1 and ELF-1 in regulating CXXC5 expression, along with our findings that CXXC5 facilitates the transition of E2-responsive, ERα-synthesizing MCF-7 cells from the G0/G1 phase to the S phase as an essential step for E2-driven cellular proliferation (Ayaz et al., 2020), we hypothesized that CXXC5 expression and/or synthesis is also regulated in a cell cycle-dependent manner.
We report here that although E2 augments both transcription and synthesis of CXXC5 in the G1 phase in synchronized MCF-7 cells, CXXC5 protein levels are primarily regulated by ubiquitination independently of cell cycle phases. To identify the ubiquitinated lysine residues of CXXC5, we employed the bioUbiquitination (bioUb) approach, which is based on the biotinylation of ubiquitin in cells (Franco et al., 2011; Ramirez et al., 2021). Our results from transiently transfected HEK293FT cells derived from immortalized human embryonic kidney cells using bioUb followed by sequential immunoprecipitation coupled with mass spectrometry analysis indicate that multiple lysine residues of CXXC5 are ubiquitinated. We find that the ubiquitinated lysine residues of CXXC5 contribute to the degradation of the protein through the ubiquitin-proteasome pathway in both MCF-7 and HEK293FT cells.
2 RESULTS
2.1 Cell cycle-dependent expression and synthesis of CXXC5
The association of RB1 and ELF1 with the core promoter of CXXC5 and the involvement of ELF1 in the regulation of CXXC5 expression (Yaşar et al., 2021) together with our observations that CXXC5 is a critical mediator of cell cycle regulation of MCF-7 cells in response to E2-ERα signaling (Ayaz et al., 2020) raise the possibility that CXXC5 expression and/or synthesis is regulated in a cell cycle-dependent manner. To address this, MCF-7 cells were synchronized in G0/G1 by the use of the hormone-withdrawal approach we described previously (Yaşar et al., 2016). To achieve this, MCF-7 cells were cultured in a growth medium containing 10% charcoal dextran-treated Fetal Bovine Serum (CD-FBS) for 72 h to remove steroid hormones, including E2. Cells were then treated without (0.01% ethanol as the vehicle control) or with 10−9 M E2, an upper physiological level of circulating hormone in adult women (Frederiksen et al., 2020) for 2–6 h intervals up to 36 h. At each time point, collected cells were processed for flow cytometry (Figure 1a and b), RT-qPCR (Figure 1c), and WB (Figure 1d) analyses. Hormone withdrawal synchronized cells at the G0/G1 phase, in which more than 80% of the cell population was accumulated. E2 treatment of cells effectively triggered cell cycle progression such that the proportion of cells began to transit into the S phase at 12 h, reaching a plateau at 21 h (65% + 3.5%) in the S phase with corresponding decreases in the G0/G1 phase population (Figure 1a and b). At subsequent time points, cells transited to the G2 phase, completing the cycle by 30 h, resulting in a desynchronized cell population at 36 h. On the other hand, ethanol as the control did not affect cycle phase distribution throughout the experimental periods; the majority (≥ 80%) of cells remained in the G0/G1 phase.
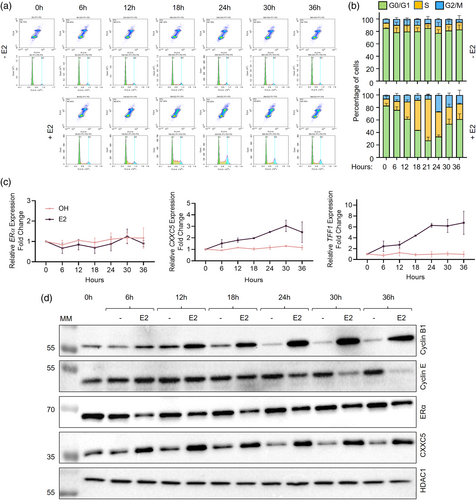
Transcript levels of ERα, which were normalized to the geometric means (Vandesompele et al., 2002) of the transcript levels of RPLP0 (Ribosomal Protein Lateral Stalk Subunit P0) and PUM1 (Pumilio RNA Binding Family Member 1), which are used as reference transcripts in breast carcinoma cell models (Lyng et al., 2008), were unaltered whether or not cells were treated with E2 (Figure 1c). Whereas E2 treatment of synchronized MCF-7 cells steadily increased CXXC5 transcript levels, reaching maximal levels by 24 h, as similarly observed with the expression of TFF1 (Trefoil Factor 1)/pS2, a well-characterized E2-ERα responsive gene (Berry et al., 1989; Métivier et al., 2003) which we used here as a control (Figure 1c).
Cell cycle progression triggered by E2 was reflected in changes in protein levels of Cyclin B1 (Figure 1d), which is the regulatory partner of cyclin-dependent kinase 1 (CDK1) essential for the transition from the G2 phase to mitosis (Yu & Yao, 2008). With the E2 treatment, Cyclin B1 levels increased in the early S phase and remained elevated until the desynchronization of cells. On the other hand, Cyclin E, as an activator of cyclin-dependent kinase 2 (CDK2) critical for the entry to and progression through the S phase (Hwang & Clurman, 2005) began to decrease at 24 h corresponding to the late S phase, reaching low levels by 36 h (Figure 1d). Diverging from ERα transcript levels, E2 treatment rapidly decreases the protein levels of ERα (Alarid et al., 1999; Wijayaratne & McDonnell, 2001). Consistent with this, E2 treatment of synchronized MCF-7 cells for 6 h reduced ERα levels to their lowest point until cell desynchronization at 36 h (Figure 1d). In contrast, CXXC5 reached the highest levels within 6 h of E2 treatment and remained at similar levels in subsequent phases (Figure 1d and Supplementary Information, Figure S1). HDAC1 levels used as the loading control were unaltered whether or not cells were treated with E2. A steady increase in CXXC5 transcript levels throughout cell cycle phases, as opposed to the protein levels, which rapidly increase and reach a plateau within 6 h post-E2 treatment corresponding to the early G1 phase, implies post-transcriptional regulation of protein levels, which could involve stabilities of transcripts and/or the CXXC5 protein.
2.2 Examination of CXXC5 protein stability upon transcription and/or translation inhibition
We then addressed whether discordant changes in transcript and protein levels of CXXC5, as observed with ERα, throughout the cell cycle involve the stabilities of transcript and/or protein. To examine this issue, we examined the effects of blocking general transcription and/or translation on transcript and protein levels of CXXC5 with RT-qPCR and WB, respectively. To block transcription, we used actinomycin D (ActD), which intercalates into DNA, thereby preventing the progression of RNA polymerases (Bailey et al., 1993). To inhibit protein synthesis, we employed cycloheximide (CHX), which disrupts the translocation step by preventing tRNA from binding to and being released from ribosomes (Colombo et al., 1965; McKeehan & Hardesty, 1969). To evaluate the minimal concentration as well as the pre-treatment duration of ActD and/or CHX in effectively blocking transcription and/or translation in MCF-7 cells, we used 5-ethynyl Uridine (5-EU) and/or L-homopropargylglycine (L-HPG) incorporation into nascent transcripts and/or polypeptide chains by chemoselective ligation followed by fluorescence microscopy (Supplementary Information, Figure S2). Based on the results, we selected to use 5 μM ActD and/or 50 μg/mL CHX in our experiments. MCF-7 cells synchronized in G0/G1 by hormone withdrawal for 72 h were treated without (0.01% ethanol as vehicle control) or with 10−9 μM E2 for 36 h to simulate a desynchronized cell population. We then incubated cells in a fresh growth medium without or with 10−9 μM E2 in the absence or presence of 5 μM ActD and/or 50 μg/mL CHX for various durations (0, 0.5, 1, 2, 4, and 8 h). Collected cells were processed for and subjected to RT-qPCR and WB analyses (Figure 2).
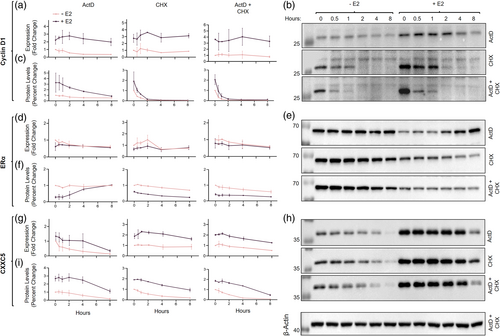
Cyclin D1, which is an estrogen-responsive gene product critical for cell cycle progression through the G1/G0 to the S phase (Doisneau-Sixou et al., 2003; Fu et al., 2004) and used here as a control, in E2-treated cells at the time of drug treatment (0 h) showed substantially augmented transcript and protein levels compared to those in untreated cells (Figure 2a–c). ActD treatment of MCF-7 cells led to a steady decline in the protein levels of Cyclin D1 with a half-life of about 3 h when E2 was present, but it did not affect protein levels in the absence of E2. CHX, on the other hand, rapidly and effectively repressed the protein levels of Cyclin D1, which were further augmented in the presence of ActD independently of E2 with a half-life of about 30 min, consistent with previous studies (Diehl et al., 1997). Thus, while steady-state levels of Cyclin D1 transcripts somewhat contribute to protein levels, Cyclin D1 is an unstable protein whose levels are primarily mediated post-translationally, whether or not cells are treated with E2.
In contrast to Cyclin D1, ERα levels, as expected, in cells treated with E2 were markedly lower than those observed in untreated cells without substantial alterations in transcript levels (Figure 2d–f). ERα protein levels showed a peculiar pattern in the presence of ActD: the reduced protein levels of ERα in the presence of E2 steadily accumulated, reaching levels comparable to those in the absence of E2, despite the similar ERα transcript levels in the absence or presence of ActD and/or CHX independently of E2 (Figure 2d). This suggests that a pool of ERα transcripts with delayed processing, as reported previously (Horwitz & McGuire, 1978), and/or stored/segregated in an intracellular sub-compartment(s) re-enters translation. On the other hand, treating cells with CHX alone and/or with ActD induced a progressive decline in ERα protein levels with a half-life of about 8 h independent of E2. These observations imply that pre- and post-translational mechanisms primarily mediate the levels of the ERα protein in MCF-7 cells.
Although E2 treatment augmented transcript levels of CXXC5, the pattern of reduction in CXXC5 transcript levels did not differ among cells treated with ActD and/or CHX independently of E2 (Figure 2g). E2 treatment also enhanced CXXC5 protein levels (Figure 2h and i), observed similarly for Cyclin D1. Protein levels of CXXC5 showed a faster decline in the absence of E2 with a half-life of 2 h as opposed to 6 h when cells were treated with E2, showing a statistically significant difference (Figure 2h and i). This suggests that E2 treatment of cells contributes to the stability of the CXXC5 protein. Thus, E2-mediated modulation of the CXXC5 protein levels in cells occurs through post-transcriptional and post-translational processes.
2.3 Degradation of CXXC5 by the ubiquitin-proteasome system (UPS)
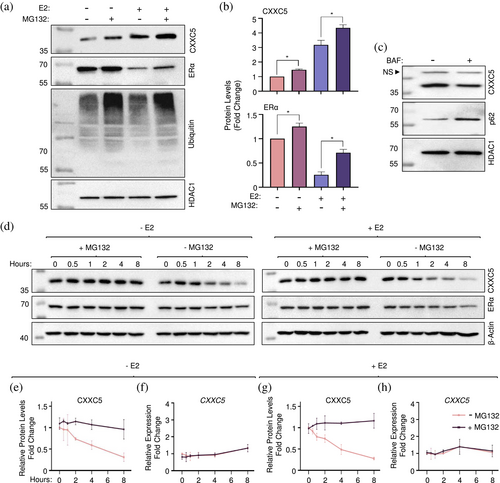
The UPS is responsible for the degradation of the majority of intracellular proteins as a multi-enzyme process encompassing the covalent conjugation of ubiquitin to substrate proteins and their recognition and degradation by the proteasome (Kwon & Ciechanover, 2017; Lecker et al., 2006). To initially examine whether or not CXXC5 protein stability involves the UPS, we used MCF-7 cells synchronized in G0/G1 with CD-FBS for 72 h and treated without or with 10−9 E2 for 36 h. Cells were then incubated in a fresh medium without (DMSO as the vehicle) or with a proteasome inhibitor MG132 at 10 μM concentration, which was based on preliminary studies (Supplementary Information, Figure S3), for 4 h (Figure 3). Collected cells were then processed for and subjected to WB. Similar to ERα, levels of CXXC5 showed an increase in the presence of MG132 (+ MG132) in cells treated without or with E2. This was correlated with the increased detection of ubiquitinated cellular proteins by a ubiquitin antibody in WB (Figure 3a and b). This together with our observation that bafilomycin A1 (BAF), which is an inhibitor of lysosome activity (Gagliardi et al., 1999; Yamamoto et al., 1998) did not restore the reduced levels of CXXC5 but effectively increased levels of the p62 protein, also known as autophagosome cargo protein sequestosome 1 (SQSTM1), a ubiquitin-binding scaffold protein degraded by lysosome (Bjørkøy et al., 2009; Zhu et al., 2016) suggests that CXXC5 is degraded by the UPS (Figure 3c). To further assess the degradation of CXXC5 by the UPS, we examined CXXC5 protein stability in the absence or presence of MG132. We synchronized MCF-7 cells at G0/G1 with the hormone withdrawal and treated cells without or with 10−9 M E2 for 36 h. Cells were then incubated in a fresh medium without (DMSO as the vehicle) or with 10 μM MG132 together with 50 μg/mL CHX to inhibit translation in the absence (− E2) or presence of 10−9 M E2 (+ E2) for 0, 0.5, 1, 2, 4, and 8 h. MG132 effectively blocked time-dependent decreases in CXXC5 levels whether or not the cells were treated with E2 (Figure 3d–h). Thus, CXXC5 is degraded by the UPS.
Since CXXC5 protein levels reached the highest levels within 6 h of E2 treatment, corresponding to the G0/G1 phase of synchronized MCF-7 cells, and remained at similar levels in subsequent phases, we asked whether the degradation of CXXC5 by UPS involves a specific cell cycle phase. To explore this issue, we used a cycle phase enrichment approach (Toker et al., 2025) of synchronized MCF-7 cells (Figure 4). To examine the CXXC5 degradation by UPS at G0/G1, we treated the G0/G1 enriched cell population following 72 h of hormone withdrawal without (Dimethyl Sulfoxide, DMSO, 0.01% as vehicle control) or with MG132 for 4 h. We treated synchronized cells with 10−9 M E2 for 21 h to obtain the S-phase enriched cell population. We then incubated cells for an additional 4 h without or with MG132 in the presence of E2. Nocodazole is an anti-mitotic agent that reversibly interferes with the polymerization of microtubules by binding to β-tubulin, thereby impairing the formation of the metaphase spindles and mitosis (Cheung & Terry, 1980). To enrich the cell population in G2 and M phases, synchronized cells treated with E2 for 21 h were incubated in fresh medium containing 0.3 μM nocodazole, the concentration of which was based on our previous observation (Toker et al., 2025), and E2 for 2 h. Cells were then treated without or with MG132 in the presence of E2 and nocodazole for an additional 4 h. Cells were collected, processed, and subjected to flow cytometry (Figure 4a) and WB (Figure 4b).
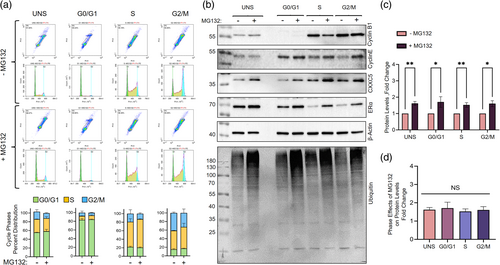
With expected phase distributions following E2 treatment of synchronized MCF-7 cells, MG132 treatment of cell populations enriched at G0/G1, S, and G2/M phases led to an increase in the detection of ubiquitinated cellular proteins with WB using a ubiquitin-specific antibody, indicating compromised proteasome functions. We observed that levels of Cyclin B1 decreased when cells were treated with MG132 in the S phase, likely due to the repression of Cyclin B1 translation (Kim et al., 2011). We also observed that when nocodazole-treated cells to block the M phase exit were also treated with MG132, Cyclin B1 levels remained unaltered, consistent with the importance of proteolysis in mitotic exit (Chesnel et al., 2006; Chow et al., 2011). On the other hand, MG132 treatment prevented the degradation of Cyclin E, ERα, and CXXC5 without an effect on β-actin levels used as control (Figure 4b). Furthermore, changes in CXXC5 levels (Figure 4c) as assessed by the ratio of protein levels in the absence and the presence of MG132 remain similar in cell cycle phases (Figure 4d). This suggests that the UPS-mediated degradation of CXXC5 is independent of cell cycle phases.
2.4 Ubiquitination of CXXC5
Degradation of CXXC5 by the UPS independent of cell cycle phases implies the ubiquitination of CXXC5 in MCF-7 cells. The ubiquitination encompasses a three-step enzymatic cascade involving E1, E2, and E3 enzymes that results in the transfer of ubiquitin to a lysine (K) residue on substrates as well as the removal of ubiquitin modifications from substrates by de-ubiquitinase enzymes, DUBs (Hershko & Ciechanover, 1998; Snyder & Silva, 2021). Due to the dynamic and spatiotemporal regulation of protein ubiquitination (Kliza & Husnjak, 2020; Sun & Zhang, 2022) together with a relatively low abundance of CXXC5 protein in MCF-7 cells, our several attempts to identify endogenously ubiquitinated CXXC5 protein with various approaches yielded no discernible results (Supplementary Information, Figure S5). To circumvent this issue, we used the bioUb approach, which is based on the use of cellular biotinylation of ubiquitin that allows the efficient isolation and enrichment of ubiquitinated proteins due to the strength and the specificity of the avidin–biotin interaction (Franco et al., 2011; Ramirez et al., 2021). The bioUb approach exploits the use of engineered six tandem ubiquitin (Ub6) peptides genetically fused to the BirA enzyme (Franco et al., 2011; Ramirez et al., 2021). Each ubiquitin peptide located amino terminally in the Ub6-BirA proprotein contains the BirA recognition sequence with a single biotin acceptor K (MGLNDIFEAQKIEWHE) (Franco et al., 2011; Ramirez et al., 2021). Upon synthesis, digestion of the Ub6-BirA protein by endogenous DUBs generates single ubiquitin peptides for biotinylation by the BirA, bioUb. Endogenous ubiquitin-conjugating enzymes then incorporate the generated bioUb into protein substrates. To explore the labeling of CXXC5 with bioUb, we used HEK293FT cells, which are E2-nonresponsive and synthesize CXXC5 at low levels (Supplementary Information, Figure S4) and show high efficiencies for transfection and heterologous protein synthesis (Meissner et al., 2001). We transiently transfected HEK293FT cells with the pCAG-(Ub)6-BirA or the pCAG-BirA expression vector (Ramirez et al., 2021) together with the pcDNA3.1(−) expression vector bearing a CXXC5 cDNA with sequences that encode amino-terminally located 3xFlag epitope, 3F, (Ayaz et al., 2021) in the growth medium containing 50 μM biotin and 1 mM ATP for 24 h. Cellular extracts were subjected to precipitation using NeutrAvidin-conjugated agarose beads followed by WB using the Flag, ubiquitin, or biotin antibody. We observed an enrichment of 3F-CXXC5 with distinct molecular masses varying from 40 to 130 kDa with a predominant CXXC5 species of about 55 kDa by the use of the Flag antibody only in the NeutrAvidin precipitated bioUb6-BirA group which also contains highly abundant bioubiquitinated cellular proteins assessed with re-probing the membrane with the ubiquitin and biotin antibodies (Figure 5a–c). As observed in MCF-7 cells (Supplementary Information, Figure S5), we are uncertain about the identity of the protein species migrating at 40 kDa (indicated by a question mark in Figure 5a). This could represent CXXC5 bound to beads, a non-specific protein bound to the beads with electrophoretic mobility similar to CXXC5, or a degraded ubiquitinated form of CXXC5. However, the latter is unlikely, as we see this protein species across all precipitated CXXC5 variants, including the variant lacking lysine residues (KNull, Figure 7). Nonetheless, these findings suggest that CXXC5 is ubiquitinated in HEK293FT cells.
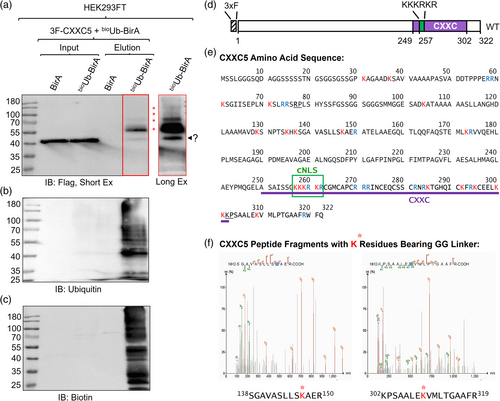
Based on this finding, we attempted to identify ubiquitinated residue(s) in CXXC5. For this, we carried out large-scale sequential immunoprecipitations (IPs) using the Flag antibody fragment-conjugated beads followed by the bioUb pull-down using NeutrAvidin-conjugated agarose beads of cell extracts transfected with expression vectors bearing 3F-CXXC5 and (Ub)6-BirA cDNAs to enrich the bioUbi-CXXC5. Samples were then subjected to SDS-10% PAGE, stained with Coomassie blue, and the stained protein bands on the gel were excised. Samples were in-gel digested with trypsin, and the generated peptides were subjected to liquid chromatography–tandem mass spectrometry (LC–MS/MS) to detect peptide remnants derived from ubiquitin.
Trypsin cleaves specifically peptide bonds at the carboxyl side of K and arginine (R) residues except for when K or R is followed by proline, P, (Manea et al., 2007). The carboxyl-terminus of the mature ubiquitin terminates with 71LRLRGG76 amino acid sequence in which the last Gly76 residue is conjugated to primarily K residues (Kelsall, 2022; McClellan et al., 2019) on target proteins (Pickart, 2001; Xu & Jaffrey, 2013). In addition to target proteins, the peptide bonds at the carboxyl side of R74 at the carboxyl-terminus of ubiquitin, when conjugated to target proteins or in free form, are also cleaved by trypsin. This cleavage leaves two glycine residues, G75G76, on the conjugated lysine residues of substrates, generating “ubiquitin signature peptides” (Kirkpatrick et al., 2005; Peng et al., 2003) with a monoisotopic mass of 114.04 Da on the modification sites. Although rare, trypsin also cleaves the peptide bond between the penultimate R72 and Leu73 (L) residues of the carboxyl-terminus of ubiquitin conjugated to a substrate. This generates an LRGG peptide with a monoisotopic mass of 383.23 Da (Kirkpatrick et al., 2005; Peng et al., 2003). These unique K-conjugated ubiquitin remnants are used to identify the ubiquitination sites through mass spectrometry. It should be noted that the G75G76 remnant on ubiquitinated peptides after tryptic digestion is not unique to ubiquitin but is shared with NEDD8 and ISG15, members of the ubiquitin-like protein family, UBL (Akimov et al., 2018). This necessitates the use of ubiquitination-specific approaches, including bioubiquination as we utilized here for identifying ubiquitinated K residue(s). CXXC5 contains ten K residues (red font in Figure 5e) and seven R residues (blue font in Figure 5e) at the amino terminus, and seven K and seven R residues in the CXXC domain at the carboxyl terminus of the protein. In addition, four K residues and two R residues are present in a region between the amino terminus and the CXXC domain of CXXC5 that contains the canonical monopartite nuclear localization signal (cNLS, 258KKKRKR262) (Lange et al., 2007). The locations of both K and R residues in CXXC5 present 34 possible trypsin cleavage sites, assessed with PeptideCutter (https://web.expasy.org/peptide_cutter/) with two exceptions: 77R and 301K, which are followed by proline residues. This potentially generates many cleaved single K residues by trypsin with possible ubiquitin signatures along with peptides with lengths ranging from two to more than 30 amino acids. Since the optimal peptide length for mass spectrometric detection of peptide fragments is 8–25 amino acids (Ahrens et al., 2022), the frequency and location of trypsin cleavage sites rendered the identification of ubiquitinated K residues of 3F-CXXC5 tryptic peptides difficult. Nevertheless, LC–MS/MS results revealed two tryptic CXXC5 peptides containing K residues that bear ubiquitin signatures: K147 in 138SGAVASLLSKAER150 and K309 in 302KPSAALEKVMLPTGAAFR319 peptides. Previous studies on the ubiquitome landscape of cell models reported that CXXC5 is one of the ubiquitinated proteins containing ubiquitinated K147 in 138SGAVASLLSKAER150 (Akimov et al., 2018; Udeshi et al., 2013) and K292 in 286TGHQICKFR294 peptide sequence (Akimov et al., 2018). Confirming the ubiquitination of K147 and identifying the unique K309 ubiquitination, our results, together with others', indicate that CXXC5 can be ubiquitinated at multiple K residues located in both the amino-terminus region and the carboxyl-terminus CXXC domain.
2.5 Verification of ubiquitination sites of CXXC5
To verify the ubiquitination of K147 and/or K309 in CXXC5 and assess the effects on protein stability, we generated CXXC5 mutants by replacing each identified positively charged K residue alone or in combination with structurally similar positively charged R residue with site-directed mutagenesis using overlapping PCR (Li et al., 2008) and cloned into the pcDNA3.1(−) expression vector. In these constructs, we used the HA tag (YPYDVPDYA) rather than the 3xFlag tag, 3F (DYKDHDGDYKDHDIDYKDDDDK) bearing a CXXC5 cDNA since 3F contains four K residues (in boldface) that could potentially be ubiquitinated, as we observed in preliminary studies (Supplementary Information, Figure S7). We transiently transfected HEK293FT cells with the expression vector bearing WT HA-CXXC5 or a K to R changed mutant HA-CXXC5 (R147, R309, or R147/309) cDNA together with the pCAG-(Ub)6-BirA expression vector in the growth medium containing 50 μM biotin and 1 mM ATP for 24 h (Figure 6a). Cellular extracts of WT, R147, R309, or R147/309 synthesizing cells assessed with WB using the HA antibody (Figure 6b) were subjected to precipitation using NeutrAvidin-conjugated agarose beads followed by WB using the HA or Biotin antibody. We observed that mutations alone or in combination did not prevent the ubiquitination of the variant proteins compared with WT HA-CXXC5 (Figure 6c). These results suggest that the remaining K residue(s) in mutant CXXC5 proteins are likely to undergo ubiquitination and/or become new targets for ubiquitination. To assess whether or not mutations alter the extent or pattern of stability of CXXC5 variant proteins, MCF-7 cells, grown in steady-state conditions, were transiently transfected for 48 h. Cells were incubated in a fresh medium with 50 μg/mL CHX for 0, 0.5, 1, 2, 4, and 8 h. Cellular extracts were subjected to WB using the CXXC5 or HA antibody (Figure 6d). Due to the molecular mass of the HA tag at the amino-termini, CXXC5 variants display slower electrophoretic migration in WB. This provides an opportunity to comparatively assess the degradation of both the endogenous (en) CXXC5 and HA-tagged CXXC5 variants synthesized in transfected cells (tr) in WB, which are also verifiable with the HA antibody. Quantitative analyses of the WB results normalized to the levels of β-actin as the loading control revealed that R147, R309, and R147/309 mutant CXXC5 proteins show stability patterns similar to that observed with the WT CXXC5 (Figure 6e). These results are consistent with our conclusion that the remaining K residue(s) in mutant CXXC5 proteins are, or become, targets for ubiquitination and subsequent changes in stability.
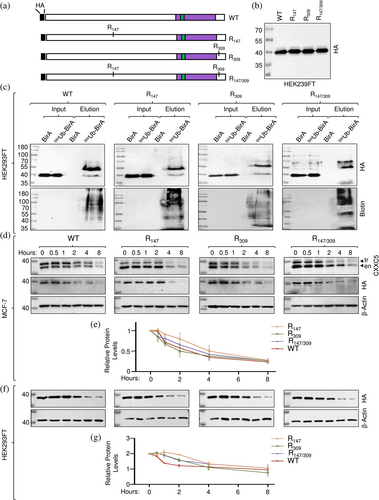
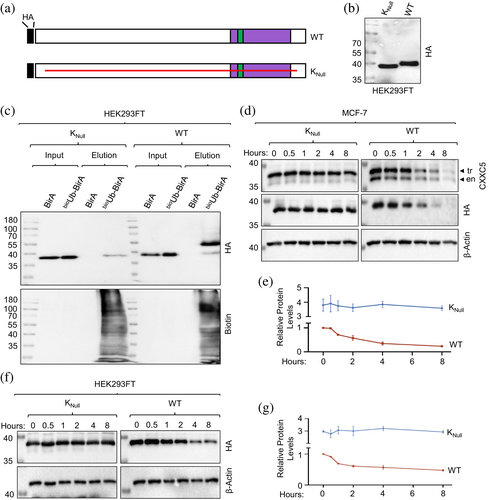
Due to the number (21) of K residues in CXXC5, the mutation of each K residue alone or in combination presents an overwhelming task. We reasoned that the positional conservation of the K residues observed in LC–MS/MS analysis alone or in combination in an otherwise K-null mutant CXXC5 protein (KNull) in which every other K residue in CXXC5 is changed to an R residue would allow us to assess the ubiquitination of a specific K residue. Since CXXC5 is predominantly localized in the nucleus and the monopartite canonical NLS (cNLS) of CXXC5 contains four K residues (258KKKRKR262), we initially assessed whether or not KNull bearing the canonical NLS sequence, KNull-cNLS, is localized to the nucleus in transiently transfected HEK293FT cells for 24 h or MCF-7 cells for 48 h, at times when transgene synthesis becomes maximal (data not shown). While, as expected, KNull-cNLS localized to the nucleus of both MCF-7 and HEK293FT cells, Knull-cNLS was also ubiquitinated in HEK293FT cells as assessed with bioUb, suggesting that K residues in the cNLS could undergo ubiquitination (Supplementary Information, Figure S8). To circumvent this issue, we initially examined whether or not changing K residues in the cNLS would alter the intracellular localization of CXXC5. For this, we generated CXXC5 mutants (Supplementary Information, Figure S6A). Of the mutants, WTM-NLS bears random amino acid replacements in the cNLS, changing the 258KKKRKR262 sequence to 258AASGGS262. We observe that WTM-NLS largely localizes to the cytoplasm in transiently transfected HEK293FT and MCF-7 cells (Supplementary Information, Figure S6B). Besides cNLS, several proteins, including capsid protein 1, VP-1 (Cheng et al., 2019), contain an NLS that bears no K but R residues critical for localization to the nucleus (Lu et al., 2021). Based on these findings, we assessed whether the 258RRRRRR262 sequence instead of the 258KKKRKR262 sequence produces a mutant CXXC5 protein that localizes to the nucleus. We transiently transfected cells with the expression vector bearing WT HA-CXXC5 (WTR-NLS) or the KNull CXXC5 mutant with the 258RRRRRR262 NLS motif (KNull) cDNA. As WTR-NLS, KNull localized to the nucleus in transiently transfected HEK293FT and MCF-7 cells (Supplementary Information, Figure S6B), suggesting that the 258RRRRRR262 sequence is a monopartite NLS motif. We further verified these results with a GFP protein that bears the MRRRRRR motif at the amino-terminus, GFPR-NLS. While the WT GFP showed diffuse intracellular staining encompassing the cytoplasm and nucleus, GFPR-NLS predominantly localizes to the nucleus (Supplementary Information, Figure S6B).
Based on these results, we assessed the ubiquitination of HA-KNull mutant CXXC5, KNull, in comparison with WT HA-CXXC5, WT, using the bioUb approach in transiently transfected HEK293FT cells (Figure 7a–e). Although the estimated molecular mass of the KNull mutant is comparable to that of WT, KNull shows an electrophoretic migration somewhat faster than the WT in WB using the HA antibody (Figure 7b). Precipitation of protein extracts of transiently transfected HEK293FT cells with the bioUb approach using NeutrAvidin-conjugated agarose beads followed by WB using the biotin antibody indicates that KNull is not ubiquitinated in contrast to WT HA-CXXC5 (Figure 7c). Furthermore, we find in transiently transfected MCF-7 cells treated with 50 μg/mL CHX for 0, 0.5, 1, 2, 4, and 8 h that KNull is stable compared to WT HA-CXXC5 (tr) or endogenous CXXC5 (en) assessed with the CXXC5 or HA antibody (Figure 7d and e), as we similarly observed in transiently transfected HEK293FT cells assessed with the HA antibody (Figure 7f and g). These results suggest that K residues of CXXC5 are targets for ubiquitination and are critical for the stability of the protein.
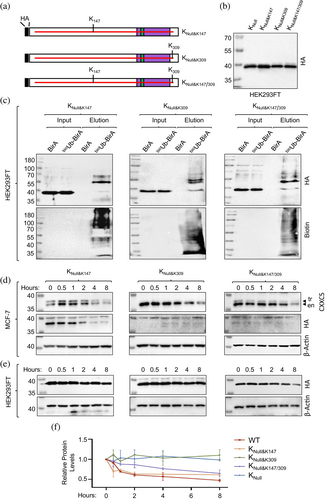
We then wanted to verify the ubiquitination of K147 and/or K309 by changing R147 and/or R309 residues in the KNull mutant back to K residue(s) (Figure 8a–f). We precipitated synthesized CXXC5 variants (Figure 8b) in cellular extracts from HEK293FT cells transiently co-transfected with expression vectors bearing bioUb and CXXC5 variant cDNAs for 24 h with NeutrAvidin-conjugated agarose beads, followed by WB using the HA or Biotin antibody. Results show that mutant CXXC5 protein bearing K147 (KNull&K147), K309 (KNull&K309), and K147/309 (KNull&K147/309) indeed undergo ubiquitination (Figure 8c) similar to WT but in contrast to KNull (Figure 7). To examine the effects of ubiquitination on the stabilities of K147, K309, and K147/309 proteins, we transiently transfected MCF-7 cells, grown in steady-state conditions, for 48 h. Cells were then incubated in a fresh medium with 50 μg/mL CHX for 0, 0.5, 1, 2, 4, and 8 h. Cellular extracts were subjected to WB using the CXXC5 or HA antibody. We utilized protein levels of β-actin for the loading control in WB using the β-actin antibody (Figure 8d). Quantitative analysis of WBs indicates that KNull&K147 exhibits a degradation pattern similar to that of the endogenous CXXC5. However, we observed little to no synthesis of K309 and KNull&K147/309 in WBs (Figure 8d), whether or not the cells were treated with MG132 for 8 h (Supplementary Information, Figure S9A). As in MCF-7 cells, KNull&K147 was similarly degraded in HEK293FT cells, whereas KNull&K309 was notably stable (Figure 8e and f). In contrast, KNull&K147/309 is degraded more slowly than KNull&K147 (Figure 8e and f). These findings suggest that ubiquitination at K147 is essential for CXXC5 degradation, whereas K309 alone does not influence degradation but mitigates the impact of K147 on CXXC5 degradation in HEK293FT cells. We did not detect KNull&K309 and KNull&K147/309 proteins even in simultaneous transient transfections in MCF-7 cells under the same conditions with identical reagents carried out with HEK293FT cells. The failure of the detection of proteins was not due to the absence of transcripts (Supplementary Information Figure S9B). This suggests that KNull&K309 or KNull&K147/309 are insufficiently translated or undergo co-translational degradation in MCF-7 cells. These findings also indicate that the ubiquitination of specific K residue(s) involved in CXXC5 degradation is cell-type dependent.
3 DISCUSSION
Our results using the bioUb approach followed by sequential IP coupled MS analyses indicate that CXXC5 is a ubiquitinated protein, and the ubiquitination contributes to the stability and degradation of the protein by the UPS.
Our observations that despite a steady increase in CXXC5 transcript levels in response to E2 throughout cell cycle phases, the augmented CXXC5 protein levels reach a plateau in the early G1 phase suggest a post-transcriptional regulation, which is critically involved in the modulation of CXXC5 levels. It is well established that dynamic and integrated multistep processes modulate the abundance of proteins. Although transcription, splicing, polyadenylation, export, storage, and stability of transcripts, as well as cis-regulatory elements within transcripts and trans-acting factors interacting with cis-elements, including ribosomes, are critical contributors to protein abundance, the dynamics of degradation in response to various physiological conditions ultimately determine protein levels in cells (Corbett, 2018; Rusilowicz-Jones et al., 2022). Referred to as translational buffering or translational offsetting, emerging evidence suggests that protein levels can be maintained at constant levels despite changes in mRNA abundance (Larsson et al., 2010; Lorent et al., 2019) due to distinct features of transcript cis-elements, including 5' untranslated regions and activity of modified tRNAs as trans-factors (Lorent et al., 2019). The structural and functional characteristics of CXXC5 transcripts remain unclear. However, specific features of CXXC5 transcripts could enable rapid and efficient protein synthesis in response to E2. This could involve a selective entrance of available CXXC5 mRNAs into the translational process and/or a selective increase in the translation efficiency of the transcripts. It is also possible that the release of stored CXXC5 transcripts from cellular compartments, such as membrane-less cytoplasmic ribonucleoprotein (RNP) assemblies, including processing bodies (PBs), upon the presence of E2 may facilitate CXXC5 protein synthesis independently of transcription. This possible mechanism may also explain our observation that, when transcription is blocked, the reduced ERα protein levels in response to E2 gradually accumulate, eventually reaching levels similar to those seen in the absence of E2. Although CXXC5 overexpression has been reported as an unfavorable prognostic indicator in breast cancer (Ayaz et al., 2020; Fang et al., 2018; Knappskog et al., 2011; Li et al., 2023; Wang et al., 2023), our findings together with a previous work (Wang et al., 2023) suggest that CXXC5 protein levels may also serve as a useful prognostic marker in this disease.
Our findings that the rapid rise in CXXC5 levels within 6 h in response to E2 corresponds to the G1 phase levels off in subsequent phases despite increases in CXXC5 transcript levels also imply post-translational control, including the ubiquitination of the protein, as we show here. Ubiquitination is a dynamic and spatiotemporally modulated co- and post-translational modification that regulates cellular processes through proteolytic and nonproteolytic mechanisms, including proteasomal degradation and proteostasis, selective autophagy, cell signaling cascades, protein trafficking, DNA repair and genome integrity, cell cycle control, and programmed cell death (Hershko & Ciechanover, 1998; Snyder & Silva, 2021). Conjugation of ubiquitin as a monomer to one K residue on substrate proteins generates monoubiquitination; whereas, multi-monoubiquitination is the attachment of a single ubiquitin to multiple K residues on a substrate protein. Conversely, the attachment of ubiquitin molecules in a linear fashion to a single lysine (K) residue on a substrate protein results in the formation of a polyubiquitin chain. Due to the seven K residues of ubiquitin, polyubiquitination can generate linear or branched chains with different topologies. Polyubiquitination primarily marks proteins for post-translational degradation through UPS; mono- and multi-monoubiquitinated proteins can also be degraded by proteasomes (Braten et al., 2016). Proteins are also degraded co-translationally. Co-translational ubiquitination is a robust process, and a substantial fraction of polyribosome-associated nascent polypeptides are ubiquitinated during translation as a reflection of a protein quality control system that monitors protein folding during translation. This can trigger the initiation of degradation of a protein before its synthesis is complete (Ha et al., 2016; Wang, Durfee, & Huibregtse, 2013).
Our LC–MS/MS results indicate that K147 and K309 of CXXC5 are ubiquitinated. The possible cell-type-specific K ubiquitination notwithstanding, previous studies on the ubiquitome landscape of cell models reported that in addition to K147 (Akimov et al., 2018; Udeshi et al., 2013), the K292 residue of CXXC5 is also ubiquitinated (Akimov et al., 2018). These findings collectively suggest that CXXC5 can undergo ubiquitination at K147, K292, and K309 residues. However, the extent of CXXC5 ubiquitination at these K residues is likely underestimated by current approaches. As discussed in the Results section, this underestimation is due to 1) the number of K residues in CXXC5, 2) the potential cleavage sites by trypsin, influenced by the locations of K and R residues that are susceptible to enzymatic digestion, 3) the generation of single K residues upon trypsin digestion, which may carry ubiquitin signatures, along with generated peptide fragments ranging from two to more than 30 amino acids in CXXC5. These factors likely made the identification of ubiquitinated K residues in CXXC5 difficult through LC–MS/MS, which is most effective for detecting peptide fragments ranging from 8 to 25 residues (Ahrens et al., 2022). Nevertheless, we also find here that K147 and/or K309 residues of CXXC5 are ubiquitinated. Although the type or nature of ubiquitination is unclear, these ubiquitinated K residues are involved in the stability of the protein in a manner specific to cell type. We observed that the KNull mutant bearing K147, KNull&K147, is ubiquitinated and undergoes degradation by UPS similar to the WT CXXC5 in both HEK293FT and MCF-7 cells. On the other hand, the mutant CXXC5 proteins bearing K309 alone, KNull&K309, or together with K147, KNull&K147/309 that are ubiquitinated are rapidly degraded in MCF-7 cells, in contrast to HEK293FT cells wherein both mutant proteins are relatively stable compared to WT CXXC5. Although the mechanism(s) is unclear, the folding of carboxy-terminally located K309 could differ dramatically from other KNull variants, leading to the recognition by the protein quality control system of MCF-7 cells that initiates ubiquitination and rapid degradation of the variant. Whatever the mechanism might be, our findings indicate that the ubiquitination of specific lysine residue(s) on CXXC5 may serve as a key factor in regulating the protein's rapid degradation when it is not needed or when its levels surpass the physiological threshold necessary for proper function in MCF-7 cells.
Previously, we suggested that E2-responsive CXXC5 binds to unmethylated CpG dinucleotide and acts as a nucleation factor in modulating target gene expressions critical for E2-mediated cellular proliferation in cell models synthesizing ERα (Ayaz et al., 2020; Yaşar et al., 2016). Gene expression relies on TFs that bind to specific sequences in gene promoters or enhancers. Insightful kinetic studies using a well-characterized E2-responsive TFF1/pS2 promoter indicate that the cyclic engagement of ERα with DNA directs and achieves the sequential and combinatorial assembly of transcriptional complexes, including ubiquitin ligases and regulatory components of the proteasome on the gene promoter (Reid et al., 2003; Zhang et al., 2006). Subsequent ubiquitination and proteasome-mediated turnover of ERα, as well as associated co-factors, contribute to the duration and magnitude of transcriptional output (Reid et al., 2003; Zhang et al., 2006). It is therefore plausible that the stability of CXXC5, which increases in response to E2 during early G1, is primarily controlled by ubiquitination when CXXC5 is bound to DNA. This may occur through enhanced or reduced interactions between CXXC5 and co-regulatory proteins, ensuring the initiation of transcription and subsequently promoting the dissociation of CXXC5 from DNA and its degradation via the UPS. This process is likely essential for fine-tuning target gene expression during cell cycle transitions, ultimately influencing cell proliferation, issues that we are currently exploring.
In summary, we find that although E2 enhances both the transcription and synthesis of CXXC5 during the G1 phase in synchronized MCF-7 cells, CXXC5 levels are primarily regulated by ubiquitination, independent of cell cycle phases. We report that multiple K residues of CXXC5 can be ubiquitinated, and ubiquitinated K residues facilitate the degradation of CXXC5 via the UPS. A better understanding of the interdependency of ubiquitination and gene expressions mediated by CXXC5 could provide novel avenues for the development of new therapeutic approaches in E2-responsive ERα-synthesizing target tissues, including the breast, wherein CXXC5 critically contributes to E2-driven cellular growth.
4 MATERIALS AND METHODS
4.1 Reagents
17β-estradiol (E2; Cat # E2257) and propidium iodide (Cat # P4170) were purchased from Sigma-Aldrich Inc. (MO, USA). Nocodazole (Cat # 31430–18-9) was obtained from Merck (Darmstadt, Germany). Antibodies for ERα (Cat # sc-543), Cyclin B1 (Cat # sc-245), Cyclin E (Cat # sc-247), and HDAC1 (Cat # sc-81598) were obtained from Santa Cruz Biotechnology (SCBT, Santa Cruz, CA, USA). The antibody for the Flag tag was from Sigma-Aldrich Inc. (Cat # F3165). The antibody specific to the HA tag (Cat # 923501) was purchased from Biolegend Inc. (CA, USA). The Ubiquitin antibody (P4D1, Cat # 3936S) and MG132 (Cat # 2194) were obtained from Cell Signaling Technology Inc. (MA, USA). The antibody specific for β-Actin (Cat # ab8227) and biotin (Cat # ab53494) were obtained from Abcam, Inc. (UK). We purchased the antibody for CXXC5 (Cat # 16513-1-AP) from Proteintech Inc. (IL, USA). RNase A (Cat # EN0531) was obtained from ThermoFisher Scientific Inc. (Waltham, MA, USA). Goat anti-rabbit (Cat # R-05072) and goat anti-mouse (Cat # R-05071) secondary antibodies conjugated with horseradish peroxidase, and the WesternBright ECL kit (Cat # K-12045-D50) were obtained from Advansta Inc. (CA, USA). Triton X-100 (Cat # A4975) was purchased from AppliChem Inc. (Darmstadt, Germany). Protease Inhibitor, PI (Cat # 11836170001) and Phosphatase Inhibitor, PhosSTOP (Cat # 4906845001) were obtained from Roche Inc. (Basel, Switzerland).
The molecular mass marker was Pageruler Prestained Protein Ladder (ThermoFisher, Cat # 26616) or Pageruler Plus Prestained Protein Ladder (ThermoFisher, Cat # 26619).
E2 was dissolved in Ethanol (Sigma-Aldrich, Cat # 1.00983) to 10−3 M as the stock concentration and kept at-20°C.
4.2 Generation of mutant CXXC5 cDNAs
To assess the intracellular location, ubiquitination, and protein stabilities of CXXC5 mutant proteins, we used overlapping PCR (Li et al., 2008) with primer sets (Supplementary Information, Table for Primers) specific to the 3F- or HA-CXXC5 cDNA.
4.3 Cell growth
The growth and maintenance of MCF-7 and HEK293FT cells were described previously (Ayaz et al., 2020, 2021; Yaşar et al., 2016). In brief, MCF-7 cells were cultured in high glucose (4.5 g/L) containing Dulbecco's modified Eagle's medium without phenol red (DMEM, Sartorius, Cat # 01-053-1A) supplemented with 10% fetal bovine serum (FBS, Sartorius, Cat # 04-007-1A), 1.2% L-Glutamine (Sartorius, Cat # 03-020-1B) and 1% Penicillin–Streptomycin (Sartorius, Cat # 03-031-1B). HEK293FT cells were cultured in high glucose (4.5 g/L) in DMEM supplemented with 10% FBS, 1.2% L-Glutamine, 1% Penicillin–Streptomycin, 2% Sodium Pyruvate (Sartorius, Cat # 03-042-1B) and 2% MEM Non-Essential Amino Acids Solution (Sartorius, Cat # 01-340-1B).
4.4 Synchronization of cell cycles
To minimize the effects of mitogenic estrogens on cellular proliferation in cell cycle synchronizations, we culture MCF-7 cells in DMEM supplemented with 10% charcoal dextran-stripped fetal bovine serum (CD-FBS) as described previously (Ayaz et al., 2020). For this, MCF-7 cells (7.5 × 105) were plated in T-25 tissue culture flasks in DMEM medium without phenol red supplemented with 10% CD-FBS for 72 h with media refreshing at 48 h. Cells were then incubated in the corresponding growth medium containing 10% CD-FBS without (0.01% ethanol, EtOH) or with 10−9 M E2 for 3- to 6-h intervals up to 36 h to test the effects of E2 on cell cycle progression. At the termination, cells were collected with trypsinization. Cells were then subjected to flow cytometry, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and western blot (WB) analyses.
4.5 Flow cytometry
Cell cycle distribution was assessed with flow cytometry as we described previously (Ayaz et al., 2020; Turan et al., 2024). Briefly, cells were washed with 1× PBS and pelleted. Cells were gently re-suspended in 100 μL of 2% CD-FBS containing 1× PBS, fixed, and permeabilized with ice-cold 70% ethanol overnight. After pelleting, cells were resuspended with 200 μL of 1× PBS containing 20 μg/mL propidium iodide, 200 μg/mL RNase A, and 0.4% (v/v) Triton X-100 for 30 min at room temperature. Cell cycle analyses were carried out with flow cytometry (NovoCyte Flow Cytometer; Agilent Technologies, CA, USA). NovoExpress Software was used during sample acquisition and for data analysis.
4.6 RT-qPCR
Total RNA from MCF-7 cells was obtained with a Macherey-Nagel™ Total RNA Isolation kit (Macherey-Nagel, Germany, Cat # 740955.50) according to the manufacturer's instructions. In brief, cells were lysed with the kit's lysis buffer, and the lysate was cleaned using NucleoSpin Filter columns prewashed with ethanol. The column was desalted with a desalting buffer and on-column DNase treatment was carried out for 45 min. The column was washed, and RNA was eluted into RNase and DNase-free H2O. Total RNA quantification and purity assessment were done with a NanoDrop 2000 (Thermo Fisher Scientific, US). Total RNA from cells was used for cDNA synthesis using the RevertAid First Strand cDNA Synthesis Kit (Thermo-Fisher, Cat # K-1621). The SYBR Green Mastermix (BioRad, Hercules, CA, USA) and gene-specific primers (Supplementary Table S1) were used for RT-qPCR reactions on BioRad Connect Real-Time PCR, as we described previously (Ayaz et al., 2020, 2021; Yaşar et al., 2016). The relative quantitation of gene expressions was assessed with the comparative 2−ΔΔCT method (Livak & Schmittgen, 2001). qPCR results were normalized to the geometric means (Vandesompele et al., 2002) of the transcript levels of RPLP0 (60 S acidic ribosomal protein P0) and PUM1 (Pumilio RNA Binding Family Member 1) as reference genes in breast carcinoma cell models (Lyng et al., 2008). In RT-qPCR experiments, the MIQE Guidelines were followed (Bustin et al., 2009).
4.7 Western blot
Cells were lysed with RIPA lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH: 8.0, 1% NP-40, 0.5% Sodium deoxycholate and 0.1% SDS) supplemented with 1xProtease Inhibitor (PI, Roche, Switzerland, Cat # 11836170001) and 1xPhosphatase Inhibitor (PhosSTOP, Roche, Switzerland, Cat # 4906845001). The lysates were then sonicated for 1 min (100 mA amplitude) followed by a centrifugation at 16,000 × g for 20 min. Supernatants were collected, and proteins were quantified using Quick Start™ Bradford Protein Assay (Bio-Rad Laboratories, USA, Cat # 5000201). Equal amounts of protein extracts (30 μg) were then subjected to SDS 10% PAGE at 100 V for about 2 h. Proteins on the gel were transferred to a PVDF membrane (MilliporeSigma, USA, Cat # 3010040001) using a wet-transfer system at 100 V for 70 min. The membrane was blocked with 5% skim milk in 0.1% Tris Buffered Saline-Tween (TBS-T), followed by incubation with an antibody specific for Flag, HA, CXXC5, ERα, Ubiquitin, Biotin, HDAC1, or β-actin. Membranes were incubated with an HRP-conjugated anti-mouse or anti-rabbit (Advansta Inc., USA, Cat # R-05072-500) secondary antibody. Proteins were visualized with the WesternBright ECL kit, and images were captured with the ChemiDocTM Imaging System (Bio-Rad Laboratories). Quantifications of images were done using Bio-Rad Image Lab Software.
4.8 Monitoring RNA and protein synthesis for the determination of optimal concentration and treatment duration of the protein synthesis inhibitor cycloheximide (CHX) and the RNA synthesis inhibitor actinomycin (ActD) in MCF-7 cells
As a measure of de novo RNA synthesis, we assess the incorporation of 5-Ethynyl-uridine (5-EU) into nascent RNA (Supplementary Information, Figure S2). MCF-7 cells were seeded at 15 × 103 cells/well in 48-well plates in DMEM supplemented with 10% FBS for 48 h. The growth medium contained none (0.001% 1× PBS as the vehicle control) or 0.5, 1, 1.5, and 2 mM 5-EU for 6 h. To assess the newly synthesized protein, we used L-Homopropargylglycine (L-HPG). For this, MCF-7 cells (15 × 103 cells/well in 48-well plates) grown in DMEM medium supplemented with 10% FBS for 48 h were preincubated in methionine/cysteine-free DMEM (Thermo-Fisher, Cat # 21013024) supplemented with 10% FBS for 1 h. Cells were then incubated with the same medium containing none (DMSO, 0.001% as the vehicle control) or 3, 6, 12, and 25 μM L-HPG for 6 h. Following 5-EU or L-HPG incubation, cells were subjected to a Click-IT reaction. For this, cells were washed once with 3% BSA in 1× PBS and fixed with 3.7% Formaldehyde in 1× PBS for 15 min at RT. Cells were treated with 0.5% Triton-X in 1× PBS for 20 min at RT. Cells were washed twice with 3% BSA in 1× PBS and incubated in the Click-IT reaction buffer (20 μM Sulfo-Cy5 Azide, 100 mM sodium ascorbate, 2 mM CuSO4, and 10 mM THPTA, tris-hydroxypropyltriazolylmethylamine, in 100 mM sodium phosphate buffer, pH: 7) for 45 min at RT on an orbital shaker. Cells were washed twice with 3% BSA in 1xPBS and incubated in 1× PBS containing 20 nM DAPI solution for 10 min at RT on an orbital shaker. After the final wash twice with 1% BSA in 1× PBS, cells were visualized in the washing solution with a fluorescent microscope (Supplementary Information, Figure S2).
After the determination of the optimal concentration of 5-EU (1 mM) or L-HPG (12 mM), we assessed the optimal concentration of ActD for blocking RNA synthesis. For this, MCF-7 cells maintained in the growth medium for 48 h were treated without (DMSO, 0.001% as control for ActD) or with 0.5, 1, 2, 4, and 8 μM ActD in the presence of 1 mM 5-EU for 6 h. Cells were then subjected to the Click-IT reaction and processed for fluorescence microscopy. For the determination of optimal CHX concentration on protein synthesis, MCF-7 cells were treated without (EtOH, 0.001% as the vehicle control for CHX) or with 12.5, 25, and 50 μg/mL CHX in the presence of 12 mM L-HPG for 6 h. Cells were subjected to the Click-IT reaction and processed for fluorescence microscopy. The results indicate that the optimal concentration of ActD to prevent RNA synthesis is about 5 μM; 50 μg/mL CHX is the optimal concentration to repress protein synthesis.
To assess the pretreatment (PT) duration of MCF-7 cells with ActD or CHX required for blocking RNA or protein synthesis, cells were preincubated without (DMSO or EtOH, as the control) or with the optimal concentration of 5 μM ActD or 50 μg/mL CHX for 0, 15, and 30 min before the addition of 1 mM 5-EU or 12 μM L-HPG. Cells were then subjected to the Click-IT reaction and processed for fluorescence microscopy. The results indicate that the simultaneous addition, 0 h, of ActD with 5-EU prevents RNA synthesis or of CHX with L-HPG blocks protein synthesis, suggesting that the effects of ActD on RNA synthesis or of CHX on protein synthesis are immediate.
To ensure that CHX treatment does not affect RNA synthesis, we co-treated cells with 1 mM 5-EU and 50 μg/mL CHX for 6 h. We also co-treated cells with 12 μM L-HPG and 5 μM ActD to examine whether or not ActD affects protein synthesis for 6 h. Cells were then processed for the Click-IT reaction and subjected to fluorescent microscopy. The results indicate that CHX and ActD at the optimal concentrations do not show cross-reactivity. Co-treatment of cells with 1 mM 5-EU and 12 μM L-HPG for 6 h in the presence of 5 μM ActD and 50 μg/mL CHX blocks both RNA and protein synthesis.
4.9 Ubiquitin pull-down using the bioUb approach
HEK293FT cells were transiently co-transfected with pcDNA 3F-CXXC5 vector (0.5 μg/well of six-well plates) and pCAG expression vector (1.5 μg/well of six-well plates) bearing bioUb6-BirA or BirA cDNA in the growth medium supplemented with 50 μM biotin and 1 mM ATP to ensure biotinylation for 24 h. Cells were then collected and pelleted. Cells were lysed with a lysis buffer containing 3 M Urea, 1%SDS, 100 mM N-Ethylmaleimide (NEM), 1× PI, and 1× PhosSTOP. Following a brief sonication, the lysate was centrifuged at 16,000 × g for 20 min and the supernatant was collected. Protein content was quantified with Quick StartTM Bradford Protein Assay. Protein samples (3000 μg) were subjected to pull-down using NeutrAvidin Agarose beads (ThermoFisher, Cat # 29201) for 1 h at RT followed by an incubation for 2 h at 4°C. Beads were sequentially washed with wash buffer (WB) WB1 (8 M Urea, 0.25% SDS in PBS), WB2 (6 M Guanidine Hydrochloride in PBS), WB3 (6.4 M urea, 1 M NaCl, 0.2% SDS in PBS), WB4 (4 M urea, 1 M NaCl, 10% isopropanol, 10% ethanol, 0.2% SDS in PBS), WB5 (8 M urea, 1% SDS in PBS) and WB6 (2% SDS in PBS). Proteins bound to beads were eluted with 4X Laemmli SDS loading buffer (200 mM Tris–HCl pH 6.8, 8% SDS, 40% Glycerol, 0.8 mg/mL Bromophenol blue, and 100 mM DTT). The eluted samples were run on SDS-10%PAGE and WB was performed with the Flag, HA, CXXC5, Biotin, or Ubiquitin antibody.
4.10 LC–MS/MS of bioUb-CXXC5 sequentially immunoprecipitated from transiently transfected HEK293FT cells
To assess the ubiquitination of 3F-CXXC5 by the use of the bioUb approach, we carried out large-scale (10 × T75 flasks) transient transfections in HEK293FT cells. Cellular extracts were immunoprecipitated with the Flag antibody followed by the biotin antibody. Precipitates were then subjected to an SDS-10% PAGE, and the gel was stained with Coomassie Staining solution (0.1% Coomassie Blue G250, 1 M ammonium sulfate, 30% methanol, 3% o-phosphoric acid). Protein bands ranging between 40 and 100 kDA were excised. Gel samples were subjected to an in-gel digestion with trypsin at the EMBL Proteomics Core Facility (Heidelberg, Germany). Peptides were then analyzed using nanoAcquity UPLC (Waters) with a nanoAcquity trapping (nanoAcquity Symmetry C18, 5 μm, 180 μm × 20 mm) and analytical column (nanoAcquity BEH C18, 1.7 μm, 75 μm × 200 mm), which was coupled to an LTQ Orbitrap Velos Pro (ThermoFisher) using the Proxeon nanospray source. For data analysis, only peptides corresponding to semi-trypsin digests were considered.
4.11 Co-immunoprecipitation (IP)
MCF-7 cells were transiently transfected with the pcDNA 3F-CXXC5 vector (1 μg/well of six-well plates) in the growth medium for 48 h. Cells were collected and pelleted. Nuclear protein isolation was carried out with a NE-PER protein extraction kit (ThermoFisher, Cat # 78833) containing 1x PI and 1x PhosSTOP. Extracts were quantified with the Quick Start™ Bradford Protein Assay. Protein lysates (500 μg) were subjected to precleaning using 25 μL Protein A (New England Biolabs, Cat # S1425S) and G (New England Biolabs, Cat # S1430S) conjugated magnetic beads for 4 h at 4°C. After discarding beads, samples were incubated with 1 μg of the CXXC5 followed by 1 μg of rabbit IgG, or 5 μg of the Ubiquitin antibody (Cell Signaling Technology, Cat # 2729S) followed by 5 μg mouse IgG (Cell Signaling Technology, Cat # 2729) for 16 h at 4°C. Lysates were then incubated with 12 μL Protein A and G conjugated magnetic beads for 1 h at 4°C. The beads were collected, washed with an IP wash buffer (150 mM NaCl, 10 mM HEPES pH 7.5, 10 mM MgCl2, 0.5% Igepal), and re-suspended with 2× SDS buffer (4%, w/v, SDS; 0.2%, w/v, bromophenol blue; 20%, v/v, glycerol, 150 mM DTT and 10% β-mercaptoethanol). Samples were loaded onto an SDS-10% PAGE, and WB was performed with the CXXC5 or Ubiquitin antibody.
4.12 Assessing the stabilities of transcripts and proteins
To evaluate the stability of transcripts and proteins of CXXC5, ERα, and Cyclin D1, MCF-7 cells were synchronized at G0/G1 with hormone withdrawal for 72 h. Cells were then treated without (ethanol control) or with 10−9 M E2 for 36 h. Cells were incubated with the growth medium containing 5 μM ActD and/or 50 μg/mL CHX in the absence or presence of E2 for 0, 0.5, 1, 2, 4, and 8 h. Cellular extracts were prepared for and subjected to RT-qPCR for CXXC5, ERα, Cyclin D1, or RPLP0 transcript levels or WB using the antibody specific for CXXC5, ERα, Cyclin D1, or β-actin. To assess the degradation of CXXC5 via UPS, MCF-7 cells were synchronized at G0/G1 with hormone withdrawal for 72 h. Cells were then treated without (ethanol control) or with 10−9 M E2 for 36 h. Cells were incubated with the growth medium containing none (DMSO control as the vehicle) or 10 μM MG132, a proteasome inhibitor, together with 50 μg/mL CHX in the absence or presence of E2 for 0, 0.5, 1, 2, 4, and 8 h. Cellular extracts were prepared for and subjected to RT-qPCR or WB using primer sets specific to CXXC5, ERα, or RPLP0 and WB with antibody specific for CXXC5, HA, ERα, or β-actin.
4.13 Evaluating the ubiquitination of CXXC5 and CXXC5 variants
To determine the ubiquitination of 3F-CXXC5, HA-CXXC5, and its variants, we used the bioUb approach. For this, transiently transfected HEK293FT cell extracts were subjected to NeutrAvidin Agarose beads. Bead-bound proteins were eluted with 4X SDS buffer, and the eluted samples were subjected to WB using the Flag, HA, Biotin, or Ubiquitin antibody.
4.14 Statistical analysis
Otherwise indicated, all experiments were repeated for a minimum of two independent times. Results were presented as the mean ± standard deviation (SD). The significance of results with more than two biological replicates was determined using GraphPad Prism (version 8.0), with a p-value threshold of 0.05 set for significance.
ACKNOWLEDGMENTS
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) under grant number 121Z346 (MM). We thank TUBITAK for their support. We express our gratitude to Drs. Ugo Mayor and Juanma Ramirez at the UPV/EHU, Spain, for the kind gift of pCAG-(bioUb)6-BirA and pCAG-BirA expression vectors. We thank Dr. Umut Şahin at Boğaziçi University, Türkiye, for the initial guidance in ubiquitination studies. We thank Mert Kaan Çadır, Öykü Sağlık, and Sude Bildi for their technical support throughout the studies.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




