Heat-sterilizable antibody mimics designed on the cold shock protein scaffold from hyperthermophile Thermotoga maritima
Review Editor: Aitziber L. Cortajarena
Abstract
Antibodies and antibody mimics are extensively used in the pharmaceutical industry, where stringent safety standards are required. Implementing heat sterilization during or after the manufacturing process could help prevent contamination by viruses and bacteria. However, conventional antibodies and antibody mimics are not suitable for heat sterilization because they irreversibly denature at high temperatures. In this study, we focused on the refolding property of the cold shock protein from the hyperthermophile Thermotoga maritima (TmCSP), which denatures at elevated temperatures but regains its native structure upon re-cooling. We designed and constructed a mutant library of TmCSP in which amino acid residues in its three surface loops were diversified. From the library, mutant TmCSPs that bind to each of eight target proteins were selected by phage and yeast surface display methods. We confirmed that the secondary structure and binding affinity of all the selected mutants were restored after heat treatment followed by cooling. Additionally, freeze-drying did not impair their binding affinity. The crystal structure of a mutant TmCSP in complex with its target, the esterase from Alicyclobacillus acidocaldarius, revealed specific interactions between them. These results clearly demonstrate the feasibility of creating heat-sterilizable antibody mimics using TmCSP as a scaffold.
1 INTRODUCTION
Antibody mimics are protein molecules with antigen-specific binding capabilities, designed to mimic the variable regions of antibodies. To date, various types of antibody mimics, such as monobodies, DARPins, and anticalins, have been developed using different protein scaffolds (Sha et al. 2017; Zhao et al. 2016). Compared to antibodies, antibody mimics are smaller, have shorter development times, can be mass-produced in Escherichia coli, and function in reducing environments like intracellular spaces because they lack disulfide bonds. Due to these advantageous properties, antibody mimics are expected to be useful not only as diagnostic agents and research tools in medicine and biology, but also as biopharmaceuticals (Amesaka et al. 2024; Fujita et al. 2023; Hantschel et al. 2020; Miller et al. 2021; Nakamura et al. 2023; Wallon et al. 2022). For their application in the medical field, ensuring a high level of safety is crucial. One way to enhance the safety of antibody mimics during manufacturing process is by introducing heat sterilization, which helps prevent contamination from viruses and bacteria. This method also reduces disposal risks and offers the advantage of processing large quantities at low cost. However, conventional antibodies and antibody mimics are not suitable for heat sterilization, as they irreversibly denature at high temperatures.
To address this issue, we focused on the excellent refolding property of the cold shock protein from the hyperthermophile Thermotoga maritima (TmCSP). TmCSP denatures at high temperatures but recovers its native structure upon re-cooling (Wassenberg et al. 1999), though the structural factors responsible for this remarkable refolding ability remain unclear. The three-dimensional structures of a family of cold shock proteins (CSPs) including TmCSP are similar to that of the antibody variable region, with a robust β-sheet structure and multiple surface loops (Kremer et al. 2001). TmCSP's small molecular weight of approximately 7 kDa allows for precise targeting, and like other antibody mimics, it can function in reducing environments since it lacks disulfide bonds. Furthermore, TmCSP has high thermal stability, with a melting temperature (TM) around 80°C (Wassenberg et al. 1999), which satisfies the common benchmark for non-antibody scaffolds (TM > 60°C) (Skerra 2007). Even if its thermal stability decreases after mutagenesis, it is expected to remain stable at the typical operating temperatures of common antibody mimics (4–37°C). Moreover, TmCSP can be easily mass-produced in E. coli (Welker et al. 1999), offering lower production and storage costs compared to conventional antibodies. Based on these characteristics, we envisioned that TmCSP would be an ideal scaffold for creating heat-sterilizable antibody mimics.
In this work, we conducted mutation analysis to identify amino acid residues in the surface loop regions of TmCSP that maintain structural stability when diversified. Based on these results, we designed and constructed a mutant library. Using phage and yeast surface-display methods (Gai and Wittrup 2007; Smith and Petrenko 1997), we selected TmCSP mutants that bind to eight different target proteins. The selected mutants were analyzed for their functional and refolding properties. The crystal structure of a mutant TmCSP in complex with its target, the esterase from Alicyclobacillus acidocaldarius, was also determined at a resolution of 2.0 Å, revealing specific interactions between them. We also compared the crystal structure with predicted models generated by AlphaFold3 (Abramson et al. 2024) to assess the usefulness of structural prediction tools in predicting mutant TmCSP–target interactions.
2 RESULTS
2.1 Identification of TmCSP positions permissive to amino acid diversification
Antibody mimics are generated by diversifying the amino acid residues on the surface of scaffold proteins. We initially attempted to identify positions within TmCSP that are tolerant to amino acid diversification, allowing us to design libraries that avoid mutating positions critical for structural stability. TmCSP contains five β-strands (βA, βB, βC, βD, and βE) and four surface-exposed loops (AB, BC, CD, and DE loops) (Figure 1a). The amino acid sequences of these loops are as follows: S9KK11 (AB loop), K18DEGG22 (BC loop), H27WSAIEMEGFKTLKEGQ43 (CD loop), and Q50EGKKGP56 (DE loop) (Figure 1b). Following previous work on the tenth human FN3 domain (FNfn10) (Batori et al. 2002), we introduced a series of substitutions and insertions into the loops of TmCSP and evaluated the thermal stability of the mutant proteins (Figure 1c). For this mutational analysis, instead of conventional alanine-scanning, we opted for serine-scanning (Pál et al. 2005). Serine substitutions, due to serine's small, hydrophilic nature, are considered less disruptive compared to more hydrophobic alanine residue. Serine is also effective in removing specific side-chain interactions without introducing new intra- or inter-molecular interactions (e.g., dimerization). The CD loop, composed of 17 residues, contains several side chains, A30, I31, and L39, that are buried within the core of the TmCSP structure. Mutations were introduced into the CD loop while preserving these buried residues. For the DE loop, mutants were constructed with and without substitution of P56, to assess its contribution to the structural stability of TmCSP. A summary of all mutant proteins is presented in Table S1, Supporting Information.
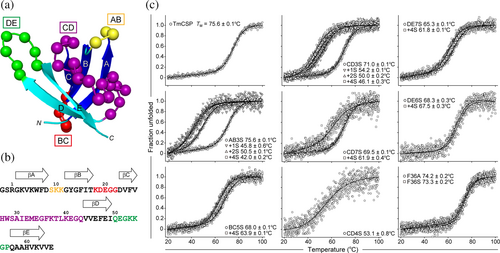
Poly-serine substitutions resulted in only marginal destabilization for all loops (AB3S, BC5S, CD3S, CD7S, DE7S, and DE6S), with the exception of CD4S (Figure 1c and Table S1). The TM value of DE7S was approximately 3°C lower than that of DE6S, indicating that P56 contributes somewhat to the conformational stability of TmCSP. Next, we evaluated the destabilization by the extension of the loops by Ser-insertion. Insertion of poly-serine residues into BC5S, CD7S, DE6S, and DE7S led to destabilization, but these mutants still retained the TM of above 60°C. In contrast, poly-serine insertions into AB3S and CD3S resulted in significant destabilization, with TM values reduced by over 20°C after the insertion of only two serine residues (Figure 1c and Table S1). These results indicate that basically all the surface loops in TmCSP are permissive to substitution mutations, with the exception of the K40EGQ43 region of the CD loop. Additionally, the BC and DE loops, as well as the E32MEGFKT38 region of the CD loop, are tolerant to insertions.
2.2 Phage and yeast surface display of TmCSP
Efficient display of scaffold proteins on phage particles is essential for the successful selection of binding proteins in phage display. However, highly stable and rapidly folding proteins pose challenges in this process. Phage display requires the displayed protein, fused to a phage coat protein, to be translocated into the periplasm of E. coli. Proteins with highly stable and rapid folding are often inefficiently translocated across the E. coli inner membrane when using classical post-translational Sec-dependent secretion signals, such as the PelB and OmpT leaders. One solution to this issue is to use a co-translational signal recognition particle (SRP)-dependent secretion signal, such as the DsbA leader (Steiner et al. 2006). This was demonstrated with TmCSP, where the DsbA-based system achieved significantly higher levels of display on the phage compared to the Sec signal-based system (Figure S1a), with display levels approaching those of monobodies under the same system (Figure S1b). For yeast surface display of TmCSP, we employed a standard Aga2-mediated system, which robustly displayed the protein, as confirmed by flow cytometric analysis (Figure S2).
2.3 Library design
Based on the aforementioned results, we diversified residues D8SKK11 in the βA and AB loop, H27WS29 and E32 in the CD loop, and Q50EGKKG55 in the DE loop. Although D8 is part of the β-strand, it is positioned at the base of the AB loop with its side chain exposed to the solvent and without interactions with other residues, making it a prime candidate for diversification, as it is likely to play a key role in target binding. These positions form contiguous loops oriented in the same direction and are distant from the N- and C-termini, which are often targeted for chemical modifications (Figure 2a). Additionally, we varied the length of the DE loop by one to four residues (Figure 2b). Notably, since the surface-exposed hydrophobic F36 might negatively impact solubility in some clones, we used the F36A mutant as an additional template scaffold. The F36A point mutation had minimal effect on the stability and solubility of TmCSP (Figure 1c and Table S1). The library was constructed using the degenerate NNC codon, which encodes 15 out of the 20 amino acids (excluding Glu, Gln, Trp, Met, and Lys) and avoids introducing stop codons. The theoretical size of the library ranged from 1614–18 = 7.2 × 1016–4.7 × 1021 DNA sequences, encoding 1514–18 = 2.9 × 1016–1.5 × 1021 protein sequences. The phage-displayed TmCSP library was generated using Kunkel mutagenesis (Huang et al. 2012; Kunkel 1985) with primers containing the NNC codon. Measurement of titers immediately after transformation of E. coli SS320 cells with the Kunkel reaction product showed 3.7 × 109 transformants. DNA sequencing of 12 randomly picked transformants revealed that all had unique sequences, suggesting that the library contains very few, if any, duplicate mutants.
2.4 Selection of TmCSP mutants toward target proteins
To evaluate the performance of the library, we screened it against eight target proteins with varying molecular sizes, three-dimensional structures, and electric charges. These target proteins included: the esterase from Alicyclobacillus acidocaldarius (AacEst) (Takano et al. 2013), the enhanced green fluorescent protein (EGFP), the maltose-binding protein from E. coli (MBP), the phosphoenolpyruvate carboxylase from E. coli (EcPEPC) (Matsumura et al. 2002), the ribonuclease H2 from E. coli (EcRNH2) (Ohtani et al. 2000), a mutant of the adenylate kinase from E. coli (EcAdktm; A55C/C77S/V169C) (Nakamura et al. 2023), the yeast small ubiquitin-like modifier (ySUMO), and the N-terminal domain of human selenoprotein P (hSeP-Ndom) (Mizuno et al. 2023). In the library screening against EcPEPC, 10 mM L-aspartate, an allosteric inhibitor known to enhance the structural stability of EcPEPC (Matsumura et al. 2002), was included. Four rounds of phage display selection were performed, with phage recovery increasing at each round, confirming successful phage enrichment. By the fourth round, the relative enrichment of phages binding in the presence of the target protein versus without the target protein was approximately 30-fold or greater for each target (Table S2), indicating substantial enrichment of TmCSP mutants that specifically bind to the respective targets. For the MBP binders, DNA sequencing of 16 clones identified only two unique protein sequences, and as a result, further selection against MBP was discontinued.
After phage display selection, clones in the enriched population for AacEst, EGFP, EcPEPC, EcRNH2, Adktm, ySUMO, and hSeP-Ndom were subjected to gene shuffling, where the N-terminal segment containing the AB and CD loops and the C-terminal segment containing the DE loop were shuffled. The resulting gene pool was then transferred into a yeast-surface display format for additional rounds of selection. After yeast-surface display selection, each mutant was monocloned and sequenced, yielding a total of 47 unique sequences across the eight target proteins. The sequences of all selected TmCSP mutants are provided in Table S3.
2.5 Biophysical and functional characterization of the generated TmCSP mutants
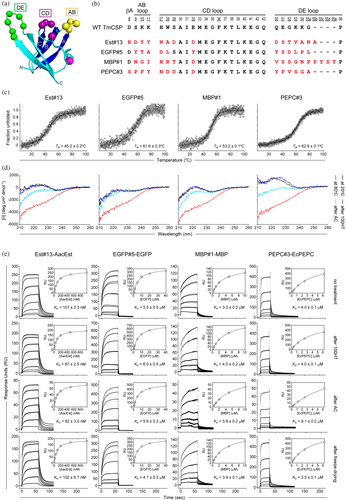
The biophysical and functional properties of four TmCSP mutants, Est#13, EGFP#5, MBP#1, and PEPC#3, were analyzed (Figure 2b). These mutants were selected based on their high expression yield as soluble proteins in E. coli and the absence of cysteine residues, which could undergo oxidation or form disulfide bonds.
We first examined whether the four TmCSP mutants aggregate upon heat treatment. Monobodies and DARPins, which are extensively studied antibody mimics, were used as controls. For the monobody control, we selected four monobodies, Mb(Ec/KpFtsZ_S1) (Fujita et al. 2023), Mb(S4) (Amesaka et al. 2024), Mb(OP-4) (Nakamura et al. 2023), and Mb(Ndom_S2) (Mizuno et al. 2023), which are previously generated and reported by our research group. For the DARPin control, we used DARPin-3G124nc, which is characterized by exceptional structural stability (TM > 92°C) and high affinity for EGFP (KD = 303 pM) (Hansen et al. 2017). Additionally, we included two Sso7d-based binders, Sso7d-BSA-2A8 and Sso7d-GFP-6B9, derived from the highly thermostable Sso7d scaffold, whose thermal stabilities and binding affinities has been previously reported (Zhao et al. 2016). Aggregation of these binding proteins after heat treatment was assessed using the aggregation index (AI = 100 × (OD340)/(OD280-OD340)), calculated from spectroscopic data (Katayama et al. 2005). Higher AI values indicate a greater extent of aggregation, while AI values below 10 suggest minimal aggregation. Heat treatment was applied under two conditions: 100°C for 10 min (abbreviated as 100HT) and autoclaving (121°C, 2 atm for 20 min; abbreviated as AC). The results indicated that Mb(Ec/KpFtsZ_S1), Mb(S4), Mb(Ndom_S2), DARPin-3G124nc, and Sso7d-BSA-2A8 exhibited high AI values and formed aggregates after 100HT, as well as after AC (Figure S3a and Table S4). In contrast, all TmCSP mutants, Mb(OP-4) and Sso7d-GFP-6B9, maintained AI values below 10, even after AC, indicating that almost no aggregation occurred (Figure S3a and Table S4). The presence of insoluble aggregates was further confirmed by centrifuging 100 μL of the heat-treated samples at 15,000g for 10 min at 20°C. Insoluble aggregates were observed in Mb(Ec/KpFtsZ_S1), Mb(S4), Mb(Ndom_S2), and Sso7d-BSA-2A8 after both 100HT and AC, but no such aggregates were detected in any of the TmCSP mutants, Mb(OP-4), DARPin-3G124nc, and Sso7d-GFP-6B9, even after AC (Figure S3b,c).
Next, we investigated the refolding properties of the TmCSP mutants after heat treatment, which is a key aspect of this study. Prior to analyzing the mutants, we reconfirmed that wild-type TmCSP refolded into its native structure after both 100HT and AC, as indicated by Far-UV CD spectra (Figure S4). For the TmCSP mutants, refolding was assessed from both structural and functional perspectives. For structural assessment, we measured the Far-UV CD spectra before and after heat treatment to evaluate the extent of structural recovery. For functional assessment, we employed surface plasmon resonance (SPR) to measure the binding affinity before and after heat treatment, confirming functional recovery. The TM values for Est#13, EGFP#5, MBP#1, and PEPC#3 were 45.0°C, 61.6°C, 53.2°C, and 62.9°C, respectively (Figure 2c), indicating that all the TmCSP mutants were in a denatured state at temperatures of 80°C and above. Our data demonstrated that all TmCSP mutants nearly completely refolded to their native structures after cooling to 20°C following 100HT (Figure 2d). After AC, structural recovery was observed when cooled to 20°C, though the degree of refolding varied between mutants (Figure 2d). Of the mutants, EGFP#5 was almost fully refolded even after AC.
We then analyzed the binding affinity of the heat-treated TmCSP mutants for their target proteins using SPR (Figure 2e). First, we assessed whether the TmCSP mutants fully recovered their function after heat treatment by immobilizing them as ligands on an NTA chip via a His10-tag and flowing the target proteins as analytes. All TmCSP maintained their binding affinity after 100HT (Figure 2e, second row), consistent with the structural assessment results. After AC, EGFP#5 maintained both the SPR response and affinity (Figure 2e, third row), indicating that most of its molecules fully refolded, in line with the structural assessment. Est#13 showed a reduced SPR response, but its affinity was still maintained, suggesting that while some molecules fully refolded, not all did. In contrast, MBP#1 and PEPC#3 exhibited reduced SPR responses and weakened affinity, indicating incomplete refolding. To further evaluate refolding efficiency, we reversed the ligand-analyte roles in SPR experiments (Figure S5). AacEst or EGFP was immobilized as a ligand, and Est#13 or EGFP#5 was flowed as an analyte. Changes in KD values were used as indicators of misfolded analytes. Post-100HT, both binders retained their KD values (Figure S5, second row), suggesting complete refolding. After AC, Est#13's KD approximately doubled, indicating that about 50% of its molecules correctly refolded (Figure S5, third row). In comparison, EGFP#5 exhibited minimal changes in KD, reflecting a higher proportion of correctly refolded molecules. Binding was further confirmed by size exclusion chromatography for Est#13, which had the highest affinity, and EGFP#5, which had the lowest affinity. In both cases, binding was observed as the elution peak shifted to a higher molecular weight upon forming a complex with the target protein (Figure S6).
Finally, we assessed the stability of TmCSP mutants against freeze-drying, an important factor for storage and transport of drug formulations. All TmCSP mutants maintained their binding affinity and SPR response post-freeze-drying (Figures 2e and S5, bottom row), suggesting that they retain their structure even after this process.
2.6 Crystal structure of the Est#13–AacEst complex confirms binding mode
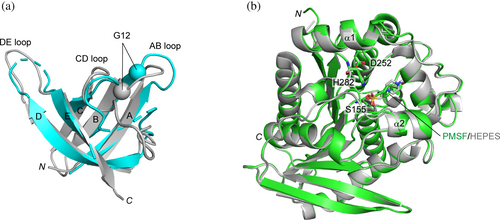
To investigate how TmCSP mutants recognize their targets, we determined the crystal structure of the Est#13–AacEst complex at a resolution of 2.0 Å (Figures 3 and 4 and Table 1). Est#13 was selected for this analysis due to its highest affinity in the SPR analysis. The root-mean-square deviation (RMSD) was assessed using PDBeFold (Figure 3a,b). Superposition of Est#13 in the complex onto wild type TmCSP, while mostly overlapping, exhibited a slightly elevated RMSD of 1.28 Å for the Cα atoms of the backbone, excluding loops including diversified residues (Figure 3a). Manual inspection of the superposed structures revealed that the Cα of Gly12, located at the base of the AB loop, was displaced by 4.2 Å. When Gly12 was excluded, the RMSD was reduced to 1.00 Å, indicating a better alignment between the backbones of Est#13 and TmCSP. Gly12 may contribute to target binding by providing greater flexibility to the AB loop, potentially adjusting the loop structure to fit a given target, similar to what has been reported for the third complementarity-determining region of the antibody heavy chain (CDR-H3) (Birtalan et al. 2008; Zemlin et al. 2003). AacEst in the complex exhibited a transition state analogue, where a sulphonyl derivative (PMSF) was covalently bound to the catalytic Ser155, and the substrate binding pocket was covered by a cap formed by two separate helical regions, α1–α2 and α6–α7 (Figure 3b) (Simone et al. 2000). The structure was in good agreement with a previously reported transition state analogue of AacEst (PDB ID: 1EVQ) (Simone et al. 2000), with an RMSD of 0.52 Å for the Cα atoms of the backbone, suggesting no major structural changes upon Est#13 binding.
| Est#13–AacEst complex | |
|---|---|
| Data collection | |
| Wavelength (Å) | 1.0 |
| Temperature (°C) | −173 |
| Detector | EIGER X 16M |
| Resolution range (Å) | 46.65–1.52 (1.61–1.52) |
| Space group | P21 |
| a, b, c (Å) | 45.82, 46.65, 101.26 |
| α, β, γ (°) | 90, 93.99, 90 |
| Total no. of reflections | 286,957 (45,369) |
| No. of unique reflections | 65,449 (10,348) |
| Multiplicity | 4.38 (4.38) |
| ⟨I/σ(I)⟩ | 11.13 (0.73) |
| Completeness (%) | 99.2 (100.0) |
| Rmeas (%) | 6.4 (134.9) |
| Rmerge (%) | 5.6 (118.1) |
| CC1/2 | 0.999 (0.54) |
| Solvent content (%) | 54.43 |
| Refinement | |
| Refinement resolution range (Å) | 45.71–2.0 (2.13–2.0) |
| No. of reflections | |
| Working set | 28,793 (4653) |
| Test set | 886 (146) |
| Rwork | 0.170 (0.171) |
| Rfree | 0.207 (0.204) |
| No. of non-H atoms | |
| Protein/water/other | 2839/176/23 |
| RMS deviations | |
| Bonds length (Å)/bond angles (°) | 0.008/0.88 |
| Average B factors (Å2) | |
| Protein | 32.20/31.94a |
| Water/other | 41.56/32.23 |
| Ramachandran plot | |
| Favored/allowed/outliers (%) | 96.3/3.7/0 |
| PDB accession code | 9JU4 |
- Note: Values in parentheses are for the highest-resolution shell.
- a Average B-factors of the AacEst/Est#13.
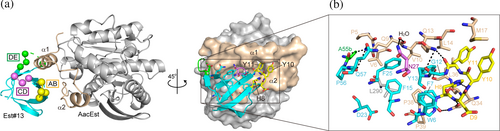
The solvent accessible surface area (SASA) buried at the interface between Est#13 and AacEst in the complex, determined using PDBePISA, was 729.6 Å2 for Est#13 and 746.1 Å2 for AacEst, which falls within the range of a sufficiently large antigen-interacting surface (600–800 Å2) (Genst et al. 2006). Est#13's three diversified loops, along with a flat surface formed by strands βA–βC, extensively interacted with a hydrophobic patch between α1 and α2 of AacEst (Figure 4a). His8, Tyr10, and Tyr11 in the AB loop of Est#13 were deeply embedded into the valley between the helices (Figure 4a, right panel). Trp6 in βA, Tyr13 and Phe15 in βB, and Phe25 in βC of Est#13 formed broad hydrophobic interactions with Val6, Val10, Leu14, Leu36, Pro38, Leu290 of AacEst (Figure 4b). Trp6 of Est#13 and Pro38 of AacEst formed a face-to-face CH–π interaction. A detailed inspection of the interaction interface identified 11 hydrogen bonds (Figure 4b). This complementary binding mode suggests a key-and-lock mechanism, where Est#13 specifically binds to AacEst. Supporting this, Est#13 displayed on the yeast surface did not bind to off-target proteins, demonstrating its specificity for AacEst (Figure S7a). It is noted that specificity tests for EGFP#5, MBP#1, and PEPC#3 confirmed that each mutant was target specific as well (Figure S7a,b).
The 10th and 11th residues in the AB loop of all TmCSP mutants that bind to AacEst are aromatic amino acids, such as Tyr and Phe (Table S3). In Est#13, the Tyr residues at positions of 10 and 11 appear to be critical for binding, as they are deeply embedded within the AacEst structure. This suggests that all AacEst-binding mutants may interact with AacEst via the same epitope–paratope as the Est#13–AacEst complex. Conversely, the involvement of the CD and DE loops in the binding of Est#13 to AacEst was minimal, involving only N27 and Ala55b, respectively. However, the sequences of the CD and DE loops are variable among the AacEst binders (Table S3), indicating that the overall backbone structure of TmCSP is maintained despite changes in these loop sequence. This variability suggests that the CD and DE loops could potentially contribute to binding when searching for clones that bind to different target proteins. Interestingly, surface-exposed aromatic residues on strands βA–βC were strongly involved in binding to AacEst, although these residues were not diversified in this study. It would be interesting to diversify the amino acid residues on the β-strand with outward-facing side chains in future experiments, similar to the “side and loop” library of Monobody (Koide et al. 2012). However, Trp6, Tyr13, Phe15, and Phe25 form a hydrophobic cluster and may be involved in refolding properties, so mutations of these residues should be carefully considered.
2.7 Comparison of the crystal structure and predicted structure of the Est#13–AacEst complex
Structure prediction tools may significantly accelerate the design of amino acid mutations in a scaffold protein that impart affinity and specificity for a given target. To explore this possibility with TmCSP-based antibody mimics, we assessed the accuracy of complex structure predictions generated by AlphaFold3 (Abramson et al. 2024) by comparing them to the actual crystal structure. Predictions were performed using the Alphafold3 web server (https://alphafoldserver.com/), and the input sequences are listed in Table S5. The accuracy of these predictions was evaluated using the pTM and ipTM scores, which measure the quality of protein structure predictions (Evans et al. 2022; Xu and Zhang 2010; Zhang and Skolnick 2004). The pTM score provides an overall assessment of the predicted structure's similarity to the true fold of the complex, while the ipTM specifically evaluates the accuracy of the predicted interface in protein–protein complexes. A pTM score above 0.5 suggests that the overall predicted fold may be reasonably accurate, and an ipTM score above 0.8 indicates high confidence in the predicted interface. For the Est#13–AacEst complex, the structure prediction scores were as follows: model_0: pTM 0.86, ipTM 0.46; model_1: pTM 0.86, ipTM 0.44; model_2: pTM 0.85, ipTM 0.39; model_3: pTM 0.85, ipTM 0.37; and model_4: pTM 0.84, ipTM 0.36. In all five predicted models, the AB loop of Est#13 was inserted deeply between α1 and α2 of AacEst, with the overall binding interface closely resembling that of the crystal structure (Figure S8). However, the main chains and side chains of the interacting residues were not completely superposed. In particular, the orientation of the side chains varied significantly, resulting in a mismatch between the predicted and observed positions of the 11 hydrogen bonds (Figure S8). Given these discrepancies, particularly in side chain orientations, designing amino acid mutations on a scaffold protein solely on the prediction tools would still be challenging. These findings are consistent with the lower iPTM scores for the Est#13–AacEst complex predictions.
3 DISCUSSION
We have successfully developed TmCSP-based antibody mimics with target-specific binding, while maintaining the high refolding ability of wild-type TmCSP. These TmCSP-based binders exhibit exceptional resistance to heat sterilization, with all clones tolerating temperatures up to 100°C and certain clones up to 121°C, without losing functionality. When exposed to these sterilizing conditions, the binders temporarily adopt a denatured state but revert to their native conformation as the temperature returns to ambient levels, restoring their binding affinity. This property allows for the integration of heat sterilization into the manufacturing process, which is particularly beneficial for pharmaceutical production, where stringent safety standards are established. Furthermore, TmCSP-based technology could have a potential to revolutionize production practices in developing countries, where stable production pipelines are often lacking. We also confirmed that freeze-drying does not impair the function of these TmCSP mutants, further supporting their ease of storage and transportation.
The crystal structure of the Est#13–AacEst complex reveals specific interactions between Est#13 and AacEst. Compared to the complex structure predicted by AlphaFold3, a tool whose accuracy has significantly improved in recent years, AlphaFold3 could predict the approximate positions of the paratope and epitope but struggled with the detailed spatial relationships of main chains and side chains, such as hydrogen bond positioning. This suggests that while AlphaFold3 is effective at making a mark on interacting regions, it is less reliable for designing mutations aimed at imparting binding affinity or specificity of a scaffold protein, which may still require experimental structural analysis.
As proof-of-concept research, we developed heat-sterilizable antibody mimics using a bacterial CSP scaffold. However, for biotherapeutic applications, TmCSP-based binders would face two significant challenges. The first challenge is protease resistance and immunogenicity. The low protease resistance of TmCSP-based binders may result in rapid degradation in the bloodstream, leading to a very short half-life. Moreover, the bacterial origin of the scaffold poses a risk of immunogenicity in humans. To address these issues, we are exploring the use of D-amino acids. D-proteins have favorable properties as therapeutic agents, including enhanced in vivo stability and low immunogenicity compared to L-proteins. Successful examples of D-amino acids-based VHHs and monobodies have been reported (Aoki et al. 2023; Aoki et al. 2024; Iwamoto et al. 2023; Uppalapati et al. 2016). Although D-proteins currently require chemical synthesis due to the limitations of recombinant expression techniques, the small size of TmCSP, like VHH and monobody, makes it particularly well-suited for this approach. The second challenge is binding affinity. The current affinity of the TmCSP-based binder is insufficient for practical applications. Adopting mRNA display could address this limitation, as it enables screening of libraries exceeding 1012 variants (Norman et al. 2021), which is over 100 times larger than the libraries used in this study, significantly increasing the likelihood of obtaining high affinity binders. Additionally, trimer phosphoramidite-based technology (TRIM technology) offers a complementary approach by enabling precise control over amino acid distributions at desired positions during library construction (Koide et al. 2012; Zhang 2023). This allows biasing toward favorable amino acids, optimizing codons for E. coli, and avoiding stop codons.
We have coined the term “HSbodies” for these CSP-derived synthetic binding proteins. In conclusion, HSbodies are resistant to both heat sterilization and freeze-drying, and are expected to hold significant potential for pharmaceutical applications.
4 MATERIALS AND METHODS
4.1 Plasmid construction
The cDNAs of TmCSP, DARPin-3G124nc, Sso7d-BSA-2A8, and Sso7d-GFP-6B9, optimized for E. coli codon usage by the DNAWorks tool (Hoover and Lubkowski 2002), were purchased from Integrated DNA Technologies (IDT, Coralville, IA). The gene sequences are provided in Table S6. The genes were amplified by PCR using primers designed to add BamHI and XhoI restriction sites to the 5′ and 3′ ends of the gene, respectively (Table S7). The genes of Sso7d-based binders were amplified by PCR using T7P and T7T (Table S7). The BamHI/XhoI-digested fragments were cloned into the pHFT2 vector (Fujita et al. 2023). Plasmids for the TmCSP mutants were prepared using the inverse PCR method with appropriate primers. For MBP, EcPEPC, EcRNH2, Adktm, and hSeP-Ndom, previously published plasmids for in vivo biotinylation were used (Fujita et al. 2023; Mizuno et al. 2023). The cDNAs of AacEst, EGFP, and ySUMO were PCR-amplified from either purchased or in-house constructed vectors and each of the specific primers (Table S7). The BamHI/XhoI-digested fragments were then cloned into the pHBT2 vector (Fujita et al. 2023), which is designed for in vivo biotinylation. DNA sequences were confirmed using T7P and T7T primers (Table S7).
4.2 Protein expression and purification
The E. coli BL21(DE3) strain transformed with the TmCSP-coding pHFT2 plasmid was cultivated in 3 mL of LB medium containing kanamycin (50 μg/mL) at 37°C for 16–18 h. This culture was then added to 1 L of 2 × YT medium containing kanamycin (50 μg/mL) and further cultivated at 37°C until the absorbance at 600 nm reached 0.5. After cooling the medium in an ice bath, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.2 mM to induce protein expression at 18°C for 16–18 h. The culture was centrifuged at 8000g and 4°C for 10 min, and the resulting pellets were stored at −80°C. The cell pellets were resuspended in 30 mL of 20 mM Tris–HCl pH 8.0, followed by addition of lysozyme (0.5 mg/mL final), PMSF (2 mM final), MgCl2 (5 mM final), DNase I (10 μg/mL final), and RNase A (10 μg/mL final). The mixture was incubated at 37°C for 30 min before adding NaCl (500 mM final). After sonication, the suspension was centrifuged at 18,000g and 4°C for 30 min. The supernatant was supplemented with imidazole (20 mM final) and loaded onto a 5 mL HisTrap HP column (Cytiva, Washington, DC). The column was washed with buffer A (20 mM Tris–HCl, pH 8.0, containing 500 mM NaCl and 20 mM imidazole) for 5 column volumes (CV), and target proteins were eluted with a linear gradient from buffer A to buffer B (20 mM Tris–HCl, pH 8.0, containing 500 mM NaCl and 500 mM imidazole) over 5 CV. The elution peak fractions were desalted by dialysis overnight at 4°C in buffer C (20 mM Tris–HCl, pH 8.0). The desalted protein solution was loaded onto a 5 mL HiTrapQ HP column (Cytiva) and washed with buffer C for 5 CV. Target proteins were eluted with a linear gradient from buffer C to buffer D (20 mM Tris–HCl, pH 8.0, containing 1M NaCl) over 5 CV. The elution peak fractions were further purified using a ProteoSEC 3–70 kDa Preparative Resin column (Protein Ark, Rotherham, UK) with buffer E (20 mM Tris–HCl, pH 8.0, containing 150 mM NaCl). The purified protein was stored at −20°C in the presence of 40% (v/v) glycerol. Biotinylated proteins of MBP, EcPEPC, EcRNH2, Adktm, and hSeP-Ndom were prepared as previously described (Fujita et al. 2023; Mizuno et al. 2023). For biotinylated AacEst, EGFP, and ySUMO, the proteins were produced and purified using the same protocol and buffers as for biotinylated MBP, except that lysozyme, PMSF, MgCl2, DNase I, and RNase A were added to the cell suspension as described above. The purified proteins were stored −20°C in the presence of 40% (v/v) glycerol. For crystallization, the affinity tag for each protein was removed using TEV protease. The yields of the TmCSP-based binders ranged from 4 to 28 mg/L of culture (without affinity tags).
4.3 CD spectra measurement
Far-UV CD spectra (200–260 nm) were measured at 20 and 80°C using a J-725 spectropolarimeter (JASCO, Tokyo, Japan). Samples were diluted with PBS (137 mM NaCl, 2.7 mM KCl, 1.8 mM KH2PO4, 10 mM NaH2PO4, pH adjusted to 8.0 with NaOH) or buffer E to a concentration of 0.1–0.4 mg/mL and measured using a quartz cell with a 1- or 2-mm optical path length. For thermal stability analysis, denaturation curves were obtained by monitoring a change in CD values at 210, 218, or 227 nm, depending on the clones. The temperature was linearly increased from 5 to 100°C at a rate of 1°C/min. The melting temperature (TM) was calculated using the thermal denaturation analysis program (JASCO).
4.4 Phage display, yeast-surface display, and library construction and screening
Experiments related to phage display, yeast-surface display, and library construction and screening were performed using protocols essentially identical to those used for monobody (Fujita et al. 2023). Full details on the materials and methods used are provided in Data S1.
4.5 Heat treatment and freeze-drying
Heat treatment was applied under two conditions: 100°C for 10 min (abbreviated as 100HT) and autoclaving (121°C, 2 atm for 20 min; abbreviated as AC). After heat treatment, samples were allowed to cool to 20°C. The 100HT was conducted using a MiniT-C dry bath incubator (Allsheng, Hangzhou, China) and AC was done using a BS245 autoclave (Tomy Seiko, Tokyo, Japan). Freeze-drying was performed using a Freeze dryer VD-500F (TAITEC, Aichi, Japan) and an oil rotary vacuum pump GLD-136 (ULVAC Technologies, Tokyo, Japan). After freeze-drying, the sample was stored at −20°C and reconstituted in sterile water before measurement.
4.6 Aggregation index determination
Aggregation in the monobodies and TmCSP mutants was assessed using an aggregation index (AI) value calculated from spectroscopic measurements. The monobodies and TmCSP mutants were each prepared at 0.35 mg/mL, and absorbance was measured using a Beckman DU730 spectrophotometer (Beckman Coulter, San Jose, CA).
4.7 Surface plasmon resonance
SPR measurements were performed in 10 mM HEPES-NaOH, pH 8.0, containing 150 mM NaCl and 0.005% (v/v) Tween 20 (with 10 mM L-aspartate for EcPEPC) at 25°C on a Biacore T100 or T200 instrument with control Software ver. 1.1.1 (Cytiva). The TmCSP mutant was immobilized via a histidine tag on a Series S Sensor Chip NTA (Cytiva). Tag-removed proteins of AacEst, EGFP, MBP, and EcPEPC at varying concentrations (0, 5, 10, 20, 50, 100, 200, 500, and 1000 nM for AacEst; 0, 0.5, 1, 2, 5, 10, 20, and 40 μM for EGFP; 0, 0.2, 0.5, 1, 2, 5, and 10 μM for MBP; 0, 0.15, 0.3, 0.75, 1.5, 3, and 6 μM for EcPEPC, respectively) were flowed over the sensor chip at a rate of 20 μL/min and the binding signal was monitored. For experiment involving a reversed ligand-analyte configuration, AacEst and EGFP were immobilized on the NTA chip as ligands via a His10-tag, and tag-removed Est#13 and EGFP#13 were flowed as analytes (0, 5, 10, 20, 50, 100, 200, 500, and 1000 nM for Est#13, and 0, 0.5, 1, 2, 5, 10, and 25 μM for EGFP#5, respectively). Equilibrium responses were recorded 4 s before the end of the analyte injection. The KD values were determined by fitting the titration curve of equilibrium responses versus analyte concentrations to a 1:1 interaction model using Biacore T100 evaluation software ver. 1.1.1 (Cytiva).
4.8 SEC analysis
A 500 μL of sample in buffer E was applied to a Superdex 75 Increase 10/300 GL column (Cytiva) or Superdex 200 Increase 10/300 GL column (Cytiva) with buffer E as the elution buffer at room temperature (flow rate: 0.5 mL/min). A mixture of the target protein with an excess of the corresponding TmCSP mutant (1.2 equiv.) was applied to SEC to determine binding by observing the shift in the elution peak compared to the elution peak of the target alone.
4.9 Crystallization
AacEst was mixed with a 2-fold molar excess of Est#13 in buffer E, and then applied to a HiLoad 16/60 Superdex 200 prep grade column (Cytiva) equilibrated with buffer E at room temperature (flow rate: 1.0 mL/min). The elution peak was analyzed by SDS-PAGE, and the eluted fractions containing both AacEst and Est#13 were collected (Figure S9), concentrated to a total protein concentration of 10.0 mg/mL, and used for crystallization. Est#13–AacEst were crystallized in 0.1M sodium citrate, pH 5.0, and 20% (v/v) PEG 8000 at 19°C by sitting-drop vapor diffusion method. Crystals were frozen in a mixture of 75% mother liquor and 25% glycerol.
4.10 Data collection, processing, and refinement
X-ray diffraction data were collected at a wavelength of 1.0 Å on the micro-focus beamline BL41XU at SPring-8, Hyogo, Japan, using an EIGER X 16M detector (Dectris, Baden-Daettwil, Switzerland). The datasets for Est#13–AacEst were integrated and scaled using the KAMO system (Yamashita et al. 2018), which automates the processing with BLEND (Foadi et al. 2013), XDS, and XSCALE (Kabsch 2010). The phases were determined by molecular replacement using PHENIX (Adams et al. 2010) with the previously determined structure of AacEst (PDB ID:1EVQ) (Simone et al. 2000) as the search model. Several rounds of refinement were performed using phenix.refine in PHENIX, with manual adjustments using Coot (Emsley et al. 2010). The refined structures were validated with MolProbity (Chen et al. 2010). Data collection and refinement statistics are summarized in Table 1. Molecular graphics were generated using PyMOL (www.pymol.org).
AUTHOR CONTRIBUTIONS
Hiroshi Amesaka: Conceptualization; data curation; investigation; validation; formal analysis; funding acquisition; writing – original draft; writing – review and editing. Marin Tachibana: Data curation; investigation; validation; formal analysis. Mizuho Hara: Data curation; formal analysis; validation; investigation. Shuntaro Toya: Data curation; formal analysis; validation; investigation. Haruki Nakagawa: Data curation; formal analysis; validation; investigation. Hiroyoshi Matsumura: Data curation; formal analysis; investigation; validation; writing – review and editing. Azumi Hirata: Writing – review and editing; data curation; formal analysis; validation; investigation. Masahiro Fujihashi: Writing – review and editing; data curation; formal analysis; validation; investigation. Kazufumi Takano: Supervision; writing – review and editing. Shun-ichi Tanaka: Conceptualization; data curation; formal analysis; investigation; validation; funding acquisition; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Numbers JP21K05386 (to Shun-ichi Tanaka), JP23H04559 (to Shun-ichi Tanaka), JP24K08717 (to Shun-ichi Tanaka), and JP23KJ1820 (to Hiroshi Amesaka), the Sasakawa Scientific Research Grant from the Japan Science Society 2022-4052 (to Hiroshi Amesaka), and the leave a nest research grand from Leave a Nest Co., Ltd. (to Hiroshi Amesaka). This work has been performed under the approval of the SPring-8 Program Advisory Committee (Proposal Nos. 2020A2536 and 2021A6623). The SPR experiments were performed at the Kyoto Integrated Science & Technology Bio-Analysis Center (KIST-BIC). We thank Dr. D. Fujita and Dr. Y. Nakura (Institute for Integrated Cell-Material Sciences at Kyoto University) for access to the flow cytometry used for yeast cell sorting.
CONFLICT OF INTEREST STATEMENT
Kyoto Prefectural University has filed a patent application (Japan Patent Application No. 2022-007417) for technologies related to CSP-based antibody mimics described in this paper. The inventors listed on the patent are Hiroshi Amesaka, Kazufumi Takano, and Shun-ichi Tanaka.




