Lysine carbamoylation during urea denaturation remodels the energy landscape of human transthyretin dissociation linked to unfolding
Review Editor: Aitziber L. Cortajarena
Abstract
Chemical denaturants such as urea have become indispensable in modern protein science for measuring the energetics of protein folding and assembly. Denaturants bind to and preferentially stabilize denatured states, folding transition states, and folding intermediates over the native state, allowing experimental access to free energies of folding and insights into folding mechanisms. However, too little attention is paid to the established chemical instability of aqueous urea, that is, its decomposition into the reactive electrophile ammonium cyanate or isocyanic acid depending on the solution pH. Protein carbamoylation by cyanate/isocyanic acid can change the dissociation and/or unfolding free energy landscape of the protein under study with time. This problem is exemplified using the human blood protein transthyretin (TTR), a kinetically stable transporter of thyroid hormone and holo-retinol binding protein. The dissociation, misfolding, and aggregation of TTR are associated with a prominent human amyloid disease. We demonstrate that modification of TTR by cyanate reshapes the energy landscape of TTR tetramer dissociation and unfolding on multiple time scales. Like certain halide anions and the more chemically inert thiocyanate anion, cyanate binds weakly and non-covalently to the thyroid hormone binding interface in the TTR tetramer. The close proximity of the bound cyanate ion to the pKa-perturbed lysine 15 ε-amino side chain nucleophile in the thyroid hormone binding sites of TTR favors carbamoylation of this nitrogen. Lysine 15 ε-amino carbamoylation substantially slows down TTR tetramer dissociation mediated by urea denaturation, thus introducing kinetic heterogeneity early in the unfolding reaction. Slower carbamoylation of the subpopulation of other, less pKa-perturbed lysine ε-amino groups hastens tetramer unfolding, leading to non-exponential, sigmoidal unfolding trajectories. We thus demonstrate that lysine carbamoylation in urea solutions can strongly alter protein unfolding energetics and the mechanism of unfolding.
1 INTRODUCTION
Urea is one of the most widely employed chemical denaturants for extracting the energetics of protein dissociation and/or unfolding. Unlike guanidinium salts, urea is a neutral molecule, eliminating contributions to folding energetics from electrostatics and charge screening due to ion-protein interactions (Hammarstrom et al., 2001). An often-overlooked disadvantage of using urea as a denaturant is its chemical instability, that is, its established decomposition into ammonium cyanate at pH 7.5 (Kraus et al., 1994; Stark et al., 1960). Cyanate is a reactive electrophile that reacts with primary amino groups, such as a protein's N-terminus (Stark & Smyth, 1963), or the ε-amino side chain groups of lysine (Lys) (Kraus et al., 1994), affording covalent carbamoylated structures (post-translational sequence modifications), which are highlighted in Figure 1a. This conversion of Lys into homocitrulline increases the size of the side chain and eliminates a positive charge on the ε-amino group, which could perturb the electrostatic balance of the protein, change (dis)assembly and/or (un)folding kinetics, alter the unfolding pathway (mechanism), and/or impair the protein's biological function.

Decomposition of a freshly prepared urea solution into ammonium cyanate at pH 7.5 is a relatively slow process that, depending on temperature, can take from hours to days (Dirnhuber & Schütz, 1948; Stark et al., 1960). Thus, for a typical single-domain protein that (un)folds on the millisecond-to-seconds time scale, carbamoylation can be ignored in the first approximation. However, carbamoylation may become an unwanted protein side reaction with kinetically stable proteins that unfold on time scales comparable to or slower than urea decomposition into ammonium cyanate (neutral pH)/isocyanic acid (acidic pH), especially at 25 or 37°C where carbamoylation is faster (Anderson et al., 2023; Colón et al., 2017; Dirnhuber & Schütz, 1948; Kraus et al., 1994; Nixon et al., 2021; Otzen, 2003; Stark et al., 1960; Xia et al., 2007; Xia et al., 2012).
Transthyretin (TTR) is a kinetically stable protein in vertebrates (Colon & Kelly, 1992; Hammarstrom et al., 2003). Transthyretin is secreted by the liver into the blood stream and by the choroid plexus into the cerebral spinal fluid, where it predominantly functions as a thyroid hormone (T3 and T4) and holo-retinol-binding protein transporter. TTR is a homo-tetramer, wherein the two most stable dimer substructures interact via short loop regions, creating two thyroid hormone binding sites per tetramer that are interconverted by a C2 axis of symmetry (Figure 1b). Tetramer dissociation along the T4-hormone binding interface into transient and kinetically unstable dimer subunits (orange and red/aqua and blue pairings in Figure 1b) is necessary and rate-limiting for TTR unfolding in denaturant solutions (Foss, Kelker, et al., 2005; Foss, Wiseman, & Kelly 2005). It has been shown that transthyretin dissociation is rate-limiting for TTR aggregation into non-native structures that drive the proteotoxicity in transthyretin amyloidosis (ATTR), the third most prominent human amyloid disease behind Alzheimer's and Parkinson's diseases (Benson et al., 2018; Berk et al., 2013; Coelho et al., 2012; Colon et al., 1996; Colon & Kelly, 1992; Kelly, 2020; Maurer et al., 2018). Specifically, the TTR tetramer dissociates into metastable dimers that further dissociate into monomers, which can misfold, as required for non-native TTR misassembly or aggregation, leading to neurodegeneration and organ deterioration in humans (Coelho et al., 2012; Foss, Kelker, et al., 2005; Foss, Wiseman, & Kelly, 2005; Jiang et al., 2021; Maurer et al., 2018; Schonhoft et al., 2017).
Both electrostatics (Hörnberg et al., 2005) and impaired protein hydration (Zhou et al., 2022) have been proposed to contribute to transition state energetics and hence the kinetic stability of TTR: binding of chloride and iodide ions to the T4-hormone binding site of the TTR tetramer is kinetically stabilizing (Hammarstrom et al., 2001). In addition, replacing Lys 15, whose charged side chain projects into the T4-hormone binding interface (Figure 1b), with an uncharged Ala (methyl group) side chain greatly stabilizes the tetramer against unfolding in urea or unfolding upon acidification (Hammarstrom et al., 2001). The gain in kinetic stability of the Lys15Ala TTR variant has been attributed to a reduction in the unfavorable charge–charge repulsion between the juxtaposed Lys 15 ε-amino side chain groups (Figure 1b) that are hypothesized to destabilize the dimer–dimer interface comprising the T4-hormone binding site in the wild-type TTR tetramer (Hammarstrom et al., 2001). The Lys 15 ε-amine is unusual in that it is highly reactive toward electrophiles such as A2, a designed, fluorogenic small molecule that binds to the T4-binding interface in the TTR tetramer and then selectively reacts with the Lys 15 ε-amino group of TTR at physiological pH (Choi et al., 2010). The resulting A2 fragment-TTR conjugate fluoresces only after the conjugation reaction is complete (Choi et al., 2010).
Herein, we show that the Lys 15 ε-amino side chain of TTR is pKa-perturbed based on its reaction kinetics with thioester A2, thus explaining its high nucleophilicity at physiological pH. Thus, the Lys 15 ε-amino side chain reacts fastest with the urea degradation product cyanate, affording neutral homocitrulline that kinetically stabilizes the tetramer, as reflected by dramatically slowed tetramer dissociation (analogous to the Lys15Ala TTR mutation). The amino terminus of TTR also reacts rapidly with isocyanate, carbamoylating this site without apparent energetic consequences. Slower cyanate-mediated carbamoylation of the remaining, less reactive Lys ε-amino side chains comprising TTR lowers the barrier for tetramer dissociation linked to urea-mediated denaturation and results in further slow kinetic phases, ultimately rendering TTR amenable to urea denaturation.
2 RESULTS
2.1 Design of a TTR variant for studying chemical modification by carbamoylation
Residues Cys 10 and Met 13 in wild-type TTR are known to be prone to oxidative modification in both human plasma and in vitro after purification from recombinant expression hosts. Met 13 can be oxidized by oxygen to Met 13-sulfoxide, while Cys 10 can be oxidized to -sulfenic, −sulfinic, and -sulfonic acids by successive addition of one oxygen atom (Zhang & Kelly, 2003; Zhang & Kelly, 2005). Cys 10 can also be oxidized, affording mixed disulfide bonds with glutathione, homocysteine, or the like (Zhang & Kelly, 2003; Zhang & Kelly, 2005).
To study how TTR ε-amino carbamoylation influences the energy landscape of tetramer dissociation in the absence of unwanted oxidative modifications at Cys 10 and Met 13, we used a TTR variant wherein we replaced the oxidation-prone Cys 10 and Met 13 residues with Ala and Val residues, respectively, generating the Cys10Ala/Met13Val variant of TTR (Table 1). We previously verified that the Cys10Ala variant is both thermodynamically and kinetically isoenergetic to wild-type TTR (McCutchen & Kelly, 1993). Val has a higher β-sheet propensity than Met and is thus better suited for occupying position 13 within β-strand A, consistent with the X-ray crystallography-based structure of the Cys10Ala/Met13Val TTR variant homotetramer (Figure 1b), showing that it is largely indistinguishable from the wild-type TTR homotetramer.
| TTR(C10A/M13V) | |
|---|---|
| PDB code | 7THA |
| Data collection Temp. (K) | 100 |
| Beam/detector | Rigaku MicroMax/Mar345 |
| Wavelength (Å) | 1.5418 |
| Space group | P 21 2 21 |
| (a/b/c) Å | 42.60/64.51/85.92 |
| (α/β/γ) (deg.) | 90/90/90 |
| Resolution range | 42.96–1.75 (1.84–1.75) |
| Unique reflections | 24,607 (3541) |
| Completeness (%) | 100 (100) |
| Rmerge | 0.067 (1.551) |
| Rp.i.m. | 0.026 (0.593) |
| CC (1/2) | 0.999 (0.730) |
| I/σ (I) | 17.3 (1.6) |
| Redundancy | 7.7 (7.6) |
| Wilson B-factor (Å2) | 23.7 |
| Refinement | |
| Total no. reflections | 23,283 (1682)a |
| Rwork | 0.181 (0.305) |
| Rfree | 0.211 (0.309) |
| RMS bond length (Å) | 0.01 |
| RMS bond angle (deg.) | 1.7 |
| Coordinate ESU (Å) | 0.115 |
| Mean B value | 30.6 |
| Protein B value | 29.9 |
| Water B value | 37.4 |
| Ramachandran favored (%) | 97.9 |
| Ramachandran allowed (%) | 1.7 |
| Ramachandran outliers (%) | 0.4b |
- a In the table above, values in parentheses are calculated for resolutions in the highest-resolution shell.
- b In chain B of the model, the loop containing residue Ser100 is involved in a crystal packing contact, and while the electron density for this region is somewhat weak/discontinuous, the modeled conformation of Ramachandran outlier residue Ser 100 represents the best apparent fit to the density.
Purified Cys10Ala/Met13Val TTR elutes as a single, symmetric peak from a C8 reversed phase column (denaturing organic solvent-based mobile phase; Figure S1), revealing no evidence of chemical modification. Mass spectrometry verifies the correct molecular weight, establishing identity (Figure S1). The Cys10Ala/Met13Val TTR variant was used throughout this study as the host sequence for the additional point mutations discussed in this text and is therefore referred to as wild-type' TTR.
2.2 The Lys 15 side chain is pKa-perturbed and is a reactive nucleophile at physiological pH
A2 is a fluorogenic small molecule that binds to the T4-hormone binding site of the TTR tetramer (Choi et al., 2010; Rappley et al., 2014). A2 shows no fluorescence emission when only bound reversibly to the TTR tetramer. The bound active ester of A2 reacts with one of the two Lys 15 ε-amino groups in each of the two thyroid hormone binding pockets, affording an amide-linked (A2 fragment)2-wild-type' TTR conjugate that is fluorescent, hereafter referred to as the A2-based TTR conjugate. A2 can thus be used as a sensitive and highly specific probe that reports on the chemical reactivity of Lys 15 ε-amino groups, the most reactive ε-amino Lys side chain composing TTR.
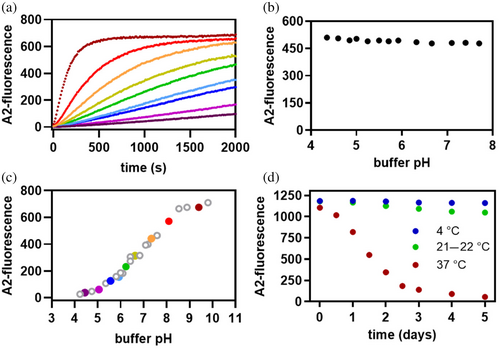
At pH 8.8, where the deprotonated Lys 15 ε-amino groups predominate over the ε-ammonium groups, A2 (20 μM) reacts with wild-type' TTR (1.5 μM tetramer) in less than 10 min (Figure 2a, bright red), as reflected by the A2-based TTR fluorescence emission at 1200 s of reaction time. Lowering the pH-value of the solution from 8.8 shifts the equilibrium toward the Lys 15 protonated ε-ammonium population, the chemically non-reactive form of the Lys 15 side chain, thus slowing down A2-consumption (Figure 2a). Since wild-type' TTR dissociates and slowly (hour time scale) misfolds and aggregates at pH-values below 4.2 (Lai et al., 1996), we restrict ourselves to pH-values above 4.2, to be sure that TTR remains tetrameric during the relatively short time course of the conjugation reaction. Control experiments assured that the fluorescence emission intensity of the A2-based TTR conjugate is only weakly dependent on pH over the pH range explored here (Figure 2b). A plot of the A2-based TTR conjugate fluorescence intensity at a reaction time of 1200 s versus the pH-value of the solution reveals that the chemical reactivity of Lys 15 is most sensitive to pH-changes around 6.8 (Figure 2c). This suggests that the pKa-value of the Lys 15 ε-amino group is ≈ 6.8, lower than the typical pKa-value reported for the ε-amino group of Lys side chains (pKa-value ≈ 9–10).
2.3 Lys 15 is carbamoylated by the urea degradation product isocyanic acid/cyanate
Having demonstrated in the previous paragraph that reactive, deprotonated Lys 15 ε-amino groups predominate at pH 7.4 in wild-type' TTR, we wondered whether Lys 15 also reacts with the cyanate degradation product produced in aging urea solutions. As wild-type' TTR does not unfold at equilibrium below 2 M urea (Hurshman Babbes et al., 2008), we incubated the wild-type' TTR tetramer (1.5 μM) at 4°C, at ambient temperature (21 to 22°C), or at 37°C, all in the presence of 0.5 M urea (prepared from a freshly made 2 M urea stock at 4°C). Aliquots were withdrawn from the wild-type' TTR urea stock solutions (0.5 M) at different time points as a function of temperature, reacted with an excess of A2 (20 μM), and after overnight equilibration, the A2-based TTR conjugate fluorescence was measured in a spectrofluorometer. Carbamoylation renders Lys 15 inactive toward the A2-conjugation reaction, manifesting as a decrease in A2-fluorescence intensity over time.
At 37°C, the A2-based TTR conjugate fluorescence decreases noticeably over 24 h, and essentially all conjugate fluorescence is lost after incubation for 3 days in a 0.5 M urea solution (Figure 2d) due to carbamoylation. Substantially less carbamoylation occurs at 4°C, as the A2-based TTR conjugate fluorescence signal remains relatively stable. More carbamoylation is observed at ambient temperature, but the loss of conjugate fluorescence is only ≈ 10% after a 5-day incubation period. We note that the decrease in conjugate fluorescence at 37°C is preceded by a lag phase and does not fit to a single exponential function. The lag phase most likely results from the fact that a fresh urea solution takes time to decompose into a sufficient concentration of ammonium cyanate to enable facile Lys 15 carbamoylation. Our data are consistent with earlier reports showing that urea decomposition is strongly temperature-dependent and favored at elevated temperatures (Dirnhuber & Schütz, 1948).
Strong reactivity of the Lys 15 side chain toward cyanate is also demonstrated more directly by limited proteolysis of wild-type' TTR in conjunction with mass spectrometric analysis of the digested peptides (Figure S2). Using a peptide that specifically reports on Lys 15 carbamoylation, we found that the ratio of carbamoylated peptide over non-carbamoylated peptide increased 20-fold upon exposure to 10 mM cyanate for 17 h at 37°C prior to proteolysis. We found no evidence for significant carbamoylation of the Lys 35 and Lys 70 ε-amino side chains over the same time period, suggesting that these Lys side chain are less pKa-perturbed than Lys 15. Fast carbamoylation of the N-terminal amine, or slower carbamoylation of the remaining 5 Lys residues (see Figure S3 for their location in the tetramer subunit) could not be detected using this method. Relevant peptides were either not identified by mass spectrometry (Lys 9, Lys 48, Lys 76, Lys 80) or the number of peptides was too low to be statistically significant (N-terminal amine, Lys 126) (see Tables S1–S3 for more details).
Previously, we reported that TTR can be chromatographically separated from TTR that is covalently modified by a fragment of A2 at Lys 15 by C8 reversed phase chromatography (Choi et al., 2010). We reasoned that the same assay should be applicable to monitor carbamoylation of all eight Lys side chains and the N-terminal amine, although isoelectric focusing is a potential alternative approach (Altland et al., 1999; Altland et al., 2007). At physiological pH and temperature, with the addition of the relevant urea decomposition product (10 mM potassium cyanate, a concentration reached in aged urea solutions at elevated temperature (Dirnhuber & Schütz, 1948; Stark et al., 1960)), wild-type' TTR carbamoylation occurs on two distinct time scales (Figures S4–S7). In a fast phase, completed within 24 h, the pKa-perturbed Lys 15 ε-amino group and apparently the N-terminal amine are near quantitatively carbamoylated. The N-terminal region of TTR is unstructured (not visible in the TTR X-ray structures) and should have a pKa-value around 8.0 (Thurlkill et al., 2006). A second phase of carbamoylation occurs on a time scale of days to weeks. The existence of a second modification phase is particularly obvious upon increasing the cyanate concentration from 10 to 200 mM, which greatly accelerates this phase, leading to much higher carbamoylation levels within just 24 h (Figure S6).
2.4 Cyanate binds non-covalently to the T4-hormone binding site of the TTR tetramer
Structural studies and biophysical assays demonstrate that chloride, iodide, and thiocyanate anions bind to the T4-hormone binding pockets at the dimer–dimer interface of the TTR tetramer (Hammarstrom et al., 2001; Hörnberg et al., 2005). These binding events kinetically stabilize the TTR tetramer against unfolding in urea solutions and against unfolding and aggregation at low pH in aqueous solution (Lai et al., 1997). To test whether cyanate, which is structurally related to thiocyanate, also binds to the TTR tetramer, we evaluated the rate of unfolding of TTR in 6 M urea as a function of added potassium cyanate. If cyanate binds to the tetramer prior to unfolding, we expect a decrease in the unfolding rate constant. To minimize the contribution from competing carbamoylation to unfolding kinetics, we used the Cys10Ala/Val122Ala TTR variant (Val122Ala hereafter), which dissociates and unfolds within minutes in concentrated urea solutions, much faster than Lys 15 becomes carbamoylated in concentrated urea solutions (time scale of hours). The Val 122 side chain does not directly contribute to the formation of the T4-binding site, and the TTR Val122Ala mutation should only minimally perturb the structure of the T4-hormone binding site.
Unfolding of Val122Ala TTR in urea results in an increase in the Trp fluorescence emission intensity and a redshift of the emission spectral maximum (Figures 3a and S8A). The unfolding reaction was monitored by the change in the fluorescence intensity ratio at two wavelengths (I355/I335), calculated from time-tagged fluorescence emission spectra. Control experiments ascertained that the kinetic rate constants extracted from trajectories rendered by the ratiometric method agree with rate constants from fits of fluorescence intensity trajectories collected at a single wavelength (i.e., I355) (Figure S8A). The ratiometric method, however, has the advantage that it eliminates intensity fluctuations (Figure S8B), which makes this method ideal for tracking kinetically stable proteins with unfolding times of weeks to months.
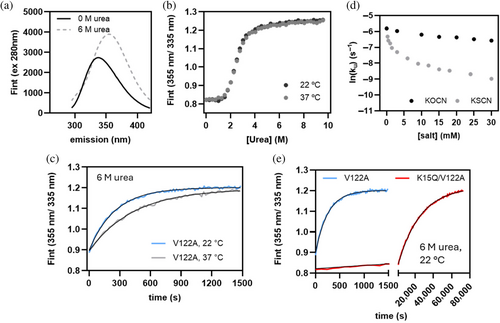
Val122Ala TTR unfolds cooperatively at equilibrium (Figure 3b) and in 6 M urea with single exponential kinetics at both ambient and at human physiological temperature (Figure 3c). We attribute the approximately twofold slower unfolding rate at 37°C (ku = 3.9 ± 0.02 s−1 at 22°C; ku = 2.3 ± 0.02 s−1 at 37°C) to an increased hydrophobic effect at elevated temperatures that helps stabilize the native tetrameric structure (Southall et al., 2002). Adding cyanate up to a concentration of 30 mM slows down unfolding at 22°C moderately (by less than one order of magnitude) (Figure 3d). Despite this rather modest effect, the mere fact that cyanate binds to the T4-hormone binding site would bring this reactive electrophile in direct contact with the nucleophilic Lys 15 side chain, which likely contributes to the susceptibility of Lys 15 ε-amino group to undergo carbamoylation. Even stronger kinetic stabilization is observed with structurally related, but chemically more inert thiocyanate (Figure 3d). The higher affinity of thiocyanate likely results from thiocyanate being the more polarizable and more hydrophobic anion. Thiocyanate is also poorly solvated in aqueous solvents, minimizing the desolvation penalty for its binding to the T4-hormone binding interface (Mason et al., 2003).
The effect of carbamoylating the ɛ-amino group of Lys15 on TTR tetramer unfolding kinetics was estimated by conducting unfolding experiments in the context of the kinetically compromised Val122Ala TTR variant in which Lysine 15 was replaced by glutamine (TTR Cys10Ala/Val122Ala/Lys15Gln). The Lys15Gln mutation retains most of the aliphatic side chain of Lysine 15 and carries the same polar terminus as carbamoylated Lys 15 and, thus, should serve as an imperfect approximation for carbamoylated Lys 15 side chain.
Like Val122Ala TTR, Val122Ala/Lys15Gln TTR unfolds with single exponential kinetics in 6 M urea and at ambient temperature. The rate constant for Val122Ala /Lys15Gln TTR (ku = 4.1 ± 0.06 s−1) corresponds to a two order of magnitude gain in kinetic stability relative to Val122Ala TTR (Figure 3e). Interestingly, we observed similar kinetic stabilization with less structurally conservative mutants, wherein Lys 15 was replaced by an Ala or Gly residue (Figure S9). Earlier work by us reported that the Lys15Ala mutation is also kinetically stabilizing in the context of wild-type TTR (harboring the Cys 10 and Met 13 residues), but because of the already enhanced kinetic stability of wild-type TTR, no rate constant could be extracted (Hammarstrom et al., 2001). We conclude that eliminating the unfavorable charge–charge repulsion between two juxtaposed dimers in the TTR tetramer is more critical for increased kinetic stability of the TTR tetramer than the size and chemical identity of the side chain that replaces the charged ε-ammonium Lysine side chain at position 15.
2.5 Carbamoylation of Lys 15 can compete with unfolding of kinetically stable wild-type' TTR
For Lys carbamoylation to kinetically compete with TTR-linked dissociation and unfolding in concentrated urea solutions, the unfolding rate of the TTR tetramer must be comparable to, or slower, than the rate at which a fresh urea solution decomposes into ammonium cyanate to carbamoylate TTR. Val122Ala TTR simply dissociates and unfolds too fast in concentrated urea solutions for Lys carbamoylation to have an impact on unfolding kinetics. We therefore focus our attention on the kinetically more stable wild-type' TTR sequence.
At low temperature (4°C), where effectively no carbamoylation occurs (Figure 2d), wild-type' TTR dissociates and unfolds over a time course of 4 days in 6 M urea, exhibiting single exponential kinetics (Figure 4a, blue filled circles, Figure S10). Notably, tetramer dissociation, rate-limiting for subunit exchange among tetrameric subunits, occurs at the same rate as urea denaturation until carbamoylation starts to alter the tetramer dissociation rate (Hammarstrom et al., 2002; Wiseman et al., 2005). In contrast, strongly non-exponential kinetics are observed at 37°C, where the fluorescence trajectory enters a plateau phase after approximately 12 h into the unfolding reaction, suggesting that linked tetramer dissociation and unfolding become significantly slowed (Figure 4a, red filled circles). The protein sample remains in this kinetically stable state for almost 2 days before further unfolding occurs and TTR denaturation goes to completion (as judged by the asymptotic (I355/I335) ratio). The resulting trajectory appears non-exponential with two unfolding processes separated by a plateau or lag phase. We note that the time range where the unfolding trajectory leaves the plateau phase and the fluorescence intensity ratio increases again coincides with the slow phase of carbamoylation of a subset of the seven Lys ε-amino side chains in each TTR subunit (not including Lys 15 which is rapidly carbamoylated) detected by the reversed phase HPLC assay (see Figures S3–S6 for details). Prior publications argue against the hypothesis that the plateau phase is caused by a kinetic uncoupling of tetramer dissociation and unfolding of released native-like dimers or monomers. In such a scenario, the rate-limiting step for TTR unfolding would be unfolding of released dimers or monomers, not dissociation of the tetramer. Engineered TTR monomers, however, unfold on the millisecond time scale (Conti et al., 2014; Jiang et al., 2001), and while stable dimeric variants of TTR have been reported, they aggregate on a much faster time scale than tetrameric wild-type TTR (Martins et al., 2024; Mizuguchi et al., 2012), suggesting also much reduced kinetic stability against dissociation and unfolding than intact tetramers. Thioflavin T binding assays were performed to rule out that transient aggregation of TTR contributes significantly to the kinetic heterogeneity observed at physiological temperature (Figure S11).
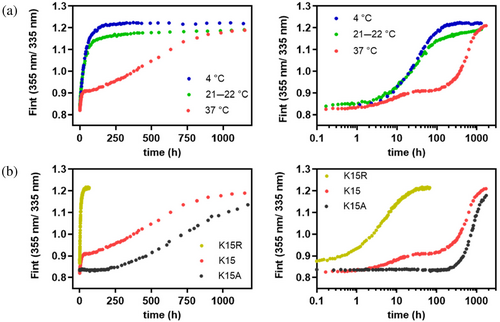
As the plateau phase is only seen at human physiological temperature, that is, under conditions where Lys carbamoylation is favored, it is reasonable to assume that the presence of a plateau phase is the manifestation of carbamoylation of the TTR tetramer. We demonstrated above that Lys 15 is pKa-perturbed (Figure 2c), more nucleophilic, and more susceptible to carbamoylation than Lys 35 and Lys 70 (Figure S2), so it is likely that carbamoylation of Lys 15 causes the plateau by significantly slowing down further dissociation linked to unfolding. Direct support for this hypothesis comes from our observation that the Lys15Gln mutation, as an imperfect but reasonable approximation for carbamoylated Lys 15, slows down unfolding of kinetically compromised TTR Val122Ala by two orders of magnitude in 6 M urea at 22°C (Figure 3e). The amplitude in the unfolding trajectory that precedes the plateau phase would then be an estimate of the fraction of tetramer that escaped Lys 15 carbamoylation prior to urea-mediated denaturation, while the amplitude of the phase that follows the plateau would correspond to unfolding of the kinetically more stable Lys 15-carbamoylated TTR subpopulation. As we elaborate in more detail in a following paragraph, unfolding of the Lys 15-modified subpopulation is likely triggered by additional carbamoylation events at a subset of the other 7 Lys ɛ-amino side chains in the TTR subunit, as the time scale of unfolding overlaps with the time scale of slow carbamoylation detected by our reversed phase HPLC assay. It is important to note, however, that if these additional Lysine side chains would be carbamoylated by cyanate with exponential kinetics, the sum of all Lysine carbamoylation reactions would still follow single exponential kinetics and no lag phase should be observed. Carbamoylation of two or more Lysine side chains may thus be required to trigger global unfolding of the Lys 15-carbamoylated TTR tetramer, which naturally explains the plateau phase in the trajectory.
At 21-22°C, the fraction of protein that unfolds in the faster phase is substantially increased (Figure 4a, green filled circles) relative to the measurement at 37°C, consistent with the slower rate of cyanate formation around 25°C. The trajectory fits to a single exponential up to 4 days, but an additional slow phase is still visible. Its amplitude, however, is too small to decide whether this phase obeys exponential kinetics or is also preceded by a plateau phase (Figure S10).
Temperature-dependent equilibrium unfolding transitions recorded after a 7-day incubation in urea quantitatively agree with the kinetics data at that time point (Figure S12), suggesting that the unfolding transition at 37°C and at day 7 has not yet reached equilibrium, and the unfolding transition contains no relevant thermodynamic information. We also note that the data obtained with wild-type' TTR in this study qualitatively agree with previous measurements on wild-type TTR that retained residues Cys10 and Met 13 (Hammarstrom et al., 2001).
The degree of deviation of wild-type' TTR from exponential kinetics in 6 M urea correlates with temperature and thus likely with the extent of carbamoylation, which is favored at higher temperatures. To demonstrate this directly, we also monitored TTR unfolding in 6 M urea at the three temperatures using our C8 reversed phase HPLC assay (Figure S13). The 6 M urea modification reaction at 37°C looks very similar to the results we obtained by modifying tetramer with 10 mM cyanate at 37°C (Figure S4). The kinetics of carbamoylation in 6 M urea, however, lags behind the kinetics of carbamoylation in 10 mM cyanate without urea, which we attribute to the time it takes a freshly made urea solution to decompose into a cyanate concentration sufficient to mediate carbamoylation. Substantially less chemical carbamoylation is detectable in 6 M urea at 21-22°C, consistent with the significantly reduced amplitude of the slow unfolding phase under these conditions detected by Trp fluorescence changes.
We next asked whether we could eliminate the plateau- and slow unfolding phase in the wild-type' TTR unfolding trajectory, which we attribute to dissociation and unfolding of the Lys 15-carbamoylated wild-type' TTR subpopulation, by replacing Lys 15 with Arg. Arginine retains the positive charge, but unlike Lys 15, it cannot undergo carbamoylation by cyanate. We found that Lys15Arg TTR unfolds completely and with single exponential kinetics in 6 M urea, even at 37°C (Figure 4b, ochre closed circles). Lys15Arg TTR thus resembles the unfolding of wild-type' TTR at 4°C, where Lys 15-carbamoylation is suppressed. Like the Lys15Gln mutation, Lys15Ala eliminates unfavorable charge–charge repulsion between two juxtaposed dimer subunits in the intact tetramer and slows down unfolding of the TTR Val122Ala tetramer in 6 M urea by two orders of magnitude (Figure S9). In the wild-type' TTR context, Lys15Ala prevents unfolding of the tetramer over the time scale where dissociation and unfolding occurs with unmodified wild-type' TTR and Lys15Arg TTR (Figure 4b, black filled circles). Lys15Ala TTR thus kinetically resembles Lys15-carbamoylated wild-type' TTR, except that the fast unfolding phase seen in wild-type' TTR is absent, and unfolding occurs in a slower phase after the plateau phase.
2.6 Sample acidification or small molecule cyanate scavenging at neutral pH reduces, but does not prevent Lys 15-carbamoylation
Protonation of the ε-amino group of Lys 15 at more acidic pH renders it less nucleophilic (Figure 2a) and should favor linked dissociation and unfolding of non-carbamoylated wild-type' TTR in 6 M urea vs competitive Lys 15 carbamoylation, which kinetically stabilizes the tetramer against dissociation. Indeed, at pH 5.50 in 6 M urea, a pH-value below the estimated pKa-value of Lys 15, the amplitude of the fast phase increases by two-fold when compared to pH 7.40 (Figure 5a, blue filled circles).
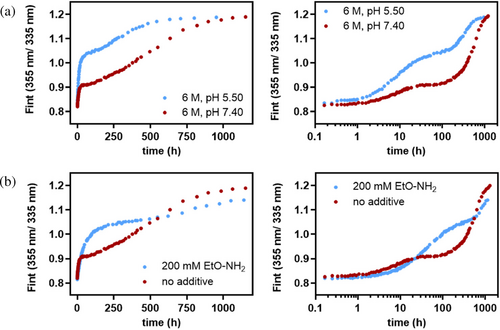
Dissociation and unfolding of unmodified tetramer over competing Lys carbamoylation is also favored at pH 7.4 by adding a stoichiometric excess (200 mM) of small molecule cyanate scavenger ethyloxyamine (pKa = 4.50) (Figure 5b, blue filled circles). Ethyloxyamine, however, requires concentrated hydrochloride acid to be added to adjust the pH of the urea/ethyloxyamine solution to a neutral pH-value. The added chloride ions bind to the T4-hormone binding interface of the tetramer and increase the kinetic stability of wild-type' TTR.
2.7 Slow carbamoylation of the less reactive Lys side chains hastens TTR tetramer dissociation and unfolding
Besides Lys 15, there are seven additional Lysine residues (Lys 9, Lys 35, Lys 48, Lys 70, Lys 76, Lys 80, and Lys 126) in the TTR subunit (Figure S3a). Lys9 and Lys126, as well as the N-terminal amine, are located in the flexible N- or C-termini and are not visible in X-ray structures of wild-type' TTR (this study) and several other structures of TTR variants (Hörnberg et al., 2000). Carbamoylation at these sites should not significantly affect the unfolding energetics of TTR. The side chains of Lys70 and 76, however, engage in side chain interactions that may help stabilize the subunit and the tetramer assembly (Figure S3). We speculated that slow carbamoylation of these Lys ε-amino groups may accelerate tetramer dissociation and subunit denaturation in urea.
To scrutinize this hypothesis, we compared the unfolding kinetics of Lys15Ala TTR with Lys15Ala TTR that had been subjected to a pre-carbamoylation pulse under native conditions prior to unfolding in 6 M urea at 37°C. We verified by reversed phase HPLC that under the carbamoylation conditions employed (200 mM cyanate, 10 h, 37°C), TTR becomes heavily modified and to a level not reached in 10 mM cyanate at 37°C, even over a period of days (Figure S7). We found that pre-carbamoylation under these conditions resulted in faster tetramer denaturation and a shortening of the plateau phase in the unfolding trajectories; that is, the unfolding trajectories appear more exponential. We take this as evidence that the slow unfolding phase that follows the plateau phase in the unfolding trajectory of Lys15Ala TTR is indeed caused by carbamoylation of non-Lys15 side chains (Figure 6a).
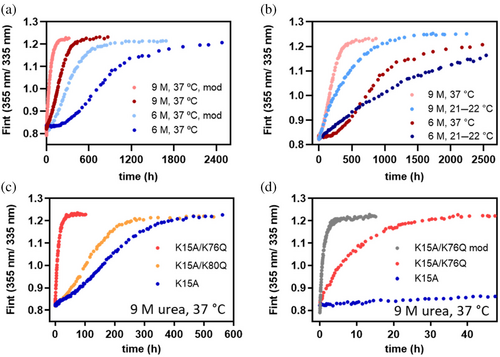
Analogously to the experiments performed with wild-type' TTR (Figure 4a), we also compared the effect of elevated temperature on the shape of the unfolding trajectories of Lys15Ala TTR without pre-carbamoylation (Figure 6b). At 21-22°C, the unfolding trajectories in both 6 M and 9 M urea can be fit to a single exponential function (Figure S14A). At 37°C, the unfolding trajectory either closely traces (9 M urea) or slightly lags behind (6 M urea) the trajectory at ambient temperature. With increasing unfolding duration, however, the unfolding trajectories at 37°C start to deviate from exponential kinetics and overtake the trajectories collected at 21-22°C, which gives the 37°C trajectories their distinctly sigmoidal shape with a visible lag phase (Figures 6b and S14B). The same trend is visible whether the trajectories are rendered ratiometrically, or from intensity changes at a single wavelength (I355) (Figure S14C).
We recall that both Val122Ala TTR and Lys15Arg TTR unfold with single exponential kinetics at 37°C, because linked tetramer dissociation and monomer unfolding kinetically outcompetes Lys 15 carbamoylation in Val122Ala TTR, and because the Arg15 residue cannot be carbamoylated in Lys15Arg TTR. Again, this is further evidence that the sigmoidal unfolding trajectories obtained with Lys15Ala TTR at 37°C result from carbamoylation of lysines other than Lys 15, which occurs after a lag-time long enough to allow buildup of a critical concentration of cyanate. Whether these carbamoylation events happen on different Lys residues within the same monomer subunit, or on the same Lysine residue but in more than one subunit in the tetramer, or both, cannot be answered from the available data.
2.8 Approximating carbamoylation of specific Lys side chains by Lys-to-Gln mutation
Lys 76 is highly conserved among the TTR family of proteins and is positioned in the single helix (residues 74–83) of the tetramer subunit, where it forms a salt bridge with the side chain of Glu 89, which is located further C-terminal to the helix (Figure S3b). Carbamoylation of Lys 76 would break this salt bridge. Lys 70 is positioned in the center of β-strand E. Its positively charged ε-amino group engages in a presumably energetically favorable cation-pi interaction with the indole ring of Trp 41 and forms a salt bridge with the side chain of Glu 72 from the same subunit. The Lys 70 ε-amino group is also positioned close to the negative side chain of Glu 92 from the same subunit and to Glu 92′ from a juxtaposed subunit in the dimer (Figure S3c). Carbamoylation of Lys 70 would break these interactions. The ε-amino groups of Lys 35, Lys 48, and Lys 80 face into solvent and do not engage in salt bridges, and residues Lys 9 and Lys 126 are located in the disordered N- and C-terminus, respectively. Their carbamoylation should have a minimal impact on the folding energetics of the TTR tetramer.
A rigorous test of the effect of carbamoylation of individual Lys residues on coupled TTR dissociation/unfolding kinetics requires replacement of individual Lys residues by homocitrulline. This necessitates chemical synthesis of the 127-residue TTR subunit, followed by the assembly of the subunits into functional tetramer, a challenge even for experts in chemical protein synthesis. While Lys-to-Gln mutations are imperfect approximations for homocitrulline, they can be produced recombinantly and were used herein to explore the effects of carbamoylation of individual Lys residues other than Lys 15.
In agreement with our structure-based reasoning, the Lys76Gln mutation in the helix of the Lys15Ala host variant greatly accelerates linked TTR tetramer dissociation and unfolding in 9 M urea at 37°C (Figure 6c). The Lys80Gln mutation serving as a surrogate for the Lys 80 carbamoylation also hastens tetramer dissociation/unfolding and largely retains the sigmoidal shape of the unfolding trajectory, but the energetic effect is attenuated relative to Lys 76 charge removal.
Pre-carbamoylation of Lys15Ala/Lys76Gln TTR under native conditions (100 mM cyanate) and prior to unfolding in 6 M urea further accelerates tetramer dissociation/unfolding (Figure 6d). However, the gain in the rate of unfolding upon pre-carbamoylation of TTR Lys76Gln is modest compared to the gain in the dissociation and unfolding rate achieved with the Lys76Gln mutation and without pre-carbamoylation. This suggests that the Lys76-Gln89 direct salt bridge is a key interaction for the kinetic stability of TTR in 6 M urea, and its disruption by Lys76 carbamoylation greatly accelerates both the unfolding of Lys15Ala TTR and Lys15-carbamoylated wild-type' TTR.
Taken together, these data strongly suggest that the apparently sigmoidal unfolding kinetics of Lys 15 carbamoylated wild-type' TTR and Lys15Ala TTR results from stochastic carbamoylation of one or more of the less pKa-perturbed Lys ε-amino side chains. Carbamoylation of the ε-amino group of Lys 70 and 76 eliminates critical side chain interactions that lead to the destabilization of the TTR tetramer. Unfolding of wild-type' TTR is therefore affected by carbamoylation on two different time scales and with opposite energetic outcomes. Early in the unfolding reaction, the pKa-perturbed reactive Lys 15 becomes carbamoylated, which greatly increases the kinetic stability of the Lys 15-modified tetramer against unfolding in 6 or 9 M urea. On a slower time scale, the Lys 15-carbamoylated protein is then destabilized again by carbamoylation at other, less reactive Lysine residues. In the Lys15Ala TTR variant, modification of the reactive Lys 15 is no longer possible, but the kinetically stabilizing effect of Lys 15 carbamoylation is simulated by the Lys15Ala mutation, which eliminates unfavorable Lys 15 ε-ammonium charge–charge repulsion in the tetramer. The unfolding trajectory of Lys15Ala TTR thus shows only one major unfolding phase that is preceded by the plateau phase.
3 DISCUSSION
A search of the Web of Science database for the topics “urea” and “folding” returned over 5900 entries. In the great majority of these studies, it is assumed that urea acts as a passive reporter of (un)folding kinetics and stability studies by tuning folding barriers and ground state stabilities, and it is assumed that urea does not actively change the underlying protein folding mechanism. Although this assumption is reasonable for proteins amenable to rapid dissociation and/or denaturation, usually little attention is given to the chemical instability of urea and its tendency to decompose into ammonium cyanate/isocyanic acid, depending on the pH of the aqueous phase, and how Lys carbamoylation by cyanate, a reactive electrophile, affects the denaturation energy landscape of the protein of interest. Cyanate at pH 7.5 reacts with both the N-terminal amine of a protein and the ε-amino group of Lys side chains, particularly those that are pKa perturbed, converting Lys side chains that are often charged into neutral homocitrulline side chains. For kinetically stable proteins, as we show here with human TTR, Lys carbamoylation can kinetically compete with coupled TTR tetramer dissociation and subunit unfolding and can mediate protein modification reactions that can drastically change the protein's dissociation and denaturation energy landscape. Carbamoylation of TTR on two different time scales, and with opposite energetic outcomes (first stabilizing, then destabilizing), complicates data analysis because the energetics of the tetramer are changing on time scale of the urea denaturation measurement—providing a dramatic example of how carbamoylation can alter the denaturation energy landscape. Carbamoylation of the most pKa-perturbed Lys ε-amino group of TTR, that is, Lys-15, affords chemically modified, kinetically stable TTR early in the urea-mediated unfolding time course, slowing down urea-mediated TTR denaturation substantially, as tetramer dissociation of TTR is rate-limiting for urea denaturation. On a much slower time scale, TTR is kinetically destabilized due to carbamoylation of the remaining, less reactive Lysine side chains (by breaking stabilizing electrostatic interactions in TTR), allowing urea denaturation to proceed more rapidly again later in the urea unfolding time course.
Cyanate derived from urea decomposition affects TTR folding energetics on at least two time scales, both through weak, non-covalent interactions and through covalent side chain modifications (Figure 7). Cyanate interacts reversibly with the intact TTR tetramer by binding to the T4-hormone binding sites. The kinetic stabilizing effect is modest and slows the rate of coupled dissociation and denaturation by less than an order of magnitude. Nevertheless, the effect is significant enough to cause subtle deviation from linearity in kinetic rate plots that could be misinterpreted as being derived from transient folding intermediates. Weak binding also brings cyanate in close proximity to the nucleophilic Lys 15 side chain, an important contributor to the high susceptibility of Lys 15 to cyanate-mediated carbamoylation. Once TTR has been Lys 15-carbamoylated, its coupled dissociation and unfolding is greatly slowed down, effectively stalled for up to 100 h in 6 M urea at human physiological temperature. Carbamoylation of TTR causes kinetic heterogeneity. The extent of partitioning of TTR into a Lys 15-carbamoylated subpopulation with enhanced kinetic stability depends on both the intrinsic TTR kinetic stability, and thus the rate of tetramer dissociation of the TTR variant under study, as well as environmental conditions, such as temperature, pH, inorganic salt ions, and other organic small molecule ligands. Urea concentration, temperature, and pH also strongly affect cyanate concentration, influencing the rate of TTR carbamoylation.
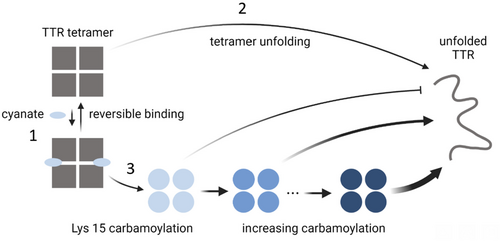
When Lys 15 carbamoylation kinetically stabilizes the TTR tetramer, the other seven Lys residues present in the subunit also become prone to slower Lys carbamoylation in the native tetramer, despite their presumably elevated, more normal pKa-values. Kinetic data from the Lys76Gln TTR variant, which eliminates a conserved salt bridge that stabilizes the single α-helical substructure in each TTR subunit, and which we used here as a proxy for carbamoylated Lys 76, reveal a hastened coupled tetramer dissociation and monomer unfolding rate. Pre-carbamoylation under native conditions and prior to unfolding in denaturant further accelerates the coupled dissociation and urea-mediated unfolding of Lys76Gln TTR, suggesting that carbamoylation of non-pKa-perturbed Lys residues in addition to Lys 76 also hastens TTR dissociation-coupled unfolding. Because of the stochastic nature of Lys carbamoylation and the possibility of non-additive effect upon carbamoylation of multiple Lys residue per tetramer, unfolding of Lys 15-carbamoylated TTR tetramer does not obey single exponential kinetics and exhibits a lag phase that precedes the slow unfolding reaction.
Thus, especially at human physiological temperature, Lys 15 carbamoylation interferes with quantitative kinetic studies. The Lys15Arg variant discussed here provides a solution, as it eliminates Lys 15 carbamoylation, but the slightly larger Arg side chain may also affect T4-hormone binding or kinetic stabilizer binding.
Reducing the reactivity of Lys residues toward carbamoylation by lowering the pH-value of the solution, or by reducing cyanate in urea solutions by adding small molecule scavengers at physiological pH-value, reduce but do not prevent carbamoylation, and both options have disadvantages. Small molecule scavengers such as ethyloxyamine require hydrochloric acid for pH-adjustment to physiological pH and cause unwanted kinetic stabilization of the tetramer as a result of chloride anion binding to the T4-hormone binding interface. A low pH-value, on the other hand, increases the tendency of TTR to misfold and aggregate, and it has the potential to change the unfolding mechanism (Lai et al., 1996).
In addition to mutations such as Lys15Arg, low temperature (4°C) also suppresses carbamoylation in wild-type' TTR, but favors tetramer dissociation in some TTR variants (Ferguson et al., 2021; Sun et al., 2018), which again may introduce unwanted kinetic heterogeneity. At ambient temperature, carbamoylation is not prevented, but reduced enough such that meaningful kinetic rate constants of unfolding can be obtained from fits to the fluorescence intensity trajectories by excluding the later part of the fluorescence unfolding trajectory. This illustrates the usefulness of temperature tuning for some kinetic folding and association studies.
In light of the results presented here, particular caution needs to be exercised when interpreting TTR kinetic unfolding data in urea solutions, especially with TTR sequences exhibiting kinetic stability similar to or exceeding that of wild-type' or wild-type TTR. Several high kinetic stability TTR variants have been reported over the years—for example, Thr119Met TTR (Hammarstrom et al., 2002; Sekijima et al., 2006), Ala108Val/Ile TTR (Sant'Anna et al., 2017), or Ile73Leu TTR (Nakagawa et al., 2022). The high kinetic stability of these variants is usually attributed to the stabilizing effect of the particular mutation they carry. The present work, however, suggests that unless the kinetic stability is verified by an alternative technique, such as subunit exchange (Rappley et al., 2014; Schneider et al., 2001), kinetically stabilizing Lys 15 carbamoylation may contribute or even dominate the measured kinetic stability of these variants in urea solutions.
Proteins with extremely long half-lives (e.g., collagens) or proteins that are not turned over in vivo (e.g., eye lens crystallins) are known to accumulate high levels of carbamoylated Lys side chains, a process that is associated with human disease and aging. TTR is an extracellular protein that is secreted by the liver into the bloodstream, where it circulates with a half-life of 2–3 days. There is ample evidence that plasma proteins, such as albumin, hemoglobin, and low-density lipoprotein undergo carbamoylation (Basnakian et al., 2010; Battle et al., 2022; Delanghe et al., 2017; Jaisson & Pietrement, 2018), so we wondered whether Lys carbamoylation of TTR, as we detect in this study in vitro, is also a relevant post-translational modification for TTR in vivo. Several proteomics studies performed on TTR isolated from human plasma reveal extensive post-translational modification of TTR at the oxidation-prone residues Cys 10 and Met 13, but surprisingly, there is no evidence that TTR becomes carbamoylated in vivo (Biroccio et al., 2006; Henze et al., 2015; Vilà-Rico et al., 2015).
Importantly, our in vitro data suggest that TTR carbamoylation can be either stabilizing (e.g., Lys 15 carbamoylation) or destabilizing (e.g., carbamoylation of Lys 76). The pKa-perturbed Lys 15 residue undergoes fast carbamoylation in the TTR tetramer, strongly kinetically stabilizing TTR. Slow carbamoylation of non-Lys 15 residues kinetically destabilizes the TTR tetramer, allowing Lys-15-carbamoylated TTR to dissociate and denature in concentrated urea solutions. Carbamoylation strongly modifies the dissociation and denaturation energy landscape of TTR, and once this is appreciated, urea can still be used effectively to probe dissociation and misfolding energetics of TTR and related kinetically stable proteins.
4 MATERIALS AND METHODS
4.1 Reagents and chemicals
Urea, potassium cyanate, potassium thiocyanate, guanidinium thiocyanate, and ammonium sulfate were obtained at the highest available purity from Sigma Aldrich. Sodium phosphate (monobasic) was purchased from MP Biomedicals. Small molecule A2 was chemically synthesized in our laboratory and dissolved at a concentration of 5 mM in dimethysulfoxide (DMSO) and stored as 50 μL aliquots at −78°C until use.
4.2 Mutant cloning, expression, and purification
TTR variants were expressed without a histidine tag. Point mutations were introduced into the sequence of wild-type' TTR using the Quick Change mutagenesis kit (Agilent) and verified by DNA sequencing of the TTR gene. For protein expression, respective plasmids were transformed into BL21 DE3 cells (New England Biolabs) and plated onto LB-agar/Kanamycin plates. Culturing of bacteria was done in Luria-Bertani broth at a 1 liter scale in 3 L flasks under constant agitation in a shaker unit at 37°C. Protein expression was induced by adding Isopropyl-beta-D-thiogalactoside (IPTG, GoldBio, St. Louis, MO) (1 mM final concentration) at a cell culture optical density of around 0.4 at 600 nm. Shaking was continued for 12 h at 24°C. Bacteria were pelleted by centrifugation, resuspended in 10 mM Tris, pH 8.0, and supplemented with protease inhibitor (Pierce). The bacterial suspension was sonicated at ambient temperature, transferred to 2 mL Eppendorf tubes, and centrifuged in a benchtop centrifuge at 12,000 rpm for 10 min. The supernatant was filtered (0.45 μm, Millex-HV) and supplemented with ammonium sulfate to 50% saturation. After gentle stirring for 2 h at ambient temperature, the solution was centrifuged (10 min, 12,000 rpm, benchtop centrifuge), and the supernatant was adjusted with ammonium sulfate to 90% saturation. After gentle stirring for another 2 h, the pellet was collected by centrifugation (10 min, 12,000 rpm, benchtop centrifuge), resuspended in 10 mM Tris, pH 8.0 (final volume <30 mL), and dialyzed against 5 L of the same buffer.
The dialyzed protein solution was filtered (0.45 μM, Millex-HV), loaded onto a Source Q column (bed volume 2.6 × 15 cm), and equilibrated in 10 mM Tris, pH 8.0. Protein elution was accomplished using a linear gradient of sodium chloride in 10 mM Tris, pH 8.0. Peak fractions of the eluted protein were collected and injected onto a semiprep-scale HiLoad 16/600 Superdex75 column (GE Healthcare) and equilibrated in 50 mM sodium phosphate, 100 mM sodium chloride, pH 7.4. Fractions containing tetrameric TTR were collected, concentrated to a volume of approximately 5 mL in a centrifugation filter unit (Millipore), and dialyzed twice against 5 L of 50 mM sodium phosphate, pH 7.4, at ambient temperature. The molecular weight of purified proteins was confirmed by mass spectrometry, and purified protein was either used immediately for biophysical and analytical experiments or stored at 4°C for no longer than 1 week.
4.3 Isocyanic acid/cyanate modification assay using C8 reverse phase chromatography
TTR carbamoylation was investigated by using an analytical, reversed phase-based chromatographic retention assay. The assay relies on previous observations made by us that conjugation of small molecule ligands to Cys10 or Lys15 in TTR leads to increased protein retention and longer elution times from the C8-resin. In the course of the present study, we found that protein column retention also increased by either mutating residue Lysine 15 (charged) to Ala (neutral), or upon exposure of TTR to electrophiles such as cyanate, which convert charged Lys side chains into neutral homocitrulline side chains by means of chemical carbamoylation.
For the TTR carbamoylation time series under native conditions, we prepared a stock solution of TTR tetramer (3.6 μM tetramer) at 37°C. At time zero, we added cyanate to the protein solution at a concentration of 10 mM. At discrete time points, we withdrew a 150 μL sample aliquot from the TTR/cyanate stock solution, flash-froze the aliquot in liquid nitrogen in a 0.5 mL Eppendorf tube, and stored the frozen aliquot at −78°C until use. Once aliquots of the entire time series were available, aliquots were thawed on ice, and immediately analyzed by C8 reversed phase chromatography. To minimize carbamoylation in the sample prior to chromatography, it was essential to process the time-tagged aliquots manually and strictly sequentially, that is, by thawing the next sample aliquot after the chromatography run of the previous sample was completed.
Chromatograms were also collected under denaturing conditions by unfolding TTR tetramer in 3 M guanidinium thiosulfate prior to addition of 10 mM cyanate. Although structurally related to cyanate, we found no evidence for thiocyanate carbamoylating Lys side chains.
To complement the tryptophan fluorescence-based kinetic unfolding experiments in urea, we analyzed TTR carbamoylation by reversed phase chromatography by diluting native TTR tetramer into 6 M urea. Sample handling under denaturing conditions was analogous to sample handling under native conditions, except that no extra cyanate was added to the solution, as cyanate was allowed to form in the denaturant solution by urea decomposition.
In the main text, we also describe experiments performed with pre-carbamoylated protein. By the term pre-carbamoylation, we refer to protein that was subject to a 10-h incubation period at 37°C in 50 mM sodium phosphate, 100 mM sodium cyanate, prior to unfolding of the modified protein in denaturant (6 M or 9 M urea). These experiments were done with TTR samples carrying the Lys15Ala mutation.
4.4 Probing Lysine 15 carbamoylation by an indirect, fluorescence-based assay
In addition to the chromatography-based carbamoylation assay described in the previous section, we also used an alternative assay that allowed us to specifically monitor carbamoylation of the reactive, pKa-perturbed Lys 15 side chain. The assay rests on small molecule A2, a non-fluorescent stilbene derivative, designed to bind specifically to the T4-hormone binding site in the TTR tetramer (see main text for details).
To specifically monitor Lys 15 modification in native TTR, we diluted a stock solution of wild-type' TTR into 50 mM sodium phosphate, pH 7.40 and 0.5 M urea (added from a 2 M urea stock solution freshly prepared at 4°C). TTR does not unfold at urea concentrations below 2 M urea (Hammarstrom et al., 2001; Jiang et al., 2001), but urea will decompose over time and depending on temperature into isocyanic acid/cyanate, reactive electrophiles that will convert nucleophilic Lys 15 into homocitrulline. Upon carbamoylation into homocitrulline, residue 15 no longer reacts with the stilbene derivate A2 when added to the protein/urea solution in stoichiometric excess (20 μM). Therefore, plotting the A2 fragment-wild-type' TTR conjugate fluorescence against the incubation time of TTR in the presence of 0.5 M urea directly reports on the kinetics of carbamoylation of Lys 15.
4.5 Chemical reactivity-based estimation of the pKa-value of the nucleophilic Lys 15 side chain
A stock solution of 150 μM of native TTR tetramer in 5 mM sodium phosphate, pH 7.40 was diluted 100-fold into aqueous buffer with a pH ranging from 4.2 to 8.8 in a 2 mL quartz cuvette and thermostated at 25°C in a spectrofluorometer. To the protein solution was added small molecule A2 at a final concentration of 20 μM (diluted from a 5 mM stock solution of A2 in DMSO), and the time-dependent increase of A2 fragment-wild-type' TTR conjugate fluorescence at 428 nm was measured (excitation: 330 nm). We noted that the kinetics of conjugation decreases with the pH-value of the solution. To estimate the pKa-value of the Lys 15 side chain, we plotted the A2 fragment-wild-type' TTR conjugate fluorescence intensity after an incubation time of 1200 s versus the pH-value of the solution. We found that the resulting curve was weakly sigmoidal shaped, with the strongest pH-dependence (inflection point of the curve that corresponds to the pKa-value of the ε-amino group of Lys15) at a pH-value of approximately 6.8. Within the limits of this reactivity-based assay, we thus conclude that the pKa-value of Lys 15 is below pH 7.0.
4.6 Chemical denaturation experiments in concentrated urea solutions
Unfolding transitions for TTR wild-type' and variants thereof were rendered by incubating native TTR tetramer (1.5 μM tetramer, in 50 mM sodium phosphate, pH 7.4) with increasing concentrations of urea (0–10 M). Protein-urea solutions were incubated for 7 days (4°C, ambient temperature (21 to 22°C), or 37°C) before samples were further analyzed in a Jasco model FP8500 spectrofluorometer. For each sample, the fluorescence emission intensity spectrum was recorded (excitation: 280 nm, emission 295–420 nm), and from each collected spectrum, we calculated the emission intensity ratio at two wavelengths (I355/I335). Unfolding transition curves in denaturant were rendered by plotting (I355/I335) vs the urea concentration.
4.7 Kinetic unfolding experiments in urea solutions
Kinetic experiments were initiated by diluting native TTR tetramer into 50 mM sodium phosphate supplemented with either 6 M urea or 9 M urea. For each experiment, a fresh stock solution of urea was prepared to minimize decomposition of urea into reactive cyanate prior to the actual unfolding experiment. The final concentration of TTR tetramer was 1.5 μM. The unfolding process was monitored as described in the previous paragraph via time-dependent monitoring of the change in (I355/I335). Except at 4°C, where carbamoylation is minimized, we find that the resulting unfolding trajectories do not fit to single exponentials, but are kinetically complex, with a lag phase that precedes the main unfolding event. Therefore, we did not attempt to extract kinetic parameters from our measurements using wild-type' TTR and TTR Lys15Ala and discuss the unfolding trajectories only qualitatively.
TTR variant Val122Ala is kinetically destabilized and unfolds on the time scale of minutes in urea solutions. Unfolding transitions were therefore collected after only 12-h incubation in Urea. Kinetic unfolding trajectories, rendered as described above, could be fitted to single exponential functions, yielding apparent rate constants of unfolding. As unfolding was done in 6 M urea, a denaturant concentration well within the post-transition baseline region, a contribution of tetramer re-association to the measured, apparent unfolding rate constant of TTR Val122Ala can be ignored. The calculated unfolding rates in 6 M urea thus reflect true microscopic rate constants of unfolding. The stabilizing effect of binding of cyanate and thiocyanate to the intact Val122Ala TTR tetramer was addressed by supplementing the unfolding buffer with aliquots from a 1 M stock solution of either potassium cyanate or potassium thiocyanate prior to adding native protein.
4.8 Verification of TTR carbamoylation by limited proteolysis and mass spectrometry
Protein samples were subjected to 10 mM cyanate at physiological pH (50 mM sodium phosphate, pH 7.40) and incubated for 0, 7, or 17 h prior to trypsin proteolysis (samples 1, 2, and 3, hereafter). The zero hour sample, in which cyanate was added immediately prior to proteolytic digestion, serves as a reference for non-carbamoylated protein. Proteolysis was done by first incubating the protein samples for 30 min in 8 M urea (in 50 mM Tris, pH 8.0, and 10 mM TCEP), then for 20 min with added 12 mM iodoacetamide. The samples were then diluted with Tris buffer solution (50 mM, pH 8.0) to a urea concentration of 2 M. Calcium chloride was added to 1 mM, and trypsin was added at a 1:50 molar ratio relative to TTR for overnight digestion at 37°C. Following trypsin digestion, peptides were acidified to pH <2 using formic acid and were then loaded onto capillary columns containing 2–3 cm C18-functionalized resin. Peptides were eluted over a gradient from 100% buffer A (95% water, 5% acetonitrile, 0.1% formic acid) to 80% buffer B (80% acetonitrile, 20% water, 0.1% formic acid) and analyzed by MS/MS. Peptide identification was carried out by searching the data against the amino acid sequence of the Cys10Ala/Met13Val variant of human transthyretin (see PDB code 7THA for sequence and structural details). Labeling sites were confirmed by searching for differential modification corresponding to carbamoylation of the N-terminal amine or the ɛ-amino group of Lys (mass increment 43.005 Da). To normalize for any absolute differences in peptide abundance among the samples, we express the extent of modification in each sample both by showing absolute numbers of counts of modified and unmodified peptides, and as a ratio of peptide counts for modified versus unmodified protein.
4.9 X-ray structure determination of TTR variant Cys10Ala/Met13Val
Crystals of TTR Cys10Ala/Met13Val were grown via sitting-drop vapor diffusion from a crystallization buffer consisting of 0.19 M CaCl2, 5% (v/v) glycerol, 0.095 M HEPES pH 7.5, and 26.6% (v/v) PEG400. Crystals were frozen directly without additional cryo-protection by plunging in liquid nitrogen. Diffraction data were collected on a Rigaku MicroMax-007HF Cu rotating anode X-ray source with Mar345 detector. Frames were indexed and integrated in XDS (Kabsch, 2010) and data were scaled in Scala (Evans, 2006). Five percent of reflections were flagged for calculation of Rfree (Brünger, 1992). The structure of TTR Cys10Ala/Met13Val was solved by molecular replacement in Phaser using PDB entry 4YDM as a search model (McCoy et al., 2007). Following structure solution, model refinement was carried out in Refmac5 (Murshudov et al., 1997). The final model has been deposited in the Protein Data Bank under accession code 7THA.
4.10 Summary on TTR variants used in this report
The side chains of Cys 10 and Met 13 in TTR wild-type are prone to oxidation. To avoid contributions from side chain oxidation to the energy landscape of tetramer dissociation and unfolding, residues Cys 10 and Met 13 were replaced with Ala and Val, respectively. The structure of TTR Cys10Ala/Met13Val was solved by X-ray crystallography and is nearly perfectly superimposable to the structure of TTR wild-type. Throughout this text, we refer to the TTR variant Cys10Ala/Met13Val also as TTR wild-type'.
We also present data for TTR variant Cys10Ala/Val122Ala. The side chain of Val 122 does not contribute to the formation of the T4-hormone binding site, but the Val122Ala mutation kinetically destabilizes the TTR tetramer against dissociation and unfolding. TTR Cys10Ala/Val122Ala unfolds in less than 1 h in 6 M urea and is therefore better suited than TTR wild-type' to quantitatively probe the effect of weak, non-covalent binding of cyanate to the T4-hormone binding site, or to probe the effect of Lys 15 mutagenesis on tetramer unfolding kinetics.
AUTHOR CONTRIBUTIONS
Marcus Jäger: Conceptualization; investigation; validation; formal analysis; writing – original draft; visualization; methodology; writing – review and editing; project administration; data curation. David E. Mortenson: Investigation; validation. Maziar S. Ardejani: Investigation; validation. Gabriel M. Kline: Methodology. Maria T. Dendle: Investigation; validation; methodology. Nicholas L. Yan: Investigation; validation. Evan T. Powers: Supervision. Martin Gruebele: Writing – review and editing; supervision; funding acquisition. Jeffery W. Kelly: Conceptualization; supervision; funding acquisition; writing – review and editing; project administration.
ACKNOWLEDGMENTS
J. W. K. and M. J. were supported by NIH grant DK46335. M. G. was supported by NSF grant MCB 2205665. D. E. M. was supported by a grant from the George E. Hewitt Foundation for Medical Research. Dr. Emily Bentley (Scripps Research) is thanked for expert editorial assistance, and Dr. Xiaoping Dai is thanked for assistance with X-ray data collection. This is manuscript # 30284 from The Scripps Research Institute.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.




