The effects of biological crowders on fibrillization, structure, diffusion, and conformational dynamics of α-synuclein
Reviewing Editor: Aitziber L. Cortajarena
Abstract
α-synuclein is an intrinsically disordered protein (IDP) whose aggregation in presynaptic neuronal cells is a pathological hallmark of Lewy body formation and Parkinson's disease. This aggregation process is likely affected by the crowded macromolecular cellular environment. In this study, α-synuclein was studied in the presence of both a synthetic crowder, Ficoll70, and a biological crowder composed of lysed cells that better mimics the biocomplexity of the cellular environment. 15N-1H HSQC NMR results show similar α-synuclein chemical shifts in non-crowded and all crowded conditions implying that it remains similarly unstructured in all conditions. Nevertheless, both HSQC NMR and fluorescence measurements indicate that, only in the cell lysate, α-synuclein forms aggregates over a timescale of 48 h. 15N-edited diffusion measurements indicate that all crowders slow down the α-synuclein's diffusivity. Interestingly, at high concentrations, α-synuclein diffuses faster in cell lysate than in Ficoll70, possibly due to additional soft (e.g., electrostatic or hydrophobic) interactions. 15N-edited relaxation measurements show that some residues are more mobile in cell lysate than in Ficoll70; the rates that are most different are predominantly in hydrophobic residues. We thus examined cell lysates with reduced hydrophobicity and found slower dynamics (higher relaxation rates) in several α-synuclein residues. Taken together, these experiments suggest that while cell lysate does not substantially affect α-synuclein structure (HSQC spectra), it does affect chain dynamics and translational diffusion, and strongly affects aggregation over a timescale of days, in a manner that is different from either no crowder or an artificial crowder: soft hydrophobic interactions are implicated.
1 INTRODUCTION
α-synuclein is an intrinsically disordered protein (IDP) which plays a vital role in cell signaling and in vesicle and neurotransmitter release. It is expressed throughout the brain in presynaptic neuronal cells (Henderson et al., 2016), but mostly in the substantia nigra. α-synuclein can aggregate inside cells, a pathological hallmark of Lewy body formation and Parkinson's disease. α-synuclein is made of 140 amino acids and composed of three regions: the N terminal domain (residues 1–60), a positively charged region which forms an α-helical structure in association with cell membranes or lipid micelles (Davidson et al., 1998); a non-amyloid β component (NAC region, 61–95) (Ueda et al., 1993); and the negatively charged C terminal domain (96–140), which is rich in acidic residues and forms a disordered structure (Chandra et al., 2003; Hnath et al., 2023). As α-synuclein is intrinsically disordered, it does not have a stable structure, and it exchanges between different conformations. However, it adopts α-helical structure when bound to a cell's membrane (Amos et al., 2021; Bartels et al., 2011; Liang & Tamm, 2018; Wang & Roberts, 2018).
The conformation of IDPs, and α-synuclein in particular, is thought to be exceptionally sensitive to macromolecular crowding. The importance of macromolecular crowding has been widely recognized (Breydo et al., 2016; Mukherjee et al., 2015; Munishkina et al., 2009; Sarkar et al., 2013; Smith et al., 2015; Wang et al., 2012), but its effects on protein conformation are not quantitively understood (Dhar et al., 2010). Intracellular environments have a high concentration of macromolecules, including nucleic acids, proteins, carbohydrates, lipids, and other biopolymers, with a total concentration between 80 and 400 g/L (Munishkina, Fink, & Uversky, 2008; Zimmerman & Trach, 1991). This macromolecular crowding results in volume exclusion as well as nonspecific interactions between crowders and biomolecules. Therefore, studying both excluded-volume crowding effects and nonspecific interactions is crucial for understanding the behavior of biomolecules in a living cell (Rivas & Minton, 2022). In particular, interactions between the crowders and proteins can dramatically affect highly dynamic proteins such as IDPs (Uversky, 2013); thus, the structural malleability of α-synuclein might make it more prone to being affected in crowded conditions. In previous studies of crowding, researchers studied proteins in various crowded in vitro and in vivo conditions to explore their effects on α-synuclein. They employed artificial crowders such as polyethylene glycol (PEG), Dextran, or Ficoll (Bai et al., 2017; Wang et al., 2012), as well as using proteins like Bovine Serum Albumin (BSA) (McNulty et al., 2006; Van Den Berg et al., 2000). However, the intracellular crowders are composed of many distinct macromolecular species of different sizes. This biocomplexity is much greater than can be achieved with synthetic polymers or protein crowders.
Trosel et al. (2023) studied the crowding effects of cell lysate on diffusivity of a model polymer, PEG. The next step toward a more biological system is to replace the probe polymer with a disordered protein, which could behave differently than a model polymer in the presence of cell lysate. In this work, α-synuclein is studied in the presence of both bacterial cell lysate and Ficoll to examine the effects of both biological and synthetic crowders on a disordered protein. Bacterial cell lysate was used as a realistic crowder to mimic the biocomplexity of the cellular environment, and Ficoll was used as a model artificial environment. Lysing bacterial cells yields a wide range of naturally occurring macromolecules, and in addition, provides the capability to manipulate the properties of the crowders, which is not feasible with in-cell studies.
2 MATERIALS AND METHODS
2.1 Protein expression and purification
A recombinant plasmid was designed (Figure S1) with (from N terminal to C terminal) a six-histidine tag, guanosine nucleotide protein beta subunit (GB-1) fusion protein, TEV cleavage site, and human α-synuclein (UniProt P37840). Uniformly 15N labeled α-synuclein was expressed in Escherichia coli BL21 cells grown in M9 media (Supporting Information) containing 15NH4Cl (Cambridge isotope Laboratories, Inc) as the only nitrogen source. The growth of bacteria was performed by incubation at 37°C with shaking at 175 RPM until the absorbance of the media reached 0.6 at OD 600 nm. At this point the protein expression was induced by adding Isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.01 M. The incubation continued under the same conditions until the absorbance reached 1.0. Then, the bacteria were harvested by centrifuging at 4690g for 15 min at 4°C. The pellets were dissolved in TBS buffer (50 mM Tris (Sigma) and 150 mM sodium chloride (Fisher)) containing 1X Halt™ Protease Inhibitor Cocktail (Fisher). The harvested cells in the buffer were lysed by Amico French press three times at 1200 psi and 4°C. Then the suspension was sonicated three consecutive times for 30 s at 4°C by using a Branson Sonifier Cell Disruptor to break large DNA molecules. Following that, the suspension was centrifuged at 100,000g for 40 min at 4°C and the supernatant, containing the expressed protein construct, was retained. The N-terminally His-tagged construct was purified with Immobilized Metal Affinity Chromatography (IMAC) (Figure S1). The IMAC column was loaded with 5 mL Sepharose fast flow resin (GE Healthcare), then it was charged with 0.2 M Nickel (II) sulfate hexahydrate (Sigma) and pre-equilibrated with loading buffer (5 mM imidazole (Sigma), 50 mM Trizma base (Sigma), 150 mM sodium chloride (Fisher); pH = 7.4). After adding the suspension of protein into the column, it was washed with loading, washing (20 mM imidazole (Sigma), 50 mM Trizma base (Sigma), 150 mM sodium chloride (Fisher); pH = 7.4), and elution (300 mM imidazole (Sigma), 50 mM Trizma base (Sigma), 150 mM sodium chloride (Fisher); pH = 7.4) buffers. After purification of His-tagged construct, the fractions with the most α-synuclein were pooled for cleavage to break the polypeptide chain between poly His-tagged GB-1 fusion and α-synuclein. EZCut Tobacco Etch Virus (TEV) protease (Biovision) was used for the cleavage. After the cleavage, the fractions were purified with IMAC to separate the α-synuclein from the fusion protein. After purification of α-synuclein, its concentration was calculated using the Beer–Lambert law and absorbance at 280 nm (A280 nm) with a molar extinction coefficient of 5960 M−1 cm−1 (De Oliveira et al., 2016) and also confirmed with a Bradford Assay. The concentration of α-synuclein was diluted to 0.2 mM for all experiments.
2.2 Crowded milieus
Four crowded milieus were prepared. First, a stock of 400 g/L Ficoll70 (Sigma) was prepared in deionized water, and the pH adjusted to 7. The Ficoll suspension was homogenized 3 consecutive times with a homogenizer (Fisher 850) at 11000 RPM for 3 min. The bacterial cell lysate was prepared by growing E. coli JM109 in LB media and lysing them using an Amico French press and using a Branson Sonifier Cell Disruptor to break down the large DNA molecules. Ultracentrifuged bacterial cell lysate was prepared by centrifuging the cell lysate using a Beckman L90K Ultracentrifuge at 100,000g and 4°C for 40 min in order to remove larger macromolecules from the cell lysate including large nucleic acids and lipid structures. The supernatant was carefully separated using a pipette and transferred to a falcon tube. Finally, a less-hydrophobic cell lysate was made by passing a 100 mL suspension of 2 mg/mL of bacterial cell lysate through a hydrophobic interaction chromatography (HIC) column filled with 3 mL Bio-Rad Methyl HIC weakly hydrophobic resin at 4°C. Two 50 mL fractions were collected, and the first fraction was used for the experiments. The pH of all bacterial cell lysate samples was adjusted to 7, and all manipulated and unmanipulated cell lysates were lyophilized using a Labconco Freezone 12 Freeze Dryer for 72 h.
The Bligh and Dyer technique (Bligh & Dyer, 1959; Breil et al., 2017) was used to assess the hydrophobicity of the cell lysate before and after manipulating its hydrophobicity. A 30 mL suspension of 0.2 g of unmanipulated cell lysate was prepared and added to a graduated cylinder. 20 mL of chloroform (Fisher) and 20 mL methanol (Caledon Laboratories Ltd) were added over the suspension. Then it was mixed well, cylinder was left for 5 min until two phases separated. The lower phase contained chloroform and hydrophobic particles, and the upper phase contains methanol and hydrophilic particles. The two phases were separated and transferred to aluminum weighing boats. All filled aluminum weighing boats were heated up on a hot plate stirrer (Corning hot plate stirrer PC-351) in a fume hood until they completely dried out. Then the dried weight boats were kept in an oven (Fisher Scientific Isotemp) at 105°C for 10 min. After that, the mass of dishes was measured and subtracted from the original mass of dishes to obtain the weight of the hydrophilic and hydrophobic particles.
Dynamic light scattering (DLS) was used to understand the effects of the weakly hydrophobic column on the cell lysate's particle size distribution. Suspensions of 1 mg/mL of the cell lysate before and after applying it to the column were prepared in water, and a Malvern Zetasizer Nano-ZS ZEN3600 was used at 25°C to measure the size distribution of particles by intensity.
2.3 Fibril formation assay
A thioflavin T (ThT) fluorescence assay was used to study the fibrillation process. Assays were conducted in 96-well black/clear bottom plates (Fisher) with a fluorescence buffer, 20 μM ThT (Fisher), 0.005% w/v sodium azide (Sigma), 20 mM Tris–HCl (Fisher), and 0.1 M NaCl (Fisher), at pH 7.4 (Horvath et al., 2021). 0.04 mM α-synuclein was used in 280 mg/mL Ficoll70 or ultracentrifuged bacterial cell lysate crowder. Measurements were made twice a day using a Synergy Max Fluorescent Plate Reader shaking at 25°C with an excitation at 440 nm and emission at 485 nm. The plates were well sealed and kept at room temperature between the measurements with continued shaking at 30 RPM.
2.4 Confocal fluorescence microscopy
ThT buffer was prepared as above but set to pH 7, and 5 μL of the ThT buffer was added to 10 μL of the samples (0.2 mM α-synuclein in the absence of crowders), in the presence of 200 mg/mL Ficoll70, and in the presence of 200 mg/mL of cell lysate after 72 h incubation at 37°C. Each sample was sealed between a microscope slide and a coverslip using a SecureSealTM imaging spacer. Images were captured by a Nikon C1 confocal microscope using a 100X (NA = 1.4) oil-immersion objective with a 488 nm (blue) laser for excitation and dichroic filter that selected for green emission.
2.5 NMR sample preparation
All NMR experiments were done on either a Bruker 500 MHz or 600 MHz spectrometer with a Triple X Inverse (TXI) probe. NMR samples contained 0.4 mM sodium 2,2-dimethyl-2silapentane-5-sulfonate (DSS) (Sigma) to directly calibrate the 1H chemical shift and 10% D2O (99.9%, Cambridge Isotope Laboratories, Inc) to lock the field frequency. NMR experiments were conducted with precision NMR sample tubes, 7″ long and 5 mm in diameter (New Era Enterprises, Inc) at 25 or 37°C.
2.6 15N-1H HSQC
The 15N-1H HSQC spectra of α-synuclein were acquired at 500 MHz at 25 and 37°C and were used to assign the peaks. The HSQC experiments were performed with 128 scans and 128 increments in the 15N dimension. The spectral width of the proton channel was 14 ppm, and that of the nitrogen channel width was 36 ppm. The HSQC spectrum was imported to NMRFAM-Sparky (Lee et al., 2015) to assign the peaks (Figure S4) based on (Sivanesam et al., 2015; Wu & Baum, 2011).
15N-1H HSQC spectra were also employed to study the structure of α-synuclein with and without the crowders. Three types of samples were prepared with 0.2 mM α-synuclein in the absence of crowders, in the presence of 200 mg/mL Ficoll70, and in the presence of 200 mg/mL of cell lysate. All samples were dissolved and diluted with milli-Q water, and the pH of all samples set to 7. 15N-1H HSQC experiments were performed immediately after preparing the samples at 500 MHz and 37°C. Samples were kept in an incubator between the measurements for 72 h at 37°C. 15N-1H HSQC measurements were performed at 0, 48, and 72 h. The HSQC spectra were processed using TopSpin 4.1.4 software and then imported into CcpNmr (Skinner et al., 2016) for further analysis, including comparing the peaks' frequencies and intensity.
2.7 DOSY NMR
Translational diffusion experiments were performed with 0.2 mM α-synuclein in the absence and presence of both 150 and 200 mg/mL Ficoll70 and cell lysate using a 15N-1H HSQC-DOSY pulse program (Shin et al., 2017) on a 500 MHz NMR spectrometer at pH 7 and 25°C. 15N-1H HSQC-DOSY experiments were done with a gradient strength range from 0.48 to 45.74 G/cm (Table S1 and S2). Diffusion data was processed in TopSpin 4.1.4, and the logarithmic scale of the attenuated signal decay was plotted versus the gradient strength parameter ( where is the gyromagnetic ratio, δ is the duration of the gradient pulse (2.2 ms), g is the gradient strength, and Δ is the delay between gradient pulses (100 ms). The plots were fitted by a biexponential function, ; (the fraction of fast diffusing species) was allowed to vary. Additional information for the diffusion measurements is explained in the Supporting Information.
2.8 Transverse relaxation (R2) NMR
HSQC-edited transverse relaxation rate (or 15N-1H HSQC-R2) experiment were obtained at 600 MHz and 25°C with a recycle delay of 1 s. The experiments collected 44 scans with 128 increments on 15N dimension processed with eight different delays of 16, 32, 64, 96, 128, 160, 240, and 320 ms. The data were processed with TopSpin 4.1.4 and then imported to NMRFAM-Sparky Software to obtain the R2 value. In Sparky, the intensities of each peak were plotted against the delay times and fitted to an exponential function.
3 RESULTS AND DISCUSSION
3.1 Fibrillization of α-synuclein in the presence of biological and synthetic crowder
A ThT fluorescence assay was used to quantify fibril formation and probe the effects of crowders on α-synuclein's fibrillization. The ThT assays were performed with α-synuclein alone and in the presence of ultracentrifuged bacterial cell lysate and Ficoll70. The experiment was initially done in the presence of unmanipulated cell lysate, but there was a high level of background fluorescent signals from the cell lysate. The ThT fluorescent can bind to β sheet rich structures, which would be expected to be present in the lysate given that it contains a large variety of proteins. To decrease these background signals, the cell lysate was ultracentrifuged to remove larger structures from the sample.
The ThT assay results (Figure 1) shows the fluorescence signal in a sample of 0.04 mM α-synuclein alone, in the presence of 280 mg/mL Ficoll70, and 280 mg/mL ultracentrifuged cell lysate. Based on the onset of a rapid increase in signal, we conclude that fibrillization occurs at about 50 h in the ultracentrifuged cell lysate, while no substantial changes happened in the presence of Ficoll. Hence, cell lysate greatly accelerates the aggregation. It should be noted that cell lysate alone also shows a (smaller) increase in signal after 50 h, possibly from small quantities of aggregated proteins in the cell lysate itself.
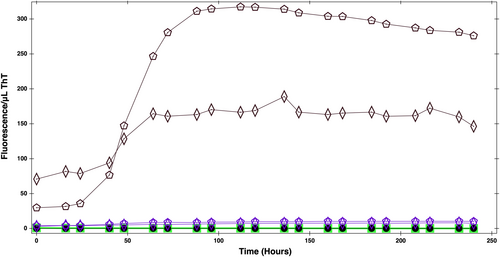
ThT combined with confocal microscopy was used to visualize α-synuclein fibrils. Figure S3 shows confocal microscopy images of α-synuclein incubated for 72 h in the absence of crowders (Figure S3A), in the presence of 200 mg/mL Ficoll70 (Figure S3B), and in the presence of 200 mg/mL bacterial cell lysate (Figure S3C). The results show no considerable fibrillization either in no-crowding conditions (Figure S3A) or in the presence of Ficoll70 (Figure S3B). Figure S3C shows, however, that in the presence of cell lysate there is an abundance of background fluorescent signal from the cell lysate, but also some clusters which are a sign of α-synuclein fibrillation. Protein crowders such as BSA and lysozyme (Breil et al., 2017; Munishkina et al., 2004; Shin et al., 2017) have been seen to promote faster fibrillation of α-synuclein than synthetic crowders. Thus, the above observations could imply that additional weak interactions present in cell lysate are important to α-synuclein's behavior. To further investigate the impact of cell lysate on α-synuclein structure and dynamics we turned to NMR.
3.2 Structure of α-synuclein with and without crowder
α-synuclein was incubated for 72 h at 37°C in three different crowding conditions: in the absence of crowders; in the presence of 200 mg/mL Ficoll70; and in the presence of 200 mg/mL bacterial cell lysate. The 15N-1H HSQC results (Figure 2a) indicate that the freshly made-up α-synuclein samples are similar in the absence and in the presence of both Ficoll and cell lysate. Quantitative comparison also shows that the spectra are similar in both their combined 15N and 1H chemical shift differences (calculated as in (Williamson, 2013)) shown in Figure 2b as a function of residue number. The r.m.s chemical shift difference for the α-synuclein residues in Ficoll70 is also ~0.02 ppm. Therefore, there is no significant difference between the frequency of the residues in the absence and presence of Ficoll. In cell lysate, five residues had moderate changes in chemical shift, but the rest had insignificant or borderline-insignificant changes and thus indicates no large-scale structural changes in cell lysate. Previous studies have also reported that α-synuclein in bacterial cells and at different concentrations of Ficoll experience small changes in chemical shifts compared to dilute conditions but still remains disordered (Bai et al., 2017; Cino et al., 2012; Li et al., 2008; McNulty et al., 2006; Morar et al., 2008; Waudby et al., 2013; Zigoneanu & Pielak, 2012). We thus believe that these minor changes in α-synuclein's chemical shift do not come from a strong effect such as structural change, but rather from the weaker effect of an α-synuclein interacting with crowding molecules.

α-synuclein's spectra in the absence of crowders and in the presence of Ficoll70 remained the same after 72 h of incubation at 37°C. However, all peaks were lost after 48 h in the cell lysate, most likely due to aggregation, which is consistent with what was found by ThT fluorescence (Figure 1 and S3). Therefore, we find that aggregation occurs faster in biological crowder than in artificial crowder.
Next, we examine α-synuclein's diffusion and internal backbone dynamics in order to better understand how soft interactions with biological crowder affects α-synuclein's conformational preferences and ability to move by translational diffusion.
3.3 15N-1H diffusion of α-synuclein
Disordered proteins, in particular, might diffuse differently in a complex crowded environment like cell lysate than in a synthetic crowder due to the presence of various repulsive and attractive forces.
15N-edited spectra (Figure 3a shows a 1D spectrum for α-synuclein without crowders) were used to obtain translational diffusion coefficients for 15N-labeled α-synuclein using HSQC-edited DOSY spectra; Figure 3b shows signal attenuation with increasing gradient strength. The signal attenuations in Figure 3b exhibit two exponential decay components: a fast decay at lower gradients that can only be consistent with single 15N-labeled residues or other 15N-labeled metabolites, and a slower decay at higher gradients that is consistent with protein diffusion. This slower component is plotted in Figure 3c for no crowder (green, 0 mg/mL) as well as for 150 and 200 mg/mL (purple is Ficoll and blue is cell lysate).
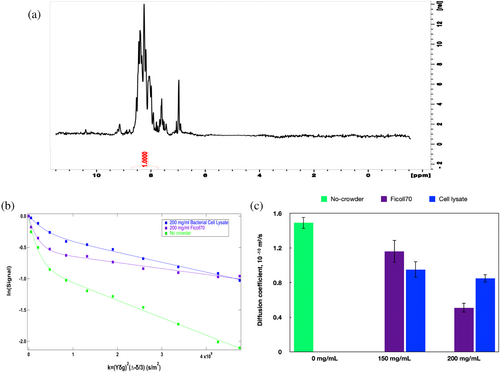
The protein diffusion value we obtained for α-synuclein (Figure 3b; green bar in Figure 3c) without crowder (using the HSQC-edited DOSY) is 1.49 × 10 −10 m2/s. We also carried out a regular 1H DOSY measurement on the same sample and obtained a diffusion value of 1.59 × 10 −10 m2/s. Wang and colleagues have previously found a value that is just over half this value, 0.78 × 10 −10 m2/s (Wang et al., 2012). Given this disparity, we confirmed that on the SDS gel, there is a single, strong band at a molecular mass consistent with 14 kDa, the expected molecular mass for α-synuclein (Figure S2).
Depending on how the diffusion coefficients are converted to hydrodynamic radii, the radii obtained from previous experiments is 3.1 nm (Wang et al., 2012) and in this work it is 1.6 nm. Uversky suggests for polypeptide chains a series of relations between hydrodynamic radius, RH, and molecular mass, M, of the form , where the values of a and b vary depending on how compact or unfolded the protein is (Uversky, 2012). Using this relation, a size of 1.6 nm would indicate a fairly compact α-synuclein (close to phenomenological eq. 6 in Uversky, 2012). Our experiments were done at the same temperature and comparable pH (7.4 in Wang et al.'s experiments vs. 7 in ours), but at different concentrations: 1 mM for Wang et al. and 0.2 mM in this study. We could not increase the concentration of α-synuclein to more than 0.2 mM since the protein aggregated quickly at higher concentrations. It is possible that α-synuclein is prone to clustering at higher concentrations. Due to the potential for clustering (von Bülow et al., 2019), and the dependence of RH on protein conformation, it is challenging to relate molecular mass to hydrodynamic radius obtained from diffusion measurements.
The diffusion coefficients indicate that α-synuclein diffuses slower in the presence of crowder compared to the no-crowder condition. As seen in Figure 3c, α-synuclein has a similar diffusion coefficient in cell lysate and Ficoll70 at a concentration of 150 mg/mL. At 200 mg/mL, however, α-synuclein diffuses substantially faster in bacterial cell lysate than in Ficoll70. The α-synuclein diffusivity decreased by around 66% in the presence of 200 mg/mL Ficoll70 in our study, as compared to an 80% decrease previously observed for 300 mg/mL Ficoll70 (Wang et al., 2012). Both our study and Wang et al., which employed protein crowders, found that α-synuclein diffuses faster in biological crowders than in Ficoll.
The faster diffusion of α-synuclein at the higher concentration of cell lysate (as compared to Ficoll70) is likely not because of structural changes. We conclude this because the location of α-synuclein's HSQC peaks do not show the large-scale changes expected for a substantial structural change (Figure 2) (Li et al., 2008; McNulty et al., 2006; Morar et al., 2008; Zigoneanu & Pielak, 2012). There are at least two explanations for this difference in the response of diffusion to the two types of crowders. First, Ficolls are compact crowders, while the cell lysate is far more heterogenous in molecular shapes and sizes. While even Ficolls have been shown to be deformable (Ranganathan et al., 2022), the diversity of molecular shape could make the lysate less obstructive at higher packings. Second, α-synuclein is negatively charged, as are the majority of bacterial proteins, DNA, and bacterial lipids in the cell lysate. Thus, another possibility is that the faster diffusion of α-synuclein in the higher concentration of cell lysate could come from the repulsion between cell lysate and α-synuclein, for example with charged molecules, which are not present in Ficoll. Consistent with our diffusion results, these repulsive interactions between cell lysate and α-synuclein could speed up the IDP relative to how it moves in Ficoll. However, these diffusivity trends are an experimental observation, and we do not currently understand their origins.
We have found in previous work that cell lysate is a non-Newtonian shear-thinning fluid that behaves like a weak gel (Trosel et al., 2023). The apparent viscosity is a variable that depends on the effective shear rate. We can assume we are in the low-shear limit, where the applied shear does not break up the underlying structure in the cell lysate. In this limit, the viscosity of Ficoll70 (at 200 g/L) is 0.006 to 0.008 Pa, while that of cell lysate is 0.76 and 0.14 Pa s in two independent cell lysate preparations. We could use one of these values or the average value of 0.45 Pa s, but regardless the value is two orders of magnitude larger than that of Ficoll70. Yet, the diffusivity of α-synuclein in cell lysate is almost 70% larger (not two orders of magnitude smaller!) than that in Ficoll70. This indicates that the bulk viscosity is not really relevant for a heterogenous fluid when the fluid viscous response comes from macromolecular constituents that are on the same length scale as the diffusing α-synuclein. The Stokes–Einstein relation is then not valid. What is important is how easily α-synuclein diffuses around the obstructions that are presented by its macromolecular surroundings.
3.4 α-Synuclein 15N-1H R2 relaxation in the absence and presence of Ficoll70 and bacterial cell lysate
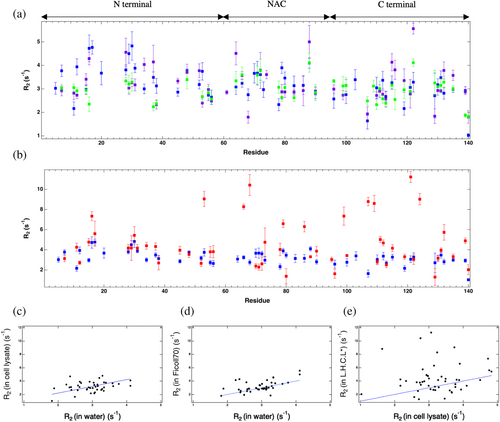
It has been suggested that α-synuclein's internal chain motions might be modified by soft interactions with crowders and converted to faster translational motion in biological crowders (Wang et al., 2012). To explore the internal chain motion of α-synuclein, we measured its 15N R2 in the absence and presence of crowders. R2 is longer, and thus more sensitive to relaxation, than longitudinal relaxation rate (R1) for large molecules, like proteins, that tumble slowly (Cavanagh et al., 2007; Rule & Hitchens, 2006) and has been shown to be sensitive to changes in IDP motion due to crowding (Cino et al., 2012). Interpreting relaxation rates of IDPs in terms of simple models is difficult compared to globular proteins as IDPs exchange between different conformations (Milles et al., 2018). Because they are flexible, disordered proteins like α-synuclein can have different correlation times for different residues (Li et al., 2008). Nonetheless, higher R2 values indicate less chain motion. Figure 4a plots the 15N-1H transverse relaxation rate as a function of residue number; the value varies in the range of 2–5 s−1. For several residues α-synuclein has a higher relaxation rate in Ficoll70 and cell lysate than in the absence of crowder, which implies lower mobility of the residues in crowded conditions compared to no crowding. Compared to no crowding, in Ficoll70 R2 was larger for 33% of α-synuclein residues, indicating less mobility and 67% were the same within error bars. However, compared to no crowding condition, 30% of α-synuclein residues in the cell lysate showed a higher R2 while 52% had the same R2 value, and 18% had lower R2 value. Unlike in Ficoll, a substantial portion, 18%, of the residues in biological crowder had increased internal motions (Figure 4c,d). The biggest mobility difference between no crowder and crowder was found in the N-terminal region (2.87 ± 0.22 s−1). The region with biggest difference in R2 between lysate and Ficoll was the C-terminal region (3.30 ± 0.27 and 3.54 ± 0.30 s−1 in the presence of Ficoll70 and cell lysate, respectively) (Table 1). Interestingly, the C-terminal is more mobile in lysate than in Ficoll; however, there is a great deal of variability in R2 within each region as will be explored further in Section 5 below.
| α-synuclein's region | No crowder (s−1) | Bacterial cell lysate (s−1) | Ficoll70 (s−1) | Less hydrophobic bacterial cell lysate (s−1) |
|---|---|---|---|---|
| N-terminal (1–60) | 2.87 ± 0.22 | 3.54 ± 0.30 | 3.30 ± 0.27 | 4.42 ± 0.58 |
| NAC (61–95) | 3.36 ± 0.40 | 3.27 ± 0.30 | 3.30 ± 0.34 | 4.55 ± 0.61 |
| C-terminal (96–140) | 2.89 ± 0.26 | 2.80 ± 0.33 | 3.05 ± 0.27 | 4.76 ± 0.44 |
- Note: Values are presented as mean standard deviation (s−1).
The R2 data show that 46% of C terminal residues in the cell lysate had a lower R2 value than in the Ficoll70 and the rest had the same relaxation rate. The C terminal is governed by electrostatic interactions in the intracellular milieu (Theillet et al., 2016), and salt was shown to affect α-synuclein C-terminal dynamics when it was crowded by lysozyme, but not by BSA, suggesting that electrostatics may play a role in crowder-C-terminal interactions depending on the nature of the crowder. We thus conjecture that electrostatic interactions between the C terminal residues and cell lysate particles lead to faster α-synuclein internal dynamics in the cell lysate than in the Ficoll. Seven residues of α-synuclein, A11, A78, K96, A107, D119, N122, and A140 move even faster in cell lysate than in water, which emphasizes the interactions and interplay of repulsive and attractive forces that can lead to faster dynamics of some residues in cell lysate. α-synuclein residues may make contacts with a number of possible crowder binding partners, contacts that form and break rapidly, leading to a push and pull between binding partners that may cause the dynamics of these regions to speed up. We note that these faster internal dynamics of different regions of α-synuclein in the cell lysate may relate to faster translational motion and possibly causes the faster translational diffusion of α-synuclein in the cell lysate compared.
3.5 α-Synuclein structure and 15N-1H R2 relaxation in the absence and presence of less hydrophobic cell lysate
Figure 4a shows that 55% of the residues whose R2 changed significantly, either higher or lower, when comparing α-synuclein in cell lysate with α-synuclein in Ficoll70 are hydrophobic. Table 2 shows the types and numbers of residues that have different (not within error bars) R2s in the cell lysate (compared to in Ficoll70). Many of the amino acids that were affected differently by cell lysate than by Ficoll70 are hydrophobic in character (alanine, valine, glycine, leucine). Thus, it directed us to focus on the hydrophobic interactions.
| Amino acid type | Number of residues whose R2 changed significantly with cell lysate (compared to Ficoll70) | Number of residues with higher R2 in the less hydrophobic cell lysate than in the unmanipulated cell lysate | Total residuesa |
|---|---|---|---|
| Alanine | 5 | 7 | 10 |
| Leucine | 1 | 1 | 4 |
| Iso leucine | - | 2 | 2 |
| Valine | 2 | 4 | 9 |
| Lysine | 4 | 2 | 5 |
| Aspartic acid | 2 | 3 | 4 |
| Glycine | 2 | 4 | 4 |
| Glutamic acid | 1 | 1 | 8 |
| Threonine | 1 | 1 | 2 |
| Glutamine | - | 3 | 3 |
- Note: Amino acids with similar physicochemical characteristics have the same shading (dark grey for hydrophobic, light grey for amphipathic/positive, no shading for all other types).
- a Total number of the identified residues.
Furthermore, most previous studies used PEG, Dextran, or Ficoll in their studies (Lee et al., 2012; Munishkina et al., 2004; Munishkina et al., 2008), which are hydrophilic polymers. However, it is interesting to use biological crowder with a variable hydrophobicity to probe the importance of hydrophobic interactions. To this end, we manipulated the hydrophobicity of the cell lysate and used a less hydrophobic cell lysate (Figure S5, Table S3). Based on the Bligh and Dyer data, the hydrophobicity of the cell lysate was decreased by around 7%.
The 15N-1H HSQC spectrum of α-synuclein in the less hydrophobic cell lysate has similar chemical shifts as unmanipulated cell lysate (Figure S6). Therefore, the less hydrophobic cell lysate, like the unmanipulated cell lysate, did not affect the structure of the α-synuclein. This is consistent with previous indications that the secondary structure of α-synuclein in a more hydrophobic milieu remains the same as that in hydrophilic milieu at pH 7.5 (Breydo et al., 2015).
Figure 4b compares the relaxation rate in unmanipulated and less hydrophobic cell lysate. The relaxation rate for different residues in the N terminal region of α-synuclein is almost the same in the presence of less hydrophobic cell lysate as in the unmanipulated lysate. However, the R2 value increased considerably for several residues in the NAC and the C terminal regions. The C terminal of α-synuclein was affected more by the less hydrophobic cell lysate, and the average relaxation rate increased 1.5 times. By lowering the hydrophobicity of the cell lysate, the residues that had faster dynamics (i.e., lower R2) in the unmanipulated cell lysate than in the water experienced a substantial decrease (i.e., higher R2) in their dynamics (Figure 4e). Looking deeper at the most affected residues in the less hydrophobic cell lysate than in the unmanipulated cell lysate, we find that 64% of the affected residues (not the same within error bars) are hydrophobic amino acids (Table 2). It is possible that reduced hydrophobic interactions between α-synuclein and crowder allows for more α-synuclein to α-synuclein hydrophobic interactions which slows down the chain motions for some residues.
4 CONCLUSION
Comparing the effects of biological and artificial crowders gives a better idea of how intracellular crowding affects α-synuclein. Our results suggest that the α-synuclein structure remains the same in either Ficoll70 or cell lysate. We find that α-synuclein diffuses faster in a high concentration (200 mg/mL) of cell lysate as compared with Ficoll70. Two regimes of crowding-induced changes in folding kinetics have been observed by Dhar et al. (2010) (using Ficoll70 as crowder): a low-concentration regime (< ~100 mg/mL) where protein folding timescales are reduced, and a high concentration regime (> ~100 mg/mL) where the timescales increase again. Interestingly, in this latter regime, which is physiologically relevant, we show that cell lysate slows α-synuclein less than Ficoll70.
We find that the α-synuclein spectrum in cell lysate (but not in Ficoll70 or in the absence of crowder) disappears after 48 h, suggesting α-synuclein aggregates in cell lysate. These samples were also evaluated by ThT fluorescence experiments (in the presence of a fluorescent stain that preferentially binds fibrils) and indeed, the cell lysate was the only one to show the presence of fluorescent micron-scale aggregates.
We also find that some residues in the C terminal regions of α-synuclein move faster in the cell lysate than in the Ficoll70. The soft interactions between cell lysate and α-synuclein may cause faster local chain motion of α-synuclein's C terminal region, and relatedly or coincidently they result in a faster translational diffusion coefficient than in Ficoll (Wang et al., 2012). Also, a modest change in the hydrophobicity of the biological crowder had a substantial impact on α-synuclein dynamics, especially in its NAC and C terminal regions. This emphasizes the importance of crowder soft interactions (including repulsive and attractive forces) with α-synuclein.
Overall, α-synuclein's translational diffusion and chain motions are faster in the presence of cell lysate than in Ficoll70. Also, several residues in the C terminal and NAC region of α-synuclein move substantially slower in the presence of less hydrophobic cell lysate compared to unmanipulated cell lysate, suggesting that hydrophobic interactions with biological crowders are important.
AUTHOR CONTRIBUTIONS
Sina Heravi: Investigation; writing – original draft; methodology; formal analysis; writing – review and editing. Jude Vincent Dobbin Power: Investigation; writing – review and editing; formal analysis; methodology. Anand Yethiraj: Conceptualization; funding acquisition; writing – review and editing; supervision; resources. Valerie Booth: Conceptualization; funding acquisition; writing – review and editing; supervision; resources.
ACKNOWLEDGMENTS
We would like to thank Memorial University's CREAIT Network, and in particular Dr. Céline Schneider, for their support with this work.
FUNDING INFORMATION
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant 204115 and 204040.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.