Structural determination and kinetic analysis of the transketolase from Vibrio vulnificus reveal unexpected cooperative behavior
Review Editor: John Kuriyan
Abstract
Vibrio vulnificus (vv) is a multidrug-resistant human bacterial pathogen whose prevalence is expected to increase over the years. Transketolases (TK), transferases catalyzing two reactions of the nonoxidative branch of the pentose-phosphate pathway and therefore linked to several crucial metabolic pathways, are potential targets for new drugs against this pathogen. Here, the vvTK is crystallized and its structure is solved at 2.1 Å. A crown of 6 histidyl residues is observed in the active site and expected to participate in the thiamine pyrophosphate (cofactor) activation. Docking of fructose-6-phosphate and ferricyanide used in the activity assay, suggests that both substrates can bind vvTK simultaneously. This is confirmed by steady-state kinetics showing a sequential mechanism, on the contrary to the natural transferase reaction which follows a substituted mechanism. Inhibition by the I38-49 inhibitor (2-(4-ethoxyphenyl)-1-(pyrimidin-2-yl)-1H-pyrrolo[2,3-b]pyridine) reveals for the first time a cooperative behavior of a TK and docking experiments suggest a previously undescribed binding site at the interface between the pyrophosphate and pyridinium domains.
1 INTRODUCTION
Vibrio vulnificus is a halophilic gram-negative bacterium living in low salinity and warm water littorals (<25% ppm NaCl and >15°C) (Hsueh et al. 2004; Phillips and Satchell 2017; Baker-Austin and Oliver 2020). It is found in the south east of Asian and in the north of American littorals, together with others Vibrio species. At the horizon 2050, expansion of Vibrio species in these regions as well as in the north of Europe is expected due to the global warming (Baker-Austin and Oliver 2018; Baker-Austin and Oliver 2020; Doktor and Fraimow 2020; Trinanes and Martinez-Urtaza 2021).
Indeed, V. vulnificus is the deadliest Vibrio specie known nowadays regarding the number of deaths per infection while V. cholerae is the most prevalent specie. It infects seafood, especially shrimp and oysters in fish farms or in sea (Hsueh et al. 2004; Phillips and Satchell 2017; Baker-Austin and Oliver 2020). The first clinical case of V. vulnificus infection happened in the 1970s. The prevalence of V. vulnificus infections still remains low; for example, 1.237 per million worldwide but it has been increasing since the 1970s (Farmer 1979; Hsueh et al. 2004). Infection by V. vulnificus is lethal in 51.6% of the cases and is the most expensive digestive infection in the US (Phillips and Satchell 2017). Two modes of infections are possible: (i) a transdermal infection where the necrotic limb should be treated using antibiotics to prevent amputation and (ii) a digestive infection that leads to septicemia with purpura and thoracic edema (without desquamations) happening in 24 h followed by fever, coma, and possibly death in 72 h (Hori et al. 2017; Park and Lee 2018). The origin of the multidrug resistance of V. vulnificus is not well known, but it could result from antibiotic rejection in waste water and further contamination of oceans and seas, combined with the capacity of this bacteria to transfer genes horizontally, in particular genes of antibiotic resistance, forming the V. vulnificus pangenome (Hsueh et al. 2004; Phillips and Satchell 2017; Baker-Austin and Oliver 2018). Therefore, new drugs directed against this bacterium should be searched. New antibiotics targets must be involved in several metabolic or cell signaling pathways, such as hub proteins, in order to inhibit important cell functions and cause pathogen death. Moreover, if the target is involved in a metabolic pathway rarely investigated (e.g., with few/no known drugs), it is likely that new molecular frameworks could be identified as lead compounds for new antibiotics. The pentose phosphate pathway (PPP) fulfills these conditions: (i) it is involved in NADPH, amino acids and nucleic acid precursors synthesis and (ii) few drugs target this pathway. The oxidative phase of PPP forms NADPH that limits the oxidative stress while the non-oxidative phase allows to regulate the biosynthesis of d-ribose-5-phosphate and d-erythrulose-4-phosphate, that are nucleic acid and amino acid precursors, respectively (Gibbs and Horecker 1954; Horecker et al. 1954; Schenk et al. 1998; Tan et al. 2019). The non-oxidative phase is totally reversible and is branched with glycolysis allowing a fine regulation of metabolites biosynthesis (Bozdech and Ginsburg 2005). Only two enzymes are involved in this phase: Transketolase (TK, E.C 2.2.1.1) and transaldolase (EC. 2.2.1.2). TK controls about 70% of the PPP and catalyzes two of the three reactions of the non-oxidative part, while only 15% of the PPP is controlled by d-glucose-6-phosphate dehydrogenase, making TK ideal for targeting the PPP (Boros et al. 1997; Cascante et al. 2000). TK was proposed as a potential target in the search of new drug against the pathogen Plasmodium falciparum (Joshi et al. 2008; Hasan et al. 2015). In Mycobacterium tuberculosis, survival and pathogenicity are dependent of arabinogalactane, a heteropolysaccharide of the cell wall, derived from d-ribose-5-phosphate synthetized in the PPP (Fullam et al. 2012). Moreover, Human TK is able to discriminate TPP from 2'methyl-thiamnie pyrophosphate while Escherichia coli TK (ecTK) is not, evidencing the difference of selectivity between both enzymes (Rabe von Pappenheim et al. 2020). These (still rare) examples from the literature strongly suggest that the PPP pathway is worth of investigation for discovery of new drugs against bacteria.
In the classical PPP, TK transfers two carbons units from a phosphorylated ketose donor (d-xylulose-5-phosphate) to a phosphorylated aldose acceptor (d-ribose-5-phosphate or d-eythrose-4-phosphate), leading to a phosphorylated aldose (d-glyceraldehyde-3-phosphate) and to a phosphorylated ketose (d-sedoheptulose-7-phosphate or d-fructose-6-phosphate (F6P)), according to a reversible substituted (ping-pong) mechanism with thiamin pyrophosphate (TPP) as cofactor (Schenk et al. 1998). During the reaction, the two carbons unit from the ketose donor is transiently grafted on the C2-carbanion of the thiazolium ring of TPP, forming α,β-dihydroxyethylthiamin pyrophosphate (DHETPP) while the aldose product is released (Fiedler et al. 2002). The two carbons unit is further transferred to the aldose acceptor from DHETPP to give the ketose product. This reaction is herein referred as the transferase reaction.
The TK of V. vulnificus (vvTK) has not been characterized up-to-date, with the exception of its uniprot entry (Q7MDD4), but yet described TKs share common structural features. They are homodimers with two active sites located in cavities formed between each monomer (Muller et al. 1993), the latter being associated with each other in an “interlocking V-shaped” structure (Mitschke et al. 2010). The two active sites bind a divalent cation (Ca2+, Mn2+, Mg2+…) linked to the pyrophosphate group of TPP (Kochetov and Philippov 1970; Muller and Schulz 1993; Schenk et al. 1998). TK monomers have three domains consisting of 5-to-6 β-strands forming a β-sheet surrounded by α-helices (Rossmann fold). The first domain binds the pyrophosphate moiety of TPP and is therefore called pyrophosphate domain (PP domain). The second domain is the pyrimidine domain (Pyr domain) and is very closed to the PP domain because it interacts with the pyrimidine ring of the TPP. PP and Pyr domains contribute together to the arrangement of the active site when TK chains interact together to form the homodimer. The last domain is the C-terminal domain. Regarding this structure, TPP is not only a cofactor but also a structural partner as it promotes homodimer association by taking place in a hydrophobic pocket formed by both chains (Lindqvist et al. 1992). It adopts a V-shape geometry characteristic for all TPP-dependent enzymes (Fiedler et al. 2002).
Several TK inhibitors have been reported in the literature and are competitive for TPP binding (oxythiamine Ki = 1.4 mM (Rask-Andersen et al. 2011), thiamine Ki = 34 mM (Li et al. 2018), pyrophosphate Ki = 0.28 mM (Li et al. 2018)), or competitive for the donor substrate binding (hydroxyphenylpyruvate (Ki = 3 mM)) for the TK from Saccharomyces cerevisiae (scTK) (Debouck and Metcalf 2000). d-arabinose-5-phosphate inhibits ecTK with a Ki of 6 mM (Šali 1998). In silico screening have allowed to identify inhibitors of the Human TK, all based on diphenyl urea derivatives (IC50 comprised between 0.1 and 0.2 mM) (Dove 1999) and a docking study on the TK from Plasmodium falciparum has led to a family of 4-anilinoquinoline triazine derivatives with IC50 comprised between 0.072 and 0.085 mM (Sharma et al. 2011) with still unknown inhibition mechanism. This highlights the fact that TK are still understudied targets in the fight against pathogens regarding the low number of available synthetic inhibitors. Obviously, this requires characterization of TK structures, kinetic mechanisms, and inhibitor mode of action, including vvTK.
Several assays have been developed to determine TK kinetic constants using a ketose/aldose substrate couple such as d-xylulose-5-phosphate/D-ribose-5-phosphate or l-erythrulose/d-ribose-5-phopshate. The d-glyceraldehyde-3-phosphate (G3P) product is then reduced by a G3P dehydrogenase (Sevostyanova et al. 2006) or an alcohol dehydrogenase using NADH as co-substrate (Hecquet et al. 1993; Bykova et al. 2001).
An electrochemical assay based on this reaction was used to screen a chemical library allowing the identification of a new inhibitor of the ecTK (I38-49, Supporting Information: Data S1) (Touisni et al. 2014; Aymard et al. 2018; Halma et al. 2017). Nevertheless, the proposed inhibition mechanism (partial mixed inhibition) was not totally satisfactory because the reaction steps involving ferricyanide are not fully understood.
In the present work, we obtain for the first time the structure of vvTK solved by x-ray crystallography. Its mechanism is further studied by steady-state kinetics using F6P and Fe(CN)63− as substrates. This study is complemented by inhibition studies using I38-49 and docking experiments. All these data are important for anticipating infection by a human pathogen which will be closely monitored in the near future.
2 MATERIALS AND METHODS
2.1 Reagents
Agar, bovine serum albumin, glycerol (≥99%), Hepes, imidazole, isopropyl β-d-1-thiogalactopyranoside (IPTG), Luria broth (LB) medium, sodium phosphate monobasic monohydrate, thiamine hydrochloride (≥99%), and thiamine pyrophosphate (TPP, ≥95%) were purchased from Sigma. Ammonium sulfate was purchased from Merck. Bradford protein assay reagent was from Bio-Rad. Potassium chloride was from BDH. d-fructose-6-phosphate disodium salt (≥98%) was purchased at ABCR. Di-sodium hydrogenophosphate, potassium hexacyanoferrate (III) (K3Fe(CN)6) and sodium chloride were purchased from Prolabo. Ampicillin was from Roth.
2.2 Plasmid and strains
pET21b(+)-vvTK recombinant vector was synthetized by GeneScript® as follow: TK gene sequence from V. vulnificus was obtained from the protein sequence (UniProt: Q7MDD4) to which a TEV protease recognition sequence (ENLYFQG) (Nam et al. 2020) was added at the C-ter extremity. The resulting sequence was inserted into pET21b(+) vector between NdeI and XhoI restriction sites at 5′ and 3′ extremities, respectively. A vector-encoded 6 × His-tag peptide sequence was present downstream of the XhoI restriction site. Codons optimization for E. coli was done by the algorithm of GenScript®, and the recombinant vector was synthetized by the same company (pET21b(+)-vvTK).
E. coli BL21(DE3) were prepared in our lab. After 10 min on ice, they were transformed by a 45 sec heat shock at 42°C using 200 ng of recombinant vector (pET21b(+)-vvTK). After 1 h of recovery in LB medium at 37°C, bacteria were centrifuged at 10,000 × g during 1 min and resuspended in 200 μL of fresh LB medium. Bacteria were then spread on LB-agar supplemented with 100 μg.mL−1 ampicillin. Clones were grown at 37°C overnight.
2.3 vvTK expression and purification
A transformed clone was used for a preculture in LB medium (37°C, overnight) in the presence of 100 μg.mL−1 ampicillin. Two-liter cultures were grown from a 1/100 dilution of the preculture in LB medium supplemented by 100 μg.mL−1 ampicillin at 30°C under agitation. Induction of the vvTK expression was done by adding IPTG (0.5 mM) and thiamine (10 μM) when the optical density at 600 nm reached a value of 0.6–0.8 (Fullam et al. 2012). Cultures were maintained at 20°C for 20 h before bacteria were harvested by centrifugation (3000 × g) at 4°C, washed with deionized water and frozen at −80°C. Bacteria pellets were suspended in 50 mM pH 8.0 sodium phosphate supplemented by 300 mM NaCl and cells were disrupted by sonication. Cell debris were eliminated by centrifugation at 6000 × g. Supernatant was clarified on Whatman #1 paper, and vvTK was purified by affinity chromatography using a nickel His-trap FF prepacked column (5 mL, GE Healthcare) mounted on an AKTA Start® chromatographic system. Elution was performed with an imidazole gradient (from 0 to 200 mM in 50 mM sodium phosphate pH 8.0 containing 300 mM NaCl). Fraction containing vvTK were collected and dialyzed overnight at 8°C against 4 L of 5 mM sodium phosphate buffer pH 8.0 containing 30 mM NaCl (8 kDa cutoff membrane, Spectra/Por 6 dialysis Membrane from Spectrum Lab). Finally, vvTK was precipitated by dialysis at 8°C against 1 L of 3 M ammonium sulfate for 5 h before storage at 4°C (De La Haba et al. 1955; Datta and Racker 1961).
Protein quantity was determined by Bradford assay, and quality of the purification was controlled by SDS-PAGE analysis using 12% (w/v) polyacrylamide separation gel and 8% (w/v) polyacrylamide stacking gel.
2.4 Enzyme kinetics
vvTK activity was measured using the assay described by Kochetov (1982) (Equation 1) and d-fructose-6-phosphate (F6P) as ketose donor: vvTK stored in 3 M ammonium sulfate was centrifuged during 2 min and, after discarding the supernatant, resuspended in 50 mM Hepes buffer pH 7.0, KCl 100 mM. Concentration was controlled by Bradford assay. Reactions were performed in 200 μL using 0–2 mM Fe(CN)63−, 0–0.5 mM F6P, 2 mM MgCl2, 0–0.2 mM TPP, and 0.9 μM vvTK in a 96-well plate (Greiner Bio-one) at 37°C. Absorbance was recorded at 420 nm during 1 h at 37°C using a Berthold Tristar 5 microtiter plate reader. Reactions were triggered by Fe(CN)63− addition and control reactions were obtained in the absence of F6P. Initial rate were corrected using control reaction (<1.0 μM.min−1). Reaction rates are expressed in μM.min−1 using response coefficient (εM420nm × l) of Fe(CN)63− (0.4725 mM−1 in these conditions). Specific activity was expressed as μmol of F6P converted to d-erythrose-4-phosphate (E4P) per minute and per milligram of vvTK.
Inhibition studies of vvTK by I38-49 (2-(4-ethoxyphenyl)-1-(pyrimidin-2-yl)-1H-pyrrolo[2,3-b]pyridine) (Dimanche-Boitrel et al. 2017) were performed using inhibitor concentrations of 0–400 μM. I38-49 was not found to react with Fe(CN)63− or Fe(CN)64−. In all experiments, the conversion was verified to be below 10% to avoid underestimation of the reaction rate. Data plotting and kinetic analysis (linear and nonlinear fitting) were done using Magicplot software (v3.0.1, www.magicplot.com).
2.5 Molecular modeling of vvTK including TEV cleavage site and 6 × his-tag
The introduced TEV sequence should form an alpha helix at the C-terminus of the recombinant vvTK. To verify that the TEV cleavage site and the 6 × His tag did not impact the secondary structure, the latter was predicted using SOPMA (https://npsa.lyon.inserm.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html).
vvTK-TEV-Histag structure was first modelized using Swiss-Model (02.01.2022 version, https://swissmodel.expasy.org/) (Waterhouse et al. 2018; Studer et al. 2020) using the ecTK structure as template (PDBid 2R8P) including the TEV cleavage site and the 6 × His-tag. Model selection was done maximizing GMQE, QSQE metrics and sequence coverage. Hydrogen atoms’ position was predicted using MolProbity (v4.5.1, https://molprobity.biochem.duke.edu/) (Chen et al. 2010; Richardson et al. 2018). Asn, Gln, and His flips were allowed and heteroatom-H bond-length were calculated on their electron-cloud. Each His residue tautomer and Asn and Gln residues rotamers were verified using PyMol (v2.5.2, https://pymol.org) to avoid steric clashes (Chen et al. 2010). Energy minimization was performed with SPDB viewer v4.10 using GROMOS96 force field in order to enhance the model quality: a first steepest descent (200 steps), followed by a conjugate gradient (200 steps) and a second steepest descent (200 steps) were performed on the vvTK model. All software options were selected for minimization (bonds, angles, non-bounded, electrostatic, torsions, improper) (Guex and Peitsch 1997). Model evaluation was performed using PROCHECK (Laskowski et al. 1993), Verify 3D (Bowie et al. 1991) and ERRAT (Colovos and Yeates 1993) (02.07.2022 versions, https://saves.mbi.ucla.edu/). RMSD were obtained using PyMol (Schrödinger, LLC).
2.6 Crystallization of vvTK structure
The vvTK stored at 4°C in 3 M ammonium sulfate was centrifugated during 2 min with a benchtop centrifuge. Supernatant was discarded, and the enzyme was resuspended in 50 mM Hepes buffer pH 7.0, 100 mM KCl before desalting by gel-filtration chromatography (Hi-Prep 26/10 desalting prepacked column from GE Healthcare mounted on an AKTA Start® system) using the same buffer to remove ammonium sulfate traces. vvTK was concentrated using Microsep Advance centrifugal device (30 kDa cutoff, Pall) at 3220 × g at 4°C during 45 min, and TPP and MgCl2 prepared in the same buffer were added to vvTK at final concentrations of 0.9 mM and 1.8 mM, respectively. Protein concentration was determined at 280 nm with a NanoDrop® system using the molar extinction coefficient of vvTK predicted by ProtParam (εM280 nm = 100,060 M−1.cm−1, 1.351 for 1 g.L−1, 02.01.2022 version, https://web.expasy.org/protparam/) (Wilkins et al. 1998).
Crystallization screening conditions were carried out using the sitting-drop vapor-diffusion method using commercial crystallization kits. For screening, a Mosquito® crystallization robot (SPT Labtech) was employed using one protein/crystallization agent ratio (100 + 100 nL drops equilibrated against 70 μL in MRC Crystallization Plates (Molecular Dimensions)). vvTK (31 mg.mL−1) led to crystal growth in 0.16 M calcium acetate, 0.08 M sodium cacodylate pH 6.5, 14.4% PEG 8000, 20% glycerol. Crystals were further cryo-protected in the same solution to which 15% ethylene glycol was added, and then harvested. X-ray diffraction data were collected at the ID23_1 beamline (ESRF, Grenoble) at a wavelength of 0.885600 Å. Data were indexed, integrated, and scaled using XDS (Kabsch 2010). The phase problem was solved by molecular replacement using phenix.phaser (Afonine et al. 2010) with the herein constructed model. The experimental model was then built using Coot (Emsley et al. 2010) and refinement was carried out using PHENIX (Emsley and Cowtan 2004; Afonine et al. 2010; Liebschner et al. 2019). Data collection and refinement statistics are shown in Table 1. PyMol (Schrödinger, LLC) was used to render the molecular structures.
| Structure-ID | vvTK |
|---|---|
| Data collection | |
| Beamline | ID23-1 |
| Wavelength (Å) | 0.8856 |
| Space group | P 1 21 1 |
| Cell dimensions | |
| a, b, c (Å) | 69.23, 75.48130.39 |
| α, β, γ (°) | 90.0, 99.87, 90.0 |
| Resolution range (Å) | 34.95–2.10 |
| Highest resolution shell (Å) | 2.1–2.2 |
| Total reflections | 245,442 |
| Unique reflections | 76,983 |
| Rmeas (%) | 8.7 (89.6) |
| CC1/2 (%) | 99.7 (67.5) |
| I/σ(I) | 11.66 (1.95) |
| Multiplicity | 3.19 (3.19) |
| Completeness (%) | 99.4 (99.6) |
| No. mol./asymm. unit | 2 |
| Refinement | |
| Rwork/Rfree (%) | 16.25/20.70 |
| No. atoms | |
| Protein | 10,356 |
| Ligand/ion | 103 |
| Water | 715 |
| Wilson B-factor (Å2) | 35.07 |
| Average B-factor (Å2) | |
| Protein | 37.30 |
| Ligand/ion | 40.85 |
| Water | 41.95 |
| r.m.s.d. | |
| Bond lengths (Å) | 0.007 |
| Angles (°) | 0.823 |
| Ramachandran | |
| Favored (%) | 97.26 |
| Allowed (%) | 2.74 |
| Outliers (%) | 0.0 |
2.7 Molecular docking of substrates and inhibitor on vvTK structure
Water molecules and nickel ion were discarded from the vvTK structure using PyMol to obtain a structure containing TPP and Mg2+. For docking experiments with DHETPP, the structure of DHETPP was obtained from the structure of S. cerevisae TK (PDBid 1GPU) and aligned with the TPP in the vvTK structure. The TPP was discarded and the resulting PDB file was saved successively in PQR and then in PDBQT format files with Open Babel (v3.1.1, https://github.com/openbabel/openbabel/releases/tag/openbabel-3-1-1) (O'Boyle et al. 2011). MMFF94 force field was used to calculate partial charges at pH 7.0. Ferricyanide SMILES code was obtained from PubChem (CID: 439210, https://pubchem.ncbi.nlm.nih.gov/) and formatted in PDB format with OpenBabel online with 3D coordinates generated at pH 7.0 (v2.3.2, https://www.cheminfo.org/Chemistry/Cheminformatics/FormatConverter/index.html). Structures of the cyclic and linear F6P were also obtained from PubChem (CID: 440641 and CID: 69507, respectively). AMDock (v1.5.2, https://github.com/Valdes-Tresanco-MS/AMDock-win) (Valdés-Tresanco et al. 2020) was used with AMBER forcefield in AutoDock 4 using following parameters: exhaustiveness = 8, number of poses = 10, energy evaluation = 2,500,000, 10 runs, cluster tolerance = 2.0, and no ligand protonation. Mg2+ cation was maintained in the active site, and pH was fixed at 7.0. For F6P docking experiment, the cube size was 42.0 Å and centered at the position x = −39.9 Å, y = 24.0 Å, z = −19.5 Å. The box used for docking Fe(CN)63−/4− on vvTK containing TPP was 32.0 Å wide and was centered on coordinates x = −38.8 Å, y = 36.3 Å, z = −22.4 Å. In the case of vvTK containing DHETPP, the box was centered at the position x = −14.60 Å, y = 59.10 Å, z = 11.10 Å (same size). The box used for the docking of I38-49 was 99 × 99 × 99 Å (maximum sized allowed by AMDock) to encompass the whole vvTK structure and to allow the binding of I38-49 at any position of the protein. In this case, the box was centered on coordinates x = 17 Å, y = 28 Å, z = −36 Å (Supporting Information: Data S1).
3 RESULTS
3.1 vvTK expression and purification
After cloning, expression in E. coli BL21(DE3) and lysis, vvTK is purified by affinity and eluted using 80 mM imidazole. The purification yield is 26% and a main band at 70 kDa (expected molecular weight is 73,891 Da) is observed on the SDS-PAGE (Supporting Information: Data S1). Around 94.0 mg of vvTK are purified from a 2 L culture with a specific activity of 0.102 μmol.min−1.mg−1 using F6P and Fe(CN)63− as substrates. When no TPP is added to the reaction media, no activity is measured meaning that vvTK is purified as an apoenzyme (e.g. without its cofactor).
3.2 Molecular modeling
vvTK was modeled by molecular threading, utilizing the structure of E. coli TK (PDBid 2R8P) by incorporating its own sequence into this structure. These two proteins share more than 30% of sequence identity ensuring a good quality of the vvTK model. Swiss-Model was the preferred tool for obtaining the vvTK dimer directly from the primary sequence. Detailed description of the modeling and metrics is provided in the Supporting Information: Data S1. As expected, the global fold of the model superimposed with the E. coli template.
3.3 Crystallization
Initial crystallization attempts were conducted in the presence of the tag. The TEV cleavage site was included with the intention of obtaining a native protein in case the tag interfered with crystallization. However, since the uncleaved protein allowed for the acquisition of a structure at a satisfactory resolution, and considering that the presence of the tag seemed to stabilize the C-terminus through its interaction with a Nickel ion, the decision was made to retain the 6 × His tag. Following the acquisition, integration, and reduction of the data, a resolution of 2.1Å is maintained. The 3D model constructed earlier prove effective as a search model in the molecular replacement process. Following subsequent refinement steps, a final crystal structure is achieved at a resolution of 2.1 Å with R factors of 16.25% (Rwork) and 20.7% (Rfree) (Figure 1, Table 1).
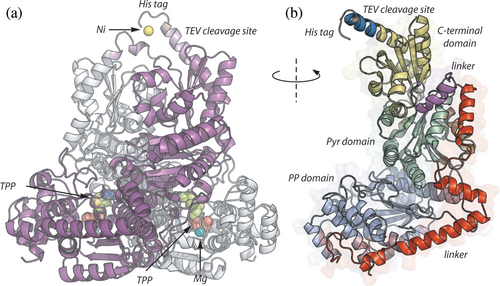
The vvTK crystal and model exhibit a closed folding with an RMSD of 0.394 Å and form the classical Canadian tent, as described for other TK with a similar fold. This structural arrangement is in close proximity to other microbial TK that have been previously crystalized (RMSD <1.0 Å), including ecTK, which was used as the template for modeling (Supporting Information: Data S1). Interestingly, all residues from Met 1 to His 678 are solved in the x-ray structure of chain A, including the 6 × His-tag together with a nickel ion (vide infra). The chain B covers the residues Met 1 to His 674. The crystal includes the vvTK cofactors (two magnesium ions and two TPP molecules) added to the protein solution before crystallization (Materials and methods). Additionally, 10 ethylene glycol molecules from the crystallization solution are found in the crystal.
As predicted by the model, the vvTK shares common features with described bacterial TK from E. coli and M. tuberculosis. It forms a homodimer in an embedded V-shaped with C-terminal extremities oriented in the same direction (Figure 1a). The two subunits have an RMSD of 1.135 Å evidencing a perfect symmetry of the protein crystal. A single vvTK chain is formed by three domains separated by two linkers (Figure 1b) (Mitschke et al. 2010; Fullam et al. 2012). The PP domain (Met 1-Leu 275) contains the interaction site with the pyrophosphate (PP) moiety of TPP. The linker region (Gly 276-Ala 354) between PP and Pyr domains is formed by three helices in vvTK vs two in M. tuberculosis TK, the first helix being split in two in vvTK (Fullam et al. 2012). The second helix (Ser 294-Tyr 315) in vvTK is lacking in H. sapiens TK. This main difference between bacterial and human TK would affect the flexibility between PP and Pyr domains. The Pyr domain (Ser 355-Phe 517) contains the interaction sites with the pyridinium and thiazolium rings of TPP and a hydrophobic pocket around both TPP rings. This pocket is also formed by the PP domain of the other monomer (vide supra, Supporting information: Data S1). The C-terminus domain (Tyr 540-Ala 663) allows the interaction between the two chains of the homodimer. All the domains have a Rossman fold as described for all other known TK (Muller et al. 1993). Two TPP molecules are resolved in the x-ray structure evidencing the localization of the active site at the interface between the two monomers (Figure 2a and Supporting Information: Data S1). As expected, the pyrophosphate moiety interacts with NH of Asp 154.B in the α6 helix, His 65.B and His 259.B in α11 helix, as well as with magnesium ion of the PP domain. The magnesium ion is coordinated by the carboxylate of Asp 154.B (α6), the amide function of Asn 184.B. and carbonyl of Ile 186.B from one side and by the pyrophosphate moiety of TPP on the other side, thus stabilizing the interaction between TPP and the PP domain. A hydrophobic pocket formed by Leu 380.B, Val 408.B, Phe 433.B, Phe 436.B, and Tyr 439.B of the Pyr domain and by Leu 115.A, Ile 188.A of the PP domain of the other monomer allows the binding of the pyridinium and thiazolium rings of TPP. Moreover, a hydrogen bond is formed between Glu410.B and the NH of the pyridinium ring.
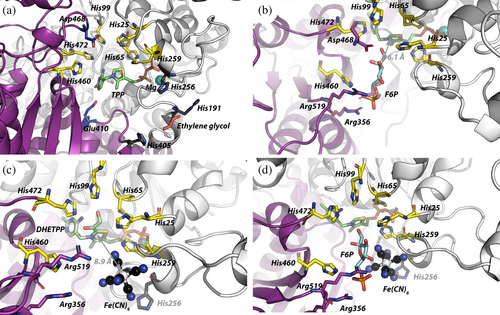
The C2 atom of the thiazolium ring of TPP, involved in the reaction as a carbanion, is at the bottom of a cavity but remains accessible to the solvent (Supporting Information: Data S1). This surface cavity is expected to be the substrate-binding site and exhibits a crown of six histidyl residues from both monomers: His 25.A, His 65.A, His 99.A, His 259.A His 460.B, and His 472.B.
These histidyl residues have been studied independently in the literature. His 69, His 103, His 263, and His 481 are shown to be involved in TPP stabilization (Robinson and Chun 1993; Fiedler et al. 2002), while His 30, His 103, and His 481 are implicated in TPP activation into carbanion, as demonstrated in scTK (Lindqvist et al. 1992). Other works focus on the stabilization of phosphorylated substrates by His 30, His 263, His 469, and His 481 in the same enzyme (Robinson and Chun 1993; Nilsson et al. 1997; Kochetov and Solovjeva 2014). Here, we attempt to attribute each histidyl residue of the crown to one of these functions. TPP activation into the reactive carbanion (e.g., deprotonation of the C2 atom of the thiazolium ring) is still not well understood but involves hystidyl residues. As proposed by Lindqvist et al. and Fiedler et al. based on the scTK (Lindqvist et al. 1992; Fiedler et al. 2002), and adapted to the vvTK structure, activation is probably initiated by a charge delocalization of the N1’ of the pyrimidine ring of TPP by Glu 410.B leading deprotonation of the amine at the C4’ position by His 472.B. The unprotonated imidazole ring readily accepts the proton from the thiazolium ring (C2 position) leading to the activated TPP (e.g., the carbanion). It is expected that protonated His 472.B is stabilized by Asp 468.B and His 99.A. It is unclear whether if His 25.A, His 65.A, and His 259.A participate to the activation despite nitrogen atoms of His 65.A and His 259.A imidazole rings being closed to the sulfur atom of the thiazolium (<3.8 Å) and probably favoring the charge delocalization in the ring. Additionally, these two residues (His 65.A and His 259.A) stabilize the pyrophosphate moiety of TPP as a clamp together with a magnesium ion.
The importance of the other histidyl residues (His 191.A, His 256.A, and His 405.B) in this region is questionable because their distance to TPP is high (>10 Å). They are located at the entrance of the substrate binding site (Figure 2a) and the triad formed by these three residues is in a cavity where one ethylene glycol molecule is found in the crystal. Moreover, these residues are not conserved in others TK. For example, in scTK (PDBid: 1GPU), His 405.B is replaced by a lysyl residue (also positively charged), and His 191.A is replaced by an alanyl residue which does not appear to be relevant for substrate interaction.
3.4 Substrate docking on vvTK
The binding of three ligands on the vvTK is of importance in this work: F6P as the phosphorylated ketose donor, Fe(CN)63− as oxidant of the DHETPP intermediate having a stoichiometry of two to complete the reaction (Equation 1), and inhibitor I38-49 (vide infra). Attempts to crystallize or co-crystallize the vvTK with these ligands by soaking have been so far unsuccessful. In the absence of vvTK crystals including these ligands, docking experiments are performed.
3.4.1 Docking of d-fructose-6-phosphate
F6P is the first substrate to bind to the enzyme in the substituted (Ping-Pong) TK mechanism and is therefore used for docking. Docking is performed with both linear and cyclic (furanose) F6P (Figure 2b and Supporting Information: Data S1). Only a limited number of poses (three) are obtained with linear F6P with energy not lower than −3.9 kcal.mol−1 and one of them do not have the ketol function properly positioned for the reaction. The other poses are located elsewhere on the vvTK with energies not compatible with protein–ligand interactions. Regarding the cyclic F6P, only one pose is found in the vvTK active site and have a free energy of −4.58 kcal.mol−1. In order to judge if this F6P pose is relevant, structures of published TK co-crystallized with substrates or products are superimposed with vvTK docked with F6P (Supporting Information: Data S1). In all these structures the distance between reactive carbonyl and the C2 of TPP (or TPP analogue) is comprised between 3.3 and 5.6 Å. This distance is slightly higher in the docking of F6P in vvTK (6.1 Å, Figure 2b) suggesting this pose is closed to the real F6P-binding position in vvTK. Interactions are observed between F6P and His 460.B, His 25.B, and His 472.B (<4 Å) of the histidyl crown. Additionally, His 460.B forms a hydrogen bond with an oxygen atom of the phosphate group of F6P, while the guanidium moieties of Arg 356.B and Arg.519.B complete the interactions with the phosphate group of F6P (<3 Å). Finally, the last interaction with phosphate is between the hydroxyl group of Ser 383.B and the C6 oxygen atom of F6P (2.9 Å). Phosphate is shifted by ~2 Å out of the binding site compared to crystalized TK with substrates but interacts with the same arginyl residues. Interactions between the furanose ring and vvTK occur between the carboxylate of Asp 468.B located at less than 3.1 Å above the F6P and the C2 and C3 hydroxyl groups of F6P, between His 472.B and His 25.A with the C1 hydroxyl group of F6P and between the C4 hydroxyl and Arg 519.B. This pose seems to correspond to an intermediate binding of F6P prior to catalysis because the distance between the C2 atom of F6P and the C2 atom of TPP is still 6.1 Å. This docking pose probably does not correspond to the true vvTK-F6P complex, but it highlights the importance of histidyl residues of the crown for substrate binding.
3.4.2 Docking of ferricyanide
Fe(CN)63− is a metallic complex with a positively charged iron atom linked to 6 negatively charged cyanide ions, giving a net negative charge to the complex. The structures of cytoglobin from Homo sapiens (PDBid: 1URV, 1UT0, 1URY, 1UX9, and 4B3W), bilirubin oxidase from Myrothecium verrucaria (PDBid: 6I3J, 6I3K), and superoxide reductase from Desulfarculus baarsii (PDBid: 1VZH, 1VZG) are the only ones obtained with Fe(CN)63−. In all these structures, Fe(CN)63− is found at the surface of the protein and never in a cavity, suggesting a relatively low affinity between these proteins and this ligand. On the other hand, Fe(CN)63− interacts strongly with homodimeric hemoglobin from Scapharca inaequivalvis through ionic interactions via a lysyl and arginyl cluster located 15 Å from the heme of this protein, allowing efficient electron transfer (Colotti et al. 1994).
In the case of the vvTK, we could expect that Fe(CN)63− binds closely to DHETPP. Another hypothesis could be that the electron transfer between DHETPP and Fe(CN)63− occurs through the histidyl crown. Several other oxidants are tried out (Ru(bpy)32+, oxidized ABTS+…) in place of Fe(CN)63− without success suggesting a selectivity of vvTK for Fe(CN)63− (Data not shown). In the docking experiment, the two redox forms Fe(CN)63− and Fe(CN)64− cannot be distinguished as far as we know, and they are refered as Fe(CN)63−/4- in this section to avoid confusion.The docking of Fe(CN)63−/4− on vvTK is done with enzyme containing DHETPP or TPP (Figure 2c). In both cases, a unique pose is obtained. Despite the required stoichiometry of two Fe(CN)63− molecules for the oxidation of one DHETPP, only one Fe(CN)63− could be docked. Fe(CN)63−/4− is found close to the nitrogen atoms of imidazole rings of His 25.A, His 259.A (both in the histidyl crown, at 4.1 Å and 3.7 Å, respectively), and in the proximity of His 256.A (4.5–4.7 Å). These distances are reported in Table 2. One cyanide moiety could also be stabilized by the hydroxyl group of Ser 383.B and nitrogen atom of Pro 382.B located at 2.7 and 4.1 Å, respectively (not shown for clarity). In this pose, the Fe(CN)63−/4− iron atom is located at 8.7 Å from the C2 of the thiazolium ring of the DHETPP. According to the Marcus equation (Gray and Winkler 2005; Kuss-Petermann and Wenger 2016), Fe(CN)63−/4− is at the optimal distance of DHETPP between 5 Å and 15 Å. His 25.A and His 259.A could also participate in the electron transfer acting as electron relays. This suggests that Fe(CN)63− should be considered as a Michaelian substrate for vvTK and mechanism elucidation should include the existence of a Fe(CN)63−-vvTK complex. It should be noted that some of the TK co-crystallized with their substrates are also assayed using Fe(CN)63− but the Fe(CN)63−-TK complexes are not investigated (Supporting Information: Data S1).
| Residue | Atom | vvTK-TPP complex | vvTK-DHETPP complex |
|---|---|---|---|
| His 25.B | NE2 | 6.2 Å | 4.1 Å |
| His 256.B | N | 2.9 Å | 4.5 Å |
| His 259.B | N | 4.5 Å | 3.7 Å |
| TPP or DHETPP | C2 | 10.3 Å | 8.7 Å |
| ΔG° (kcal/mol) | −4.51 | −5.43 |
- Note: Distance is determined between the atom of the residue and closest nitrogen atom of Fe(CN)63− except for TPP and DHETPP where it is determined using the iron atom. Free energies of dockings are calculated using AMDock.
When the docking is done with vvTK containing TPP instead of DHETPP, only few differences are observed regarding involved residues (Table 2). Nevertheless, Fe(CN)63−/4− is found closer to His 256.A and consequently further from His 25.A, His 259.A, and from the C2 atom of TPP. This suggests that Fe(CN)63−/4− could also bind vvTK containing TPP. Regarding the charge and the size of Fe(CN)63−/4−, this would be a potential competitor of F6P binding or of another Fe(CN)63−/4− molecules. Unfortunately, it was impossible to determine by docking experiments how a second Fe(CN)63−/4− could interact within an existing vvTK-DHETPP-Fe(CN)63−/4− complex because the algorithm erases the first docked Fe(CN)63−/4− molecule. The second Fe(CN)63−/4− could be considered henceforth as a substrate with a binding site affording a vvTK-(DHE)TPP-(Fe(CN)63−/4−)2 complex or simply as a chemical oxidant for the first bound Fe(CN)63−/4−. This remains to be elucidated.
Finally, the possibility for the vvTK to simultaneously bind F6P and Fe(CN)63−/4− is investigated (Figure 2d). The superposition of the docking poses for each substrate do not evidence any steric clashes. The closest distance between C4 hydroxyl of F6P and the closest nitrogen atom of Fe(CN)63−/4− is 3.9 Å. Considering the radius of these atoms (Slater 1964), these molecules are 1.05 Å apart, but additional local movements in the vvTK in solution can allow or disallow the concomitant binding of F6P and Fe(CN)63−/4−. In the transferase reaction, TKs follow a substituted mechanism (ping-pong) with successive binding of the ketose donor and aldose acceptor. In the present case, the possibility for both F6P and Fe(CN)63− to bind simultaneously on the enzyme could also lead to a sequential (ordered or non-ordered) mechanism (vide infra). In addition to the current work performed on vvTK, these results could be probably extrapolated to other TKs considering the structural homology between these enzymes.
3.4.3 Docking of I38-41, an inhibitor of ecTK
In a previous study, I38-49 is shown to inhibit the ecTK following a partial mixed mechanism when TPP concentration varies. The assay is conducted using l-erythrulose as ketose substrate together with Fe(CN)63− as oxidant (Aymard et al. 2018). I38-49 is also an inhibitor of vvTK (vide infra). In the absence of crystallographic information regarding the specific interaction between I38-49 and vvTK, we performed docking experiments in order to identify putative binding sites and to contribute to elucidate the inhibition mechanism (Figure 3, Table 3, Supporting Information: Data S1).

| Ranking | Free energy (kcal.Mol−1) |
|---|---|
| 1 | −11.20 |
| 2 | −11.14 |
| 3 | −10.97 |
| 4 | −10.85 |
| 5 | −10.17 |
| 6 | −9.98 |
| 7 | −9.60 |
| 8 | −9.11 |
| 9 | −9.11 |
| 10 | −9.04 |
Ten poses are proposed after docking I38-49 on the vvTK structure filled with TPP. The first four poses, corresponding to the more stable complexes, are found in the same cavity located at the interface between PP domain, Pyr domain and first linker (Figure 3, Supporting Information: Data S1). This cavity is formed by loops between helix α2 and sheet β1 and helices α4 and α5 of the PP domain, between helix α19 and sheet β8, and helix α21 and sheet β9 of the Pyr domain and by the loop between helices α15 and α16 of the first linker (Figure 3). In particular, the loop between helices α4 and α5 (29-residues long) must be flexible enough to accept ligands and/or to induce movements inside the PP domain and further cooperative behavior. Residues 326–329 of the first linker and Tyr 102 of the PP domain complete the closure of the cavity. Several orientations of I38-49 in this cavity are obtained but always with the 7-aza-indole and the phenyl rings in the same plane (Supporting Information: Data S1). Only the fifth pose of I38-49 is positioned at the entry of the active site, the phenyl ring occupying the position of the F6P cycle and the indole ring being at 4–5 Å of the guanidinium moiety of Arg519. The last four poses show nonrelevant interactions with the surface of the PP domain (pose #6), with the TEV protease cleavage site (pose #7) or with the C-ter domain (poses #8 and #9). The tenth pose shows I38-49 positioned on the other side of the linker compared to poses #1–4, also with possible interactions between the Oxygen O7c with Asn 402 and N7a.
According to the free energy (−9.98 to −11.20 kcal.mol−1, Table 3), no position could be definitively excluded but the number of poses in the cavity of the Pyr domain seems to indicate a preferential position. In this case, as I38-49 is found close to the crossroad of Pyr domain, PP domain and first linker, domain movement is suspected to lead to cooperativity (vide infra).
3.5 Enzyme kinetics
The assay proposed by Kochetov (1982) is used to measure vvTK activity, based on the oxidation of the DHETPP intermediate by two molecules of Fe(CN)63− (λmax = 420 nm, yellow) together with one water molecule yielding two uncolored Fe(CN)64− molecules (Equation 1). In the present work, F6P is used as ketose because the furanose configuration protect the ketone function from oxidation by Fe(CN)63−, as opposed to other available TK ketones substrates (e.g., β-hydroxypyruvate or l-erythrulose).
The exact mechanism of the reaction involving Fe(CN)63− is still not well understood probably because of its complexity: two Fe(CN)63− molecules are involved, one water molecule is necessary to respect the mass balance and the two carbons units are released as glyoxylate with unknown reaction sequence. It is nevertheless accepted that the first reaction step leads to DHETPP and to the release of E4P. Then, two ferricyanide molecules oxidize DHETPP back into TPP with a water molecule forming one molecule of glyoxylate (Equation 1). This second step is irreversible. Therefore, taking the advantage of having access to vvTK, we investigate its steady-state kinetics.
3.6 Rate versus TPP
Reaction rates (v0) are determined under F6P (0.5 mM) and Fe(CN)63− (1 mM) while TPP concentration varied (Supporting Information: Data S1). In this case, the vvTK follows a Michaelian behavior (confirmed by the Hanes-Wolf plot) with an apparent KMTPP of 2.62 ± 0.20 μM, an apparent Vm value of 11.36 ± 0.27 μM.min−1 (kcat of 0.21 ± 0.01 s−1). The KM value is similar to the one of the ecTK (1.8–4.8 μM) and close to the one of eukaryotes (0.6 μM for scTK, 7 μM for Mus musculus TK and 0.4 μM for Homo sapiens TK).
3.7 Rate versus F6P and Fe(CN)63−
The reaction rate is then evaluated as the function of [F6P] (0–500 μM) and [Fe(CN)63−] (0–2000 μM) (Figure 4). When one of the substrate concentration is constant, the rate increases with the concentration of the other substrate according to an apparent Michaelis–Menten mechanism. Therefore, F6P interacts with vvTK forming a TK-F6P complex as expected. Fe(CN)63− also forms a reversible complex with vvTK as already suggested by the docking experiments and now confirmed by the kinetic study. At this stage, it is still impossible to determine how the two Fe(CN)63− molecules interact with vvTK, for example, if they bind simultaneously or one after the other. The Lineweaver–Burk plots clearly exhibit bundles of unparallel straight lines with an intersection point far from the y-axis (1/v0 axis) (Figure 4). This could correspond to several mechanism such as steady-state ordered, Theorell-Chance or random kinetic mechanisms. In this assay, both F6P and Fe(CN)63− should bind to vvTK before the release of the product. This excludes the substituted mechanism observed when TK catalyzes the transferase reaction. When F6P concentrations varies, the Michaelis–Menten plot sometimes shows a slight sigmoidal shape suggesting some cooperativity of the vvTK for F6P. Hill coefficients are not convincing regarding the low fitting quality and/or h indices below 1.2 (Data not shown). Kochtetov et al. also suggest that some cooperativity could exist in TK but without clear evidence (Kovina and Kochetov 1998; Kochetov and Solovjeva 2014).

The apparent Vm values obtained from Michaelis–Menten and Lineweaver–Burk plots (Vmapp) are used to obtain the secondary plots Vmapp = f([Fe(CN)63−]) and Vmapp = f([F6P]) corresponding to the Michaelis–Menten plots when the other substrate is saturating. This allows to determine the KM values for both substrates (KF6Papp = 66 ± 18 μM and KFeCN6app = 139 ± 28 μM) and Vm values (12.34 ± 0.71 to 13.60 ± 1.45 μM.min−1) (Supporting Information: Data S1). This is the first determination of KFeCN6 for a TK. For comparison, apparent KF6P is found to be 600 μM for mtTK (Fullam et al. 2012) and 340 μM for hsTK (Meshalkina et al. 2011), using the same assay, probably because these values are determined under non saturating Fe(CN)63− concentrations (0.5 mM and 1.25 mM, respectively). Attempts to identify the mechanism by model discrimination using enzyme kinetic software (e.g., Dynafit) (Kuzmič 2009) or by classical analysis is yet unsuccessful without product inhibition studies.
When DHETPP is oxidized, two electrons have to transferred to Fe(CN)63− but only one molecule can bind to the vvTK active site according to the docking experiment. We strongly believe that one of the electrons could be transiently accepted by the histidyl residues of the crown, the other being accepted by Fe(CN)63−. After release of the first Fe(CN)64− (reduced), a second Fe(CN)63− could bind to accept the second electron. Alternatively, a radical product could be released and oxidized into glyoxylate outside of the enzyme active site by free Fe(CN)63− molecules. This contribution to the deciphering TK-catalyzed reaction using the Fe(CN)63− assay highlights the probable role of the histidyl crown, the existence of TK-F6P-Fe(CN)63− ternary complex and the fact that this simple and easy-to-handle assay requires much more effort to be fully understood.
3.8 Inhibition by I38-49
The I38-49 inhibitor is evaluated on vvTK against F6P binding (Figure 5). In the absence of I38-49, vvTK adopts a noncooperative behavior with kinetic parameters similar to those observed previously (Vmapp = 8.70 ± 0.41 μM. min−1, KF6P = 106 ± 7 μM). As the concentration of I38-49 increases from 25 to 400 μM, reaction rates decrease and vvTK adopts a cooperative behavior as shown by the sigmoidal shape of the curves. This indicates that the binding of I38-49 induces conformational changes on vvTK with an inhibition constant KI38-49 (e.g., Ki in usual kinetic models). Data are fitted using the Hill equation allowing the apparent Vm, K0.5 and h values to be determined for each I38-49 concentration.
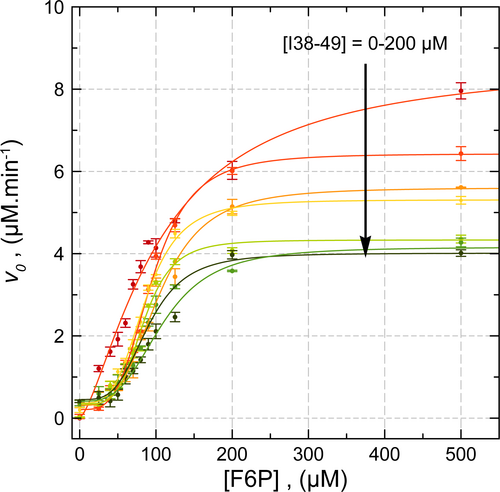
At saturating [F6P], the Vmapp value decreases with [I38-49] meaning that I38-49 is not a competitor of F6P and binds to another site that could be the one suggested by the docking experiment (Figure 3). At these high [F6P], Vmapp value decreases following a non-cooperative binding (e.g., hyperbolic) with a KI38-49 value of 30.71 ± 7.58 μM. Interestingly, at saturating [I38-49], around 35% of the vvTK activity is still observed (partial inhibition of vvTK). This was previously observed for this inhibitor with ecTK (Aymard et al. 2018). This could be explained in two ways: I38-49 binds on both vvTK monomers and induces conformational changes that decrease the kcat value of both monomers ([F6P] and [Fe(CN)63−] are in excess in this case), or I38-49 binds to one monomer and induces conformational changes inhibiting one monomer and preventing the binding of another I38-49 molecule on the other monomer, leaving the second active site ready for catalysis.
The K0.5 value ([F6P] for a rate equal to half the maximum rate) is not significantly affected by the presence of I38-49 meaning its binding does not affects the binding of F6P, considering that K0.5 approximates individual F6P dissociation constants (Supporting Information: Data S1). This confirms the noncompetitive binding of I38-49. Finally, the Hill coefficient, close to 1 in the absence of I38-49, drastically increases to 3.3–4.4 in the presence of I38-49 (Supporting Information: Data S1). This states that, once I38-49 is bound, vvTK adopts a cooperative behavior regarding F6P binding.
We could hypothesize that, in the presence of I38-49, F6P binds to one of vvTK active site but is not transformed even at low concentrations considering the noncompetitive nature of I38-49. At [F6P] above K0.5 value, the second active site could be occupied, probably leading to another vvTK conformation and activity is partially recovered.
I38-49 was previously shown to inhibit ecTK (Ki value of 3.4 μM versus TPP binding) when activity is measured with saturating L-ery and Fe(CN)63− concentrations (Aymard et al. 2018). Similarly, Vmapp value decreases as I38-49 concentration increases but ecTK cannot be fully inactivated suggesting that one ecTK active site is still available for catalysis similarly to vvTK. This can only be explained by conformational changes in the ecTK induced by I38-49. Moreover, as the varying substrate was TPP, no cooperativity was observed but should be evaluated in the near future.
4 DISCUSSION (CONCLUSION)
We have solved the structure of a new TK from Vibrio vulnificus, a human pathogen organism whose expansion zone could grow in the future, which justifies increased knowledge and monitoring. The vvTK structure is similar to previously described TK,with a small difference in the first linker between the PP and Pyr domains formed by four helices instead of two on other TK (Figure 1). We also highlight the existence of a crown formed by 6 histidyl residues around the TPP-binding site (Figure 2a). Its role, if any, has to be clarified but it is probably involved in the TPP activation into carbanion. To assess activity, the oxidation rate of the DHETPP by Fe(CN)63− is used with F6P as the donor substrate (Kochetov 1982). In the absence of other crystallographic structures, docking of cyclic F6P and Fe(CN)63−/4− suggests that both substrates could bind simultaneously to vvTK (Figure 2). This raises the question of the enzyme mechanism using Fe(CN)63−. The natural transferase reaction catalyzed by TK follows a substituted mechanism (Ping-Pong), but the docking experiments suggest that, using Fe(CN)63−/4−, a sequential mechanism is also possible. This is confirmed by steady-state kinetics with KF6Papp = 66 ± 18 μM and KFeCN6app = 139 ± 28 μM (Figure 4). In fact, this is the first kinetic study of the oxidation of DHETPP by Fe(CN)63−/4− in a TK catalyzed reaction, as far as we know and the first evidence of the existence of a vvTK-Fe(CN)63−/4- complex. Obviously, this is only a partial elucidation of the mechanism, but it is highly intriguing and would deserve much more effort to be fully understood in the future, requiring access to the kinetic release of E4P and glyoxylate, isotopic studies to understand to role of the water molecule, as well EPR experiments to study DHETPP oxidation by Fe(CN)63−.
Compound I38-49 discovered to inhibit ecTK is also active on vvTK with an unexpected mechanism. I38-49 induces a cooperative behavior regarding F6P binding (Figure 5) (Aymard et al. 2018). Cooperativity of TK was suggested in the literature but never formerly demonstrated. It is evidenced here for the first time, thanks to I38-49. However, I38-49 does not lead to full TK inhibition meaning that the second binding site is modified by binding to the first one, probably locking one monomer in an active conformation. As co-crystallization of vvTK with I38-49 is yet unsuccessful, docking is done suggesting a binding site at the interface between the PP and Pyr domains and the linker between them (Figure 3). This putative site was never described for any other TK and could be a new target for drugs or metabolites in the PPP regulation. In the future, it should be more deeply investigated in vvTK but also in other TK to enable the design specific drugs.
AUTHOR CONTRIBUTIONS
Bastien Doumeche: Writing – review and editing; funding acquisition; conceptualization; methodology; formal analysis; supervision; project administration. Rainier-Numa Georges: Conceptualization; methodology; validation; investigation; data curation; formal analysis; visualization; writing – review and editing; writing – original draft. Lionel Ballut: Methodology; investigation; validation; formal analysis; writing – review and editing. Guillaume Octobre: Methodology; writing – review and editing. Arnaud Comte: Methodology. Laurence Hecquet: Writing – review and editing. Franck Charmantray: Writing – review and editing; funding acquisition; methodology; investigation; conceptualization; formal analysis; supervision; resources.
ACKNOWLEDGMENTS
The AuRA region is greatly acknowledged for the funding of one of us (Rainier-Numa Georges). The authors thank the European Synchrotron Radiation Facility for access to MX-beamlines. Technical support from all the beamlines staff is gratefully acknowledged.
FUNDING INFORMATION
The Project CEITOP (Criblage Electrochimique d'Inhibiteur de Transcétolases d'Organismes Pathogènes) is funded by the Region Auvergne-Rhone-Alpes (AuRA).