Evidence of direct interaction between cisplatin and the caspase-cleaved prostate apoptosis response-4 tumor suppressor
Review Editor: John Kuriyan
Abstract
Prostate apoptosis response-4 (Par-4) tumor suppressor protein has gained attention as a potential therapeutic target owing to its unique ability to selectively induce apoptosis in cancer cells, sensitize them to chemotherapy and radiotherapy, and mitigate drug resistance. It has recently been reported that Par-4 interacts synergistically with cisplatin, a widely used anticancer drug. However, the mechanistic details underlying this relationship remain elusive. In this investigation, we employed an array of biophysical techniques, including circular dichroism spectroscopy, dynamic light scattering, and UV–vis absorption spectroscopy, to characterize the interaction between the active caspase-cleaved Par-4 (cl-Par-4) fragment and cisplatin. Additionally, elemental analysis was conducted to quantitatively assess the binding of cisplatin to the protein, utilizing inductively coupled plasma-optical emission spectroscopy and atomic absorption spectroscopy. Our findings provide evidence of direct interaction between cl-Par-4 and cisplatin, and reveal a binding stoichiometry of 1:1. This result provides insights that could be useful in enhancing the efficacy of cisplatin-based and tumor suppressor-based cancer therapies.
1 INTRODUCTION
The field of cancer treatment has undergone a transformative shift in recent years, with advancements in our understanding of tumor biology, and the introduction of targeted therapy and immunotherapy which have revolutionized treatment approaches (Hegde & Chen, 2020; Kwon et al., 2022; Sanmamed & Chen, 2018; Waarts et al., 2022; Xia et al., 2021; Zhang & Zhang, 2020). However, despite these notable advances, cancer remains a global public health concern, causing over 3.3 million fatalities in the United States alone in 2020 (Siegel et al., 2023). The challenges of early diagnosis and development of drug resistance continue to impede successful outcomes (Emran et al., 2022; Gupta, 2022; Tyner et al., 2022). Additionally, non-specific targeting by drugs often leads to adverse effects, while cancer recurrence and metastasis further complicate treatment (Babaei et al., 2021; Bakir et al., 2020; Birkbak & McGranahan, 2020; Fares et al., 2020; Ganesh & Massagué, 2021; Lyden et al., 2022; Sznurkowska & Aceto, 2022).
Prostate apoptosis response-4 (Par-4), a 38-kDa tumor suppressor protein, has emerged as a promising therapeutic target in cancer treatment, due to its ability to selectively induce apoptosis in cancer cells (Boghaert et al., 1997; Cernaj, 2016; Cheratta et al., 2021; El-Guendy et al., 2003). Par-4 also plays a crucial role in controlling epithelial-to-mesenchymal transition, a process facilitating dissemination from the primary site to distant tissues (metastasis) (Cheratta et al., 2021; Fares et al., 2020; Seyfried & Huysentruyt, 2013; Yeung & Yang, 2017). Metastasis is responsible for approximately 90% of cancer-related deaths (Dillekås et al., 2019; Ganesh & Massagué, 2021; Seyfried & Huysentruyt, 2013). Par-4 expression also sensitizes cancer cells to chemoradiotherapy and reduces resistance to chemotherapeutic drugs (Guo et al., 2019; Wang et al., 2010).
In humans, the Par-4 gene encodes a 340-residue protein that undergoes cleavage by the caspase-3 enzyme, resulting in two fragments: the Par-4 Amino Fragment of approximately 15 kDa and the carboxy-terminal caspase-cleaved Par-4 (cl-Par-4) of approximately 25 kDa (Chaudhry et al., 2012; Thayyullathil et al., 2013). The cl-Par-4 fragment is functionally active; it enters the nucleus and induces apoptosis by inhibiting various cell survival pathways (Chaudhry et al., 2012; Diaz-Meco et al., 1999). The cl-Par-4 fragment contains the SAC (Selective for Apoptosis in Cancer cells) domain, connected to the carboxy-terminal CC (Coiled-coil) domain through a linker region. While the SAC domain is the minimum fragment that can induce apoptosis in a cell, the CC domain is involved in many protein–protein interactions (Chaudhry et al., 2012; Goswami et al., 2008; Tiruttani Subhramanyam et al., 2017). Sequence alignment of Par-4 from rat, mouse, and human reveals a high degree of sequence conservation, particularly in the SAC and CC domains (Libich et al., 2009).
We have previously shown that cl-Par-4 forms a predominantly alpha-helical tetramer at pH 7 in the presence of high salt (Clark et al., 2019; Raut et al., 2021). The cl-Par-4 protein also forms relatively small alpha-helical particles at pH 4 (Clark et al., 2018). In contrast, at pH 7 with low salt, cl-Par-4 self-associates to form very large particles (Clark et al., 2019; Raut et al., 2021). These findings demonstrate that pH, salt, and perhaps other charge-related characteristics, play critical roles in the self-association state of cl-Par-4. The self-association state could, in turn, affect function via modulation of interactions and/or localization.
Numerous chemotherapeutic agents have also been developed to treat cancer. The platinum-containing compound cisplatin is one of the most widely employed of these agents (Bukowski et al., 2020; Qi et al., 2019). Cisplatin [cis-diamminedichloroplatinum (II)] was approved as a cancer therapeutic in 1978 (Makovec, 2019) and has shown broad-spectrum effectiveness: it has been used to treat cancers of the lung, ovaries, breast, and brain, among others (Dasari & Tchounwou, 2014; Makovec, 2019; Zoń & Bednarek, 2023). The therapeutic strategy of cisplatin is to target the rapidly dividing DNA of cancer cells, where it binds preferentially to the N7 position of purine bases. This disrupts DNA replication and transcription, ultimately leading to apoptosis or necrosis (Dasari & Tchounwou, 2014; Fuertes et al., 2003; Makovec, 2019; Moon et al., 2021).
Despite its effectiveness as an affordable standard therapy for various tumors, cisplatin's utility is limited by the development of drug resistance and its accumulation in tissues, which can lead to severe side effects such as neurotoxicity, ototoxicity, and nephrotoxicity (Martinho et al., 1898; Messori & Merlino, 2014; Mortensen et al., 2020; Peleg-Shulman et al., 2002; Zhao et al., 2014; Zoń & Bednarek, 2023). Several cisplatin analogs have been developed for use in the treatment of cancers (Dasari & Tchounwou, 2014; Kopacz-Bednarska & Król, 2022). Although some analogs demonstrate reduced adverse effects, they also exhibit reduced pharmacological efficacy (Kopacz-Bednarska & Król, 2022).
A range of natural products, combination therapies, and additional treatment procedures have been explored to counteract cisplatin's drawbacks (Mok et al., 2011; Mortensen et al., 2020; Wang et al., 2018; Yarchoan et al., 2017; Zhang & Lu, 2021). For instance, a sulfur-containing compound (sodium thiosulfate) has been used to mitigate the interaction of cisplatin with plasma proteins (Harned et al., 2008; Pinato et al., 2014; Sooriyaarachchi et al., 2012). However, sodium thiosulfate itself introduced additional toxicity (Pinato et al., 2014).
More recently, Wang et al. (2017) reported a synergistic effect when combining cisplatin with full-length Par-4. SK-NEP-1 nephroblastoma cells were used to demonstrate that cisplatin treatment combined with overexpression of Par-4 led to a significant upregulation of ATF4 and BAX, implying the activation of the endoplasmic reticulum (ER) apoptosis pathway. A mouse xenograft model was also used to show decrease in transplanted tumor size upon treatment with a combination of cisplatin and adenovirus-mediated Par-4. These effects were markedly more pronounced compared to treatment with cisplatin or Par-4 alone. This suggests that a combined cisplatin/Par-4 treatment regimen could be developed that uses less cisplatin, and thus causes less toxicity.
The above study did not, however, establish a direct interaction between cisplatin and Par-4. Also, it should be noted that Wang et al. utilized full-length Par-4 in their study. However, as the apoptotic process involves cleavage of Par-4 by caspase-3, it can be assumed that cl-Par-4 is present in the assays. Furthermore, Chaudhry et al. (2012) showed that the use of caspase-3-deficient mutant cells and caspase-3 inhibitors led to a significant decrease in cisplatin-induced apoptosis. This suggests that the cl-Par-4 fragment plays a role in the process.
In this study, we provide the first evidence and analysis of direct interaction between cisplatin and the cl-Par-4 fragment. We show that not only does cisplatin bind cl-Par-4, but it also cross-links cl-Par-4, creating higher-order oligomers, the size of which depends on the cisplatin/cl-Par-4 ratio and the solution conditions. Further investigation of the mechanistic details of the cl-Par-4-cisplatin interaction, including within tumor cells, may open new avenues for enhancing the efficacy of platinum-based therapies and tumor suppressor-based therapies.
2 RESULTS AND DISCUSSION
2.1 Choice of initial conditions for interaction studies: pH 7, 4.5% NaCl, 10:1 molar ratio
It has been reported that intraperitoneal administration of cisplatin, prepared at pH 7 in 4.5% NaCl (770 mM), reduces cisplatin-associated toxicity without compromising its antitumor activity (Mannel et al., 1989). We have previously shown that cl-Par-4 is highly helical and non-aggregated under similar conditions (Clark et al., 2019; Raut et al., 2021), and so we chose these conditions for our initial studies. We also followed previously published conditions of 10:1 cisplatin to protein molar ratio and 24-h incubation time used in a study of interaction between cisplatin and lysozyme (Ferraro et al., 2016).
2.2 Interaction of cisplatin with cl-Par-4 under initial conditions
Under the above conditions, we investigated the cisplatin/cl-Par-4 interaction via circular dichroism (CD) spectroscopy, dynamic light scattering (DLS), and UV–vis absorption spectroscopy. Our aim was not only to document the direct interaction, but also to assess the potential structural changes and cross-linking induced by cisplatin binding to cl-Par-4.
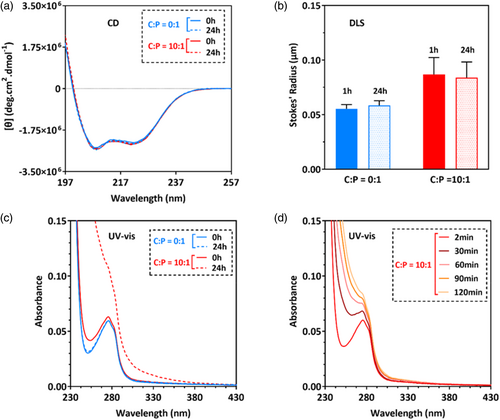
In the absence of cisplatin, cl-Par-4 displays an intense CD spectrum with minima at 208 and 222 nm (Figure 1A, blue traces), indicating a highly alpha-helical conformation. After 24 h of exposure to cisplatin, the CD intensity reduced considerably (Figure 1A, red dashed line). This intensity reduction is similar to that previously observed for cl-Par-4 under low salt conditions, which induces the formation of large particles that scatter light (Raut et al., 2021).
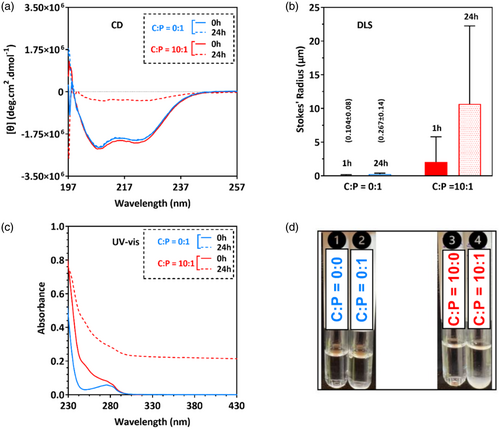
DLS analysis confirms that exposure to cisplatin does induce the formation of large particles (Figure 1B, red bars), which is entirely consistent with the reduction of CD intensity in Figure 1A. The minimal particle size increase seen in the control sample over time is consistent with our previous results with cl-Par-4, showing a small number of large particles forming over time under similar conditions (Raut et al., 2021).
Exposure of cl-Par-4 to cisplatin caused an immediate increase in UV–vis absorbance readings below 280 nm (Figure 1C, solid red line). Over time, the apparent absorbance increased significantly over the entire wavelength range (Figure 1C, dashed red line). This result is characteristic of the formation of large particles that scatter light and is consistent with the DLS and CD observations. Visual inspection of samples showed the presence of precipitation only in the tube containing the cisplatin/cl-Par-4 mixture (Figure 1D).
We conclude that at pH 7 and 4.5% NaCl, mixing of cisplatin and cl-Par-4 at a molar ratio of 10:1 results in large particles that significantly scatter light.
2.3 Interaction of cisplatin with cl-Par-4 at lower C:P molar ratios
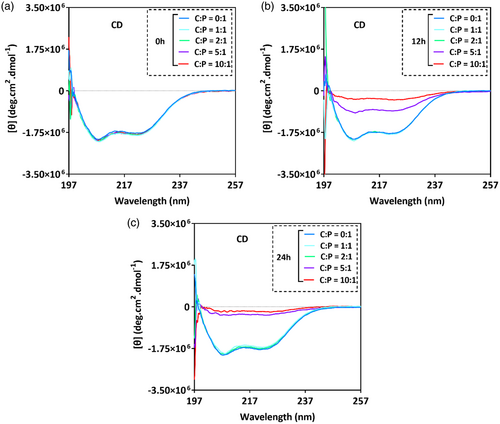
In the previous section, we have shown that a 10:1 molar ratio of cisplatin to cl-Par-4 (C:P = 10:1) causes precipitation due to large particle formation. Presumably, this is the result of cross-linking of cl-Par-4 molecules by cisplatin. We next investigated whether the interaction between cisplatin and cl-Par-4 can occur at a lower molar ratio, and whether this will result in reduced cross-linking. For this purpose, we use C:P molar ratios of 1:1, 2:1, 5:1, and 10:1.
CD spectra showed a notable reduction in intensity after 12 h (Figure 2B), and further reduction after 24 h (Figure 2C), only in the samples with C:P molar ratios of 5:1 and 10:1. This suggests that significant cisplatin-induced cross-linking occurs only at these higher molar ratios of cisplatin.
To assess whether a lower C:P ratio affects particle size, we further assayed the sample with a C:P molar ratio of 1:1. Although the CD intensity did not change significantly with time (Figure 3A), the particle size did increase (Figure 3B) and apparent UV–vis absorbance below 280 nm was seen to increase with time (Figure 3C). Most of this increase occurred during the first 30 min after exposure to cisplatin (Figure 3D, darkest trace), with the rate of change decreasing significantly by the 120-min time point, suggesting a characteristic time of the order of 1 h for the interaction. However, little change in apparent absorbance was seen above 280 nm. Taken together, these results are consistent with minimal cross-linking of cl-Par-4 by cisplatin at a C:P ratio of 1:1, insufficient to create the large particles seen at a molar ratio of 10:1.
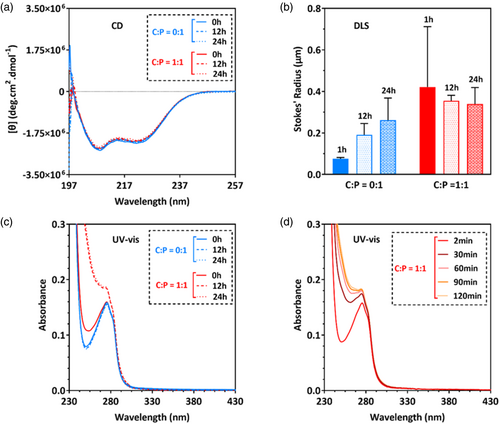
We conclude that at pH 7 and 4.5% NaCl, mixing of cisplatin and cl-Par-4 at a molar ratio of 10:1 or 5:1 result in scattering, while ratios of 2:1 or 1:1 do not initially produce significant scattering. However, with time, even the 1:1 sample produces scattering due presumably to particle size, which complicates further analysis.
2.4 Interaction of cisplatin with cl-Par-4 at pH 4
We previously reported that at pH 4 with low salt, cl-Par-4 is highly helical and stable against aggregation (Clark et al., 2018; Pandey et al., 2023). Therefore, in an attempt to avoid the scattering phenomenon seen at pH 7, we repeated the 10:1 C:P binding studies at pH 4 with low salt (50 mM NaCl). Furthermore, it should be noted that the apoptotic processes that produce the active cl-Par-4 fragment are accompanied by acidification of the cytosol (Chaudhry et al., 2012). This therefore adds to the potential utility of investigating the cisplatin/cl-Par-4 interaction under acidic conditions.
CD spectra showed no apparent change upon exposure to cisplatin (Figure 4A). DLS analysis showed somewhat increased particle size upon exposure to cisplatin (Figure 4B). However, this size increase is far less (by two orders of magnitude) than the size increase seen at pH 7 at the same C:P ratio (Figure 1B, red-dotted bar). Apparent UV–vis absorbance (Figure 4C,D) increased below 280 nm immediately after exposure to cisplatin, and continued to increase with a characteristic time of about 1 h. The Figure 4 data (pH 4, C:P = 10:1) is in fact similar to the Figure 3 data (pH 7, C:P = 1:1). Thus, both of these conditions appear to promote interaction between cl-Par-4 and cisplatin, but with little cross-linking, or not enough cross-linking to cause large particles to form. However, we did observe some increase in higher wavelength scattering at pH 4 after 24 h (see Figure 4C, red dashed line), suggesting that some cross-linking may occur with time under these conditions.
We conclude that at pH 4, upon mixing of cisplatin and cl-Par-4, particle size remains stable with time and scattering is not significant, which facilitates further analysis.
2.5 Cisplatin-induced cross-linking of cl-Par-4 at pH 4
To assess whether changes in particle size and apparent absorbance seen at pH 4 are associated with molecular cross-linking of cl-Par-4 by cisplatin, we performed non-reducing denaturing gel electrophoresis (non-reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis [nrSDS-PAGE]) as a function of C:P molar ratio at pH 4 (Figure 5). All samples were incubated for 24 h at room temperature before running the gel.
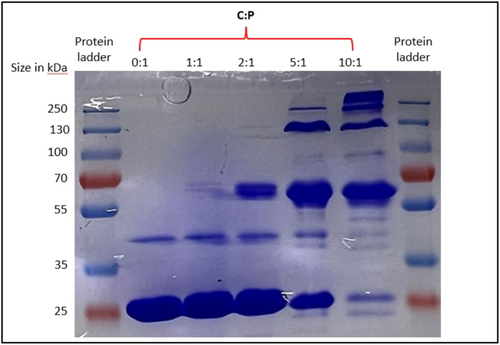
Under these conditions, in the absence of cisplatin, denatured cl-Par-4 is predominantly a monomer (Figure 5, lane 2) with a small amount of apparent dimer. Exposure to a 1:1 molar ratio of cisplatin produces slightly elevated dimer levels, and some additional higher MW bands consistent with an approximate trimeric size (lane 3). The trimeric band becomes significantly more intense at a C:P ratio of 2:1 (lane 4), and it becomes the dominant band at higher C:P ratios (lanes 5 and 6). Very little monomer (perhaps 5%) remains at C:P = 10:1. These results strongly suggest that cisplatin cross-links cl-Par-4, with degree of cross-linking increasing with molar ratio of cisplatin.
We conclude that at pH 4, increasing molar ratio of cisplatin produces an increasing degree of cross-linking.
2.6 Quantitation of bound cisplatin using ICP-OES and AAS at pH 4
Inductively coupled plasma-optical emission spectroscopy (ICP-OES) and atomic absorption spectroscopy (AAS) were used to monitor the Pt element. Both techniques provide evidence of direct binding via increased intensities in cisplatin-exposed cl-Par-4 samples inside of dialysis tubing versus dialysis buffer outside of the tubing (Figure 6, red bars). Control samples without cl-Par-4 show that unbound cisplatin is unimpeded by the membrane (Figure 6, brown bars).
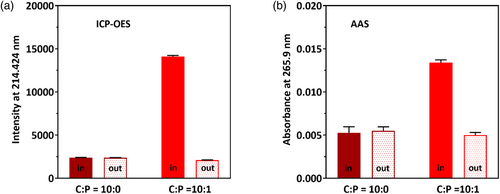
Conditions were chosen to match those of the samples in Figure 4: pH 4 and a C:P ratio of 10:1. Quantitation using direct spectroscopic ratios or using calibration of standard solutions shows that cl-Par-4 binds approximately 1.2–1.4 equivalents of cisplatin as measured by ICP-OES, and approximately 0.7 equivalents of cisplatin as measured by AAS. The difference between these ratios may be due to a variety of factors, including inherent difficulties in the use of acid solutions to dissociate and stabilize metal ions and eliminate interference in samples. Nonetheless, each analysis indicates that approximately one molecule of cisplatin binds per molecule of cl-Par-4, despite the high C:P ratio of 10:1. This finding is completely consistent with the other data under these conditions (Figure 4), confirming that cisplatin binds, but at a level insufficient to cause the formation of large particles.
We conclude that at pH 4 and a C:P molar ratio of 10:1, cisplatin and cl-Par-4 interact in a molar ratio of approximately 1:1.
2.7 Future work: Investigating the mode of cisplatin/cl-Par-4 interaction and consequences
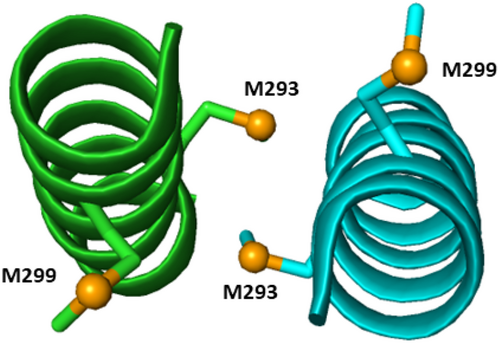
While the present study does not investigate the specific site of cisplatin interaction in the cl-Par-4 molecule, platinum (II) exhibits a pronounced affinity towards sulfur and nitrogen (Li et al., 2011). Cisplatin has been shown to form bonds with side chain groups such as thioether, sulfhydryl, and imidazole (Li et al., 2011). Therefore, the most likely potential side chain targets for cisplatin are cl-Par-4 residues C173 in the SAC domain, and M293 and M299 within the coiled-coil, where the amino acid numbering is based on full-length Par-4. The C173 position is near the nuclear localization signal (residues 145–161) and so coordination at this site could potentially affect nuclear localization. The M293 and M299 positions both fall near the center of the long C-terminal coiled-coil (residues 262–340). Note that M293 lies at the cl-Par-4 coiled-coil dimer interface (see Figure 7), while M299 is more exposed. Thus, M293 may be less accessible to cisplatin. However, if cisplatin can access this position, perhaps via a monomer/dimer equilibrium, then the interaction could help stabilize the dimer by cross-linking the two M293 positions. On the other hand, the more easily accessible M299 may play a role in the higher-order oligomerization induced by cisplatin.
Numerous studies have reported cisplatin-induced protein cross-linking, resulting in higher oligomeric states (Li et al., 2011; Turski & Thiele, 2009). In fact, it has been reported that approximately 98% of injected platinum binds to proteins within 24 h, both reducing drug efficacy and causing protein-related side effects (Kato et al., 2019; Li et al., 2011). Not all proteins, though, bind to and cross-link with cisplatin. For example, no platinum cross-linking was found with hemoglobin even when using a 5:1 molar excess of cisplatin (Li et al., 2011).
The potential exists, however, that cross-linking by cisplatin could activate certain proteins. It is well known that higher oligomeric states can in some cases be advantageous. For example, self-association is crucial for Class II transactivator (CIITA) to regulate the expression of major histocompatibility complex II (MHC II) proteins (Kretsovali et al., 2001). Similarly, self-association of the Nod1 protein helps induce NF-ƙB activation (Inohara et al., 2000), and trimerization of the Fas-transmembrane (Fas-TM) domain is crucial for Fas-mediated cell death (Fu et al., 2016). Cisplatin could, in these or other instances, stabilize higher-order oligomers that are necessary for function. This may prove to be the case with Par-4.
Localization plays a key role in Par-4 activity: caspase-induce cleavage of cytoplasmic Par-4 leads to nuclear translocation of the resulting cl-Par-4 fragment (Chaudhry et al., 2012). Also, Par-4 and cl-Par-4 can be secreted and recruited to nearby cancer cells (Hebbar et al., 2012). It will be interesting to see whether these translocation and secretion properties are altered by interaction with cisplatin. Alternatively, changes in structure, charge, or accessibility upon binding cisplatin may affect interaction with GRP78 (Hebbar et al., 2012), PKC-ζ (Díaz-Meco et al., 1996), RASSF2 (Donninger et al., 2010), or other effector molecules, altering function through interference or stabilization of interactions.
3 CONCLUSION
Our findings clearly show that cisplatin binds directly to cl-Par-4 under two very different buffer conditions (pH 7 high salt and pH 4 low salt). The observed reduction in CD intensity, increased particle size, enhanced light scattering, and visible turbidity in cisplatin-exposed samples suggest that cisplatin promotes substantial cross-linking of cl-Par-4 at a C:P ratio of 10:1 at pH 7 (Figure 1). At lower C:P ratios (e.g., C:P = 1:1), interaction is still observed, but with far less increase in particle size, and thus, presumably less cross-linking (Figures 2 and 3). Non-reducing SDS-PAGE gel electrophoresis of the cisplatin-exposed cl-Par-4 sample at pH 4 reveals somewhat similar results: increased cross-linking at higher C:P ratios (C:P ≥ 2:1) and minimal cross-linking at a C:P ratio of 1:1 (Figure 5). Increase in particle size and light scattering was found to be less pronounced at pH 4, with no apparent change in CD spectra (Figure 4). A molar binding stoichiometry of cisplatin to cl-Par-4 of approximately 1:1 at pH 4 was determined using ICP-OES and AAS (Figure 6).
The observed increase in cross-linking of cl-Par-4 with an increase of C:P molar ratio suggests that the binding of multiple cisplatin molecules to cl-Par-4 leads to a progressive increase in the oligomerization state of the protein/cisplatin complex. The oligomerization of cl-Par-4 induced by cisplatin binding could have functional implications. Oligomerization has been implicated in regulating the cellular localization, stability, and activity of various proteins (Baisamy et al., 2005; Koike et al., 2009; Singh & Jois, 2018; Torshin, 1999). In the case of cl-Par-4, the formation of higher oligomeric complexes through cisplatin binding may affect its localization and modulate its interaction with other proteins. Thus, these findings have implications for cisplatin-based chemotherapy regimens. For instance, they suggest that an in vivo concentration array of cisplatin versus cl-Par-4 (or full-length Par-4) may reveal a specific molar ratio range most conducive to selective induction of apoptosis in the targeted cancer cells.
Finally, as discussed, combination therapies are useful in that they allow reduction in the amount of the individual components, to decrease side effects. Wang et al. have clearly shown the additive effect of a combined cisplatin/Par-4 treatment of tumors (Wang et al., 2017). However, by our calculations, the data in Wang et al. does not meet the statistical definition of synergy. Nonetheless, if combination therapy is to be pursued as a therapeutic option, knowledge of direct interaction between cisplatin and Par-4 will be needed, whether the effect proves to be synergistic or merely additive.
4 MATERIALS AND METHODS
4.1 Sample preparation
4.1.1 Expression, purification, and preparation of cl-Par-4
The expression and purification of cl-Par-4 were carried out according to previously published protocols with slight modifications (Clark et al., 2018; Raut et al., 2021). In brief, BL21 (DE3) Escherichia coli cells with a codon-optimized construct of cl-Par-4 in the modified expression vector H-MBP-3C were grown in Luria-Bertani medium containing ampicillin at 37°C until an optical density at 600 nm (OD600) of 0.8–0.9 was reached (Alexandrov et al., 2001). The culture was induced by 0.5 mM isopropyl thio-β-D-galactoside (IPTG) and grown at 15°C until an OD600 of 1.5–1.6 was reached. Cells were lysed and extracted protein was purified using a HisTrap HP 5 mL column attached to an AKTA chromatography purification system (GE Healthcare, Uppsala, Sweden) under refrigeration.
For pH 7 samples, the purified cl-Par-4 protein in elution buffer (10 mM Tris, 1 M NaCl, 1 mM TCEP, and pH 7.4) was passed through a 0.22-μm filter, and then concentrated to 10 mg/mL using a Vivaspin Turbo 15 centrifugal concentrator (Sartorius, Epsom, UK) with a 10 kDa MWCO. The sample was then dialyzed against a high salt pH 7 phosphate-buffered saline (PBS) buffer (1× PBS, 1 M NaCl, 1 mM TCEP, and pH 7) overnight under refrigeration.
For pH 4 samples, the purified cl-Par-4 protein was concentrated to 10 mg/mL as described above for pH 7, and then gradually added dropwise to 100 mL of acetate buffer at pH 3.6 (10 mM sodium acetate, 20 mM NaCl, 1 mM TCEP, and pH 3.6) with continuous stirring. The pH was adjusted to 4, and the sample was passed through a 0.22-μm filter, and then re-concentrated to 10 mg/mL using a Vivaspin Turbo 15 centrifugal concentrator with a 10 kDa MWCO. The sample was subsequently dialyzed against an acetate buffer of pH 4 (10 mM sodium acetate, 20 mM NaCl, 1 mM TCEP, and pH 4) overnight under refrigeration, to precisely bring the salt content to 50 mM NaCl. Sodium Chloride is not required for stability at pH 4.0: the 50 mM NaCl concentration resulted from the above procedure, which began with dilution of the 1 M NaCl cl-Par-4 stock solution.
The pH 7 and pH 4 samples were each stored at refrigeration temperature for no longer than 1 week before use in biophysical experiments. The NaCl concentration was reduced to 4.5% for the pH 7 sample, and to 50 mM for the pH 4 sample. Samples were diluted to cl-Par-4 concentrations of 1.2 mg/mL before all incubations with cisplatin. These incubated samples were used for nrSDS-PAGE, ICP-OES and AAS. For additional spectroscopic analysis (CD, DLS, and UV–vis), samples were further diluted to 0.2 mg/mL cl-Par-4 after incubation with cisplatin. All samples were wrapped in aluminum foil and stored in the dark whenever possible, to reduce light-induced damage.
4.1.2 Cisplatin stock preparation
Cisplatin stock solutions were prepared by dissolving cisplatin powder (EMD Millipore Corp, Burlington, MA, USA) in 1× PBS with 140 mM NaCl at either pH 7 or pH 4, resulting in a concentration of 1.67 mM (500 ppm). These stock solutions were stored at refrigeration temperature (2–8°C) and enclosed in aluminum foil-wrapped tubes to minimize light exposure and maintain stability.
4.1.3 Mixing of cisplatin and cl-Par-4
Cisplatin and cl-Par-4 were mixed at different molar ratios as specified in Section 2. The mixtures were incubated at room temperature in the dark for 24 h to facilitate optimal interaction. All samples for a series of measurements were maintained at the stated condition by combining the appropriate amount of stock solutions of cisplatin, cl-Par-4, and buffer. For instance, all samples described in Figure 2 were maintained at pH 7, 1× PBS, and 4.5% NaCl.
4.2 CD spectroscopy
CD spectra were recorded in the far-UV wavelength range (190–260 nm) at a scan speed of 20 nm/min using a 1-mm path length quartz cuvettes at ambient temperature using a J-815 CD spectrometer (Jasco, Easton, MD, USA). A buffer blank spectrum was subtracted from the recorded spectra, and the spectra were subjected to smoothing using a means-movement function of 25 nm.
4.3 Dynamic light scattering
DLS data were collected at ambient temperature using a standard diode laser of 640 nm wavelength and at a scattering angle of 173° on a NanoBrook Omni particle sizer and zeta potential analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA). Five scans were recorded for each sample and mean hydrodynamic radii (Stokes' radii) were calculated from the effective diameters obtained from the summary statistical report of the NanoBrook software.
4.4 UV–vis absorption spectroscopy
UV–vis absorption spectra were acquired at ambient temperature over a wavelength range of 200–600 nm at a medium scan speed, utilizing 1 cm path length quartz cuvettes on a SHIMADZU UV-1800 spectrophotometer (Shimadzu USA Manufacturing Inc., Canby, OR, USA). For each sample, three scans were recorded. The net absorbance reading was determined by subtracting the average of the three buffer scans from the average of the three sample scans.
4.5 Non-reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Sample preparation for nrSDS-PAGE involved mixing 32 μL of cisplatin-exposed cl-Par-4 with 8 μL of 5× non-reducing SDS-PAGE loading dye. The samples were denatured by incubating at 90°C for 2 min. Aliquots of 30 μL of each sample were loaded into separate wells. Electrophoresis was conducted using 1× non-reducing SDS-PAGE running buffer at 120 V for 80 min. Gels were stained with Coomassie stain for 1 h and de-stained overnight. Gel band visualization was performed using a white light box.
4.6 Inductively coupled plasma-optical emission spectroscopy
Quantification of bound cisplatin to cl-Par-4 was performed using an Agilent 5800 ICP-OES optical emission spectrometer (Agilent, Santa Clara, CA, USA). Platinum emission intensity was measured at 214.424 nm, the characteristic peak of platinum. Before analysis, unbound cisplatin was removed by dialyzing the cisplatin/cl-Par-4 sample against pH 4 buffer (10 mM sodium acetate and 50 mM NaCl at pH 4) for 12 h in the dark at room temperature using dialysis tubing with a molecular weight cutoff (MWCO) of 12–14 kDa. To assess any potential interference caused by the dialysis tubing on the movement of cisplatin, the cisplatin buffer was also dialyzed against pH 4 buffer. Additionally, a cl-Par-4 sample without cisplatin was dialyzed.
After dialysis, an equal volume (3 mL) of each sample from inside and outside the dialysis tubing was collected separately and mixed with an equal volume of concentrated nitric acid (Conc. HNO3). The mixtures were then heated for 12 h at 100°C. After cooling to room temperature, the final volume of each sample was adjusted to 8 mL by adding DI water. Subsequently, all the samples were filtered through a 0.22-μm syringe filter before analysis to remove any particulate matter. Three measurements were conducted for each sample. The net emission intensity in the cisplatin/cl-Par-4 sample was calculated by subtracting the intensity of cl-Par-4 without cisplatin (blank). Similarly, the net emission intensity in the cisplatin buffer sample was calculated by subtracting the intensity of buffer without cisplatin (blank). The results were reported as mean ± standard deviation. Cisplatin standard solutions were prepared, acid digested, and a calibration curve was generated to determine cisplatin concentrations in the samples.
4.7 Atomic absorption spectroscopy
The quantification of bound cisplatin to cl-Par-4 was also performed using AAS with a Shimadzu AA-7000 atomic absorption spectrophotometer (Shimadzu Corporation, Kyoto, Japan) equipped with a platinum hollow cathode lamp and flame atomizer. Absorption of platinum was measured at 265.9 nm, the characteristic peak of platinum.
Sample preparation followed a similar protocol to ICP-OES with modifications. After the dialysis, an equal volume (3 mL) of each sample from both inside and outside the dialysis tubing was collected separately and mixed with 2 mL of 1% hydrochloric acid (HCl). Subsequently, all samples were filtered through a 0.22-μm syringe filter before the analysis. Three measurements were conducted for each sample. Net absorption in the cisplatin/cl-Par-4 sample was calculated by subtracting the absorption of cl-Par-4 without cisplatin (blank). Similarly, the net absorption in the cisplatin buffer was calculated by subtracting the absorption of buffer without cisplatin (blank). Results were reported as mean ± standard deviation. Cisplatin standard solutions were prepared in 1% HCl to create a calibration curve for determining cisplatin concentrations in the samples.
AUTHOR CONTRIBUTIONS
Steven M. Pascal: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – review and editing; data curation. Krishna K. Raut: Conceptualization; formal analysis; investigation; methodology; validation; visualization; writing – review and editing; writing – original draft; data curation. Samjhana Pandey: Investigation. Gyanendra Kharel: Investigation.
ACKNOWLEDGMENTS
Andrea M. Clark, Alvin A. Holder, Sandeep Kumar, James W. Lee, and Areej Malik.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.