Recent innovations in machine learning for skin cancer lesion analysis and classification: A comprehensive analysis of computer-aided diagnosis
Abstract
The global primary health concern of skin cancer emphasizes the need for quick and accurate diagnosis to improve patient outcomes. Although, it might be challenging to evaluate the possible risk of a skin spot merely by looking at it and feeling it. This review article offers a thorough overview of current breakthroughs in machine learning (ML) and computer-aided diagnostics (CAD) for the aim of analysis and classification of skin cancer lesions over the past 6 years. This paper carefully reviews the whole diagnostic process: data preparation, lesion segmentation, feature extraction, feature selection, and final classification. Analyzed are many publicly accessible datasets and creative ideas including deep learning (DL) and ML integrated with computer vision, together with their impact on increasing diagnosis accuracy. Given the variety and complexity of skin lesions, even with enormous progress, there are still major obstacles. This review rigorously assesses current methods, notes areas of great challenge, and provides recommendations to direct the next research targeted at improving early detection strategies and CAD systems.
1 INTRODUCTION
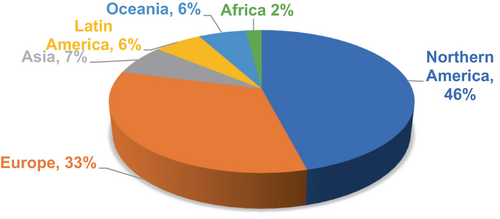
The urgent need for early identification and treatment of this deadly disease drives research on skin cancer detection and categorization. Unchecked development of atypical skin cells caused by which tumors develop skin cancer. Clinics need a thorough knowledge of the skin, which consists of three primary layers: the epidermis, dermis, and subcutaneous fat if they are to properly address and treat skin cancer. Physical screening and visual evaluations defined historically the diagnosis of skin cancer; they took a lot of time and were not always correct. Thanks in great part to major developments in dermoscopy, a non-invasive technique based on lighted, magnified images of skin lesions, diagnosis has been significantly improved. Dermoscopy has given doctors more precise diagnosis tools and better means of spotting skin cancer lesions. Notwithstanding these developments, more study is clearly needed to guarantee the timely and correct identification of maybe deadly skin malignancies.1 Early melanoma, a form of skin cancer, emphasizes the need for early identification given almost a 100% chance of survival within 5 years after diagnosis. As shown in Figure 1, the global spread of skin cancer cases in 2022 shows the general incidence of the disease and emphasizes the need for improving early classifying methods for best results.2 By way of an extensive examination of current approaches and methods in skin cancer detection research, addressing unresolved challenges, and guiding future research in this field, the proposed study intends to aid these activities.
In the past few years, machine learning (ML) techniques have become strong tools for finding life-threatening diseases like skin cancer early on. ML methods use computer programs to find complex patterns and traits in medical samples. This makes it easier for machines to automatically diagnose and sort samples. ML algorithms have shown promise in diagnosing skin cancer, making it possible to treat normal tumors quickly and stop them from turning into cancerous ones. Skin cancer includes various types, namely melanoma, squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and Merkel skin carcinoma (MCC), some example images of skin cancer are shown below in Figure 2. Each type of condition has its signs and symptoms, like a change in the color of the skin, a mole that gets bigger over time, ulcers, or the growth of lumps or cysts.3 For successful diagnosis and treatment plans to be made, symptoms must be correctly identified and put into groups. Most cases of skin cancer are caused by the environment, especially exposure to ultraviolet (UV) light. UV light is a very important part of the process of making vitamin D. Too much UV light can cause skin problems like skin cancer, changes in skin color, wrinkles, and even skin shrinkage. The link between UV exposure and different types of skin cancer shows how important it is to protect yourself from the sun and get regular medical checkups. Since skin diseases like skin cancer are becoming more common, it is important to take precautions to reduce the risks that come with being exposed to UV light.4 The suggested steps are to stay out of the sun during peak hours, wear protective clothing, and put on sunscreen every day. Regular self-examinations and visits to a dermatologist regularly are important for early detection and quick treatment, which leads to better results and lower death rates.

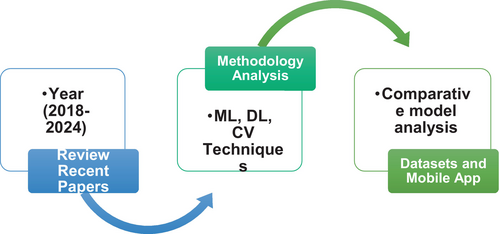
The goal of this article is to give a full look at the methods, techniques, and approaches used to analyze skin tumors for diagnostic reasons. The goal is to find and understand what is wrong with these cancers on a deeper level. This study looks at how pre-processing, segmentation, feature extraction, feature selection, and classification methods are used in computer-aided skin cancer detection tools. In this talk, we will talk about the amazing things that have been done so far, as well as the problems that have come up when trying to examine complex and rare features of skin tumors. Figure 3 shows the section of this research article. This study tries to make skin cancer detection research better and could lead to discoveries in this important field.
1.1 Motivation to improve skin cancer diagnosis
The objective of this article is to offer a thorough examination of the diverse facets and components associated with the diagnosis of skin cancer. The objective of this review is to present a comprehensive analysis of computer-aided diagnosis (CAD) techniques employed in the detection and classification of skin cancer. To accomplish this aim, this research has conducted a systematic review to evaluate and assess existing methods based on predetermined evaluation criteria. A comprehensive examination has been carried out on selected sources to gather relevant literature review regarding the methodologies utilized, the results achieved, and the dataset available in skin cancers. This research holds considerable importance, as it underscores the heightened mortality rates associated with skin cancer, both presently and in projected future scenarios.
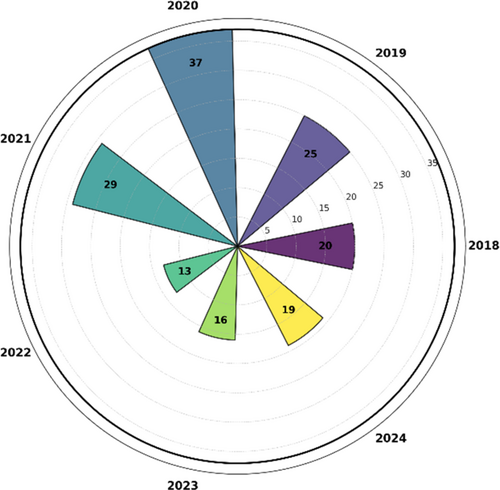
The primary aim of this review is to improve understanding of the problem domain and its proposed solutions through a comprehensive examination of a wide range of academic literature. The main goal is to optimize. The precision and effectiveness of skin cancer diagnosis, lead to enhanced patient outcomes and reduced mortality rates. Table 1 exhibits the comparison of the presented survey with further reviews of the skin cancer recognition system. We reviewed 20 papers from 2018, 25 papers from 2019, 37 papers from 2020, 29 papers from 2021, 13 papers from 2022, and 7 papers from this year 2023.
| S. no. | Analysis parameters | Current analysis | [5] | [6] | [7] | [8] | [9] | [10] |
|---|---|---|---|---|---|---|---|---|
| 1 | Comparison with existing models | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 | Discussed Machine Learning Models | Yes | No | Yes | No | Yes | Yes | No |
| 3 | Discussed Deep Learning Model | Yes | No | Yes | No | Yes | Yes | No |
| 4 | Defined Dataset | Yes | No | No | No | No | No | No |
| 6 | Methodology Discussion | Yes | No | No | No | No | No | No |
| 7 | Defined Existing App | No | No | No | No | No | No | No |
Skin cancer, classification, deep learning (DL), early diagnosis, dermoscopy, prevention, and many more are the preliminary search terms applied for paper selections. Subsequently, found 793 academic papers and conference proceedings fulfilling the given selection criteria. A total of 345 papers from the first cohort were chosen depending on their title relevance for the current research. The number of research papers dropped to 147 after a more thorough examination of the abstracts of the specified publications was then done to ascertain their fit. The chosen papers depending on their abstracts underwent extensive review to ascertain their general quality. After much review, the last collection of 126 academic publications was selected for close inspection and analysis. These final papers come from several sources and are distributed as follows: From IEEE publications, 15%; from Google Scholar, 25%; from the Association of Computing Machinery Digital Library, 11%; from Springer, 29%; from Science Direct. Figure 4. below shows the yearly paper selection. The selection process was meant to guarantee that the research articles selected for inclusion in this study came from a great range of reliable sources and academic venues. The main goal of this large collection is to guarantee the inclusion of important points of view from the current corpus of literature in this specific field of research, so laying a strong basis for further analysis and evaluation.
1.2 Scope of current research
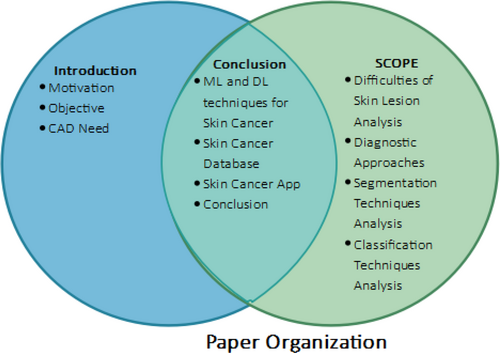
The application of automated techniques for disease identification and classification would help much in skin cancer research. The concerning rise in skin cancer cases emphasizes the need for early identification to lower death rates. The aim of this work is to evaluate current approaches for skin cancer identification and classification. The processing in this study revolves mostly around image processing, computer vision, and other types of ML. The objectives, approaches, and results of the research are introduced in the first part of this article. Section 2 breaks out the method the skin cancer detection approach employs. Section 3 covers the challenges of detection, therefore offering an understanding of the issues scientists encounter. Proceeding further, Section 4 advances in the ML portion; Section 5 details the reference datasets used to confirm the accuracy of the outcomes. Section 6 also investigates the usage of mobile apps tailored for smartphones for research on skin cancer. This paper investigates how quickly and simply mobile technologies might be applied to diagnose skin cancer. Section 7 examines closely the research techniques and provides suggestions for the further study. The research report ends with a careful review and useful recommendations for the next projects. Figure 5 below shows the overall paper theme. As researchers add to the always-growing corpus of knowledge on skin cancer detection and classification, these suggestions are supposed to act as a road map and source of inspiration.
1.3 Necessity of CAD
The current diagnostic techniques employed for skin cancer are subject to various limitations that have implications for their efficacy and precision. Figure 6 depicts the need for CAD. This section elucidates the constraints and difficulties inherent in current methodologies, providing insight into areas that necessitate enhancement.11
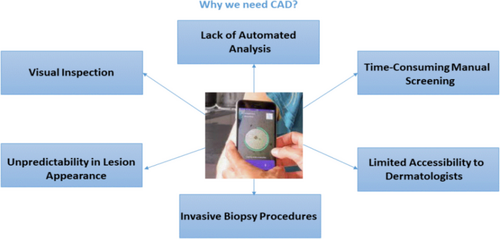
1.3.1 Visual inspection
The reliance on visual inspection by clinicians is a prominent aspect of traditional diagnostic methods. Nevertheless, it is important to acknowledge that visual interpretation is a subjective process that is susceptible to variations among practitioners. The inclusion of human judgment introduces the possibility of diagnostic errors and inconsistencies, thereby affecting the dependability of outcomes.12
1.3.2 Time-consuming manual screening
Manual screening time-consuming particularly in cases of a significant volume of skin lesions, manual screening of them might be a labor-intensive operation.13 Combining a careful estimate with an evaluation of several visual characteristics of lesions increase the length and complexity of the screening process. The occurrence of this constraint creates a challenge to the scalability and efficiency of skin cancer detection.
1.3.3 Unpredictability in lesion appearance
The variances in the presentation of skin lesions make it difficult to exactly differentiate benign from malignant lesions. Lesions add even more complexity to the diagnostic process since their size, form, pigmentation, apparent features, and other visual physiognomies display great multiplicity.14 Attaching accurate diagnosis is much hampered by the lack of uniform and established metrics for the characterizing of lesion postures.
1.3.4 Limited accessibility to dermatologists
In some geographical areas, there could be a shortage of dermatologists with specific capacity in the identification of skin cancer, which would increase the risk of either delayed or undetectable diagnosis.15 The mentioned restriction shows a biased influence on underprivileged populations and distant areas marked by restricted access to particular healthcare facilities. Patient effects depend much on the suitable availability of investigative tools.16
1.3.5 Invasive biopsy procedures
Skin cancer is often diagnosed using invasive biopsy techniques, in which case tissue samples are taken for later laboratory examination. Although the operation produces definitive results, it is an intrusive surgery that could cause pain and scarring for each person.17 Furthermore, biopsy treatments for lesions located in certain anatomical areas or for people with particular medical conditions could be limited.
1.3.6 Lack of automated analysis
The lack of automated analysis in the study and interpretation of skin lesions is related to the possibility of human mistakes and subjectivity.18 The absence of automated analysis tools and algorithms can impede the progress of diagnostic approaches distinguished by impartiality and accuracy. Automated analysis techniques could help to raise skin cancer diagnosis's consistency, precision, and dependability.19 The evolution of the field of skin cancer diagnostics depends critically on the awareness of these constraints.20 Factors that can improve the efficacy and efficiency of skin cancer detection are boosting objectivity, lowering screening length, establishing consistent criteria, improving dermatologist accessibility, studying non-invasive diagnosis methods, and including automated analysis. Acknowledging and evaluating these constraints helps one to increase the accuracy and availability of skin cancer diagnosis, hence influencing patient outcomes and more efficient healthcare delivery.21
1.3.7 Prospects of CAD in skin cancer
Incorporation of CAD technology into clinical activities offers great possibilities to transform skin cancer identification and therapy. Particularly in early melanoma and other high-risk skin disorders, CAD systems can greatly improve diagnosis accuracy by using DL models and sophisticated ML. By means of mobile and edge artificial intelligence platforms, CAD systems provide accessibility and hence guarantee the availability of diagnostic tools in remote and resource-limited places. Accurate and quick diagnosis made possible by healthcare technology democratizing itself might help to improve patient outcomes. Moreover, by offering second-opinion diagnostics and thereby lowering the possibility of misdiagnoses, CAD systems can enhance clinical expertise.22, 23 Even more, CAD systems in skin cancer detection will be able to become more scalable and capable with quantum computing for fast data processing and federated learning for cooperative model training.24
1.4 Difficulties in skin lesion analysis
Examining and analyzing skin lesions holistically calls for different challenges and nuances. This part clarifies the difficulties in microscopic analysis of skin lesions, including the resolution of natural constraints and the capture of complex characteristics.25
1.4.1 Fine texture and structure analysis
Examining skin lesions requires fine texture and structural analysis since many of them show complex textures and detailed structural patterns.26 Analyzing these minute elements by hand might be challenging since some patterns may have subtlety or lack a direct view of the unaided human vision.27 Using advanced imaging techniques and specialized algorithms able to efficiently capture and analyze small details will help one to get a precise and comprehensive study of these intricate traits.28
1.4.2 Variability in lesion appearance
In terms of appearance, skin lesions can show a great variety; they might be nodules, plaques, ulcers, or patches, each with unique visual appeal. The various lesion presentations provide difficulties for precisely diagnosing and defining the lesions.29 Variability in visual characteristics including color, form, texture, and others adds complexity to the analytical process.
1.4.3 Overlapping and irregular borders
Commonly seen in skin lesions, overlapping and uneven borders are a feature that makes precise definitions of these lesions challenging.30 In several fields, including data clustering, geographical boundaries, and image segmentation, the concept of borders or boundaries is rather important. However, these limits are sometimes challenged in accurate analysis and categorization by complexity including overlap and irregularity.31 The successful segmentation and delineation of the lesion area from its surrounding areas calls for the application of advanced image processing methods and algorithms able to efficiently manage the inherent complexity involved.32, 33
1.4.4 Subjectivity and inter-observer variability
In the framework of thorough lesion analysis, the questions of subjectivity and inter-observer variability surface since they call for the engagement of researchers or doctors who have to interpret and use judgment. Attaching results that are consistent and can be repeated is hampered by inter-observer variability, whereby several viewers may show differences in seeing and interpreting lesions.34 Subjectivity in the analysis process can cause differences that would subsequently influence the dependability and comparability of the results.35
1.4.5 Limited access to high-resolution imaging
Comprehensive analyses of skin lesions depend on the use of high-resolution imaging methods including confocal microscopy or dermoscopy. Still, the availability of these specialized imaging technologies may be limited in particular healthcare environments or geographical areas, therefore limiting the potential to do thorough research.36 The restricted availability of high-resolution pictures could hinder the capacity to precisely record and assess complex features of the lesion.37
1.4.6 Limited data availability and annotation
A major difficulty is limited data availability which makes it impossible to acquire a complete and diversified dataset including full annotations for skin lesions.38 Training and validation of ML models depend critically on the use of annotated data including accurate and trustworthy ground truth information.39 Still, the lack and complexity of particular kinds of lesions provide a great obstacle in getting enough carefully annotated data for further investigation. Imaging technologies, image processing algorithms, and standardizing of analysis techniques must all develop if we are to solve the difficulties related to detailed skin lesion analysis.40 Robust and automated approaches help to improve accuracy and dependability in the whole investigation of skin lesions.41 These approaches ought to be able to record and analyze complex features, reduce inter-observer variance, and provide smooth access to high-resolution imaging modalities.42
2 DIAGNOSTIC APPROACHES FOR SKIN LESIONS
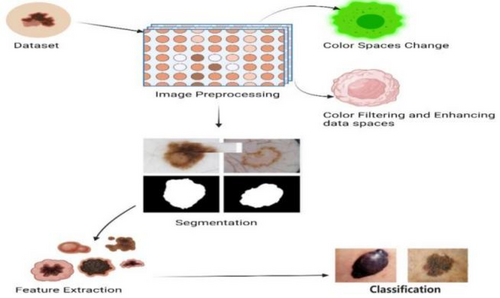
The accurate diagnosis and classification of skin lesions necessitates the successful study of these abnormalities. This section is dedicated to discussing the various methodologies and protocols employed for the analysis and decision-making process utilizing information derived from photographs of cutaneous lesions.43 The main stages of a CAD system are shown below in Figure 7 and also defined below.
2.1 Techniques for preprocessing
The process of preprocessing is of utmost importance in the analysis of skin lesions as it serves to eliminate extraneous information, enhance the quality of images, and optimize the performance of the system.44 A range of preprocessing techniques are employed to mitigate artifacts and improve visual effects. Table 2 below shows the preprocessing techniques used in recent years.
| S. no. | Paper title | Techniques | Advantages | Limitation |
|---|---|---|---|---|
| 1 | “Deep learning ensemble methods for skin lesion analysis toward melanoma detection”45 | Tri-threshold fuzzy intensification-based contrast enhancement technique | Improved accuracy, model diversity, reduced variance | Increased computational complexity, training time |
| 2 | “Segmentation of melanoma skin lesion using perceptual color difference saliency with morphological analysis”46 | Color image transformation | Perceptual color difference saliency, morphological analysis, clinical relevance | Challenges with lesion heterogeneity, dependency on image quality, sensitivity to image variations |
| 3 | “Segmentation of melanocytic lesion images using gamma correction with clustering of keypoint descriptors”47 | Gamma correction | Gamma correction for image enhancement, robustness to illumination variations | Computational complexity, potential overfitting |
| 4 | “Skin lesion classification based on deep convolutional neural networks with global attention pooling”48 | Wiener filter, Gaussian filter | Global attention pooling, deep learning capabilities | Class imbalance, interpretability |
| 5 | “Construction of saliency map and hybrid set of features for efficient segmentation and classification of skin lesion”49 | Enhanced 2D blue channel and Particle Swarm Optimization (PSO) based segmentation | Saliency map construction, hybrid feature set, improved discrimination | Dependency on image quality, overfitting risk |
| 6 | “Deep semantic segmentation and multi-class skin lesion classification based on convolutional neural network”50 | YOLOV2-squeezeNet model | Semantic segmentation, multi-class classification, end-to-end learning | Annotation challenges, data availability, and diversity |
| 7 | “FF-UNet: A U-shaped deep convolutional neural network for multimodal biomedical image segmentation”51 | Feature-fused module and attention gate mechanism | U-shaped architecture, multimodal image processing, feature fusion | Data requirements, generalization to new modalities |
2.1.1 Change of color spaces
The utilization of color space conversion techniques is of paramount importance in enhancing image quality due to the significant role that color plays in perception and preprocessing.52 In the domain of skin cancer recognition systems, it is customary to convert lesion images into different color spaces, such as CIELAB (International Commission on Illumination's lab), HSV (hue, saturation, and value), and grayscale, among other options. These transformations are implemented to enhance visual impacts and facilitate the subsequent stages of analysis in the process. Researchers can leverage the advantages offered by different color representations through the utilization of multiple color spaces. This approach facilitates a more effective and accurate analysis of skin lesions.53, 54
2.1.2 Techniques for filtering and enhancing data
Image noise and artifacts have the potential to lower the overall quality of collected and transmitted images. By employing filtering algorithms to get rid of extraneous noise.55 In addition, image enhancement methods are used to adjust an image's brightness, contrast, and sharpness. Several noise-reduction and image-enhancement techniques are put to use in the field of skin cancer identification.56 Included are algorithms like contrast-limited adaptive histogram equalization, adaptive histogram equalization, global–local contrast stretching, color constancy with shades of gray, adaptive gamma correction, gamma correction, and more.57 With the use of these preprocessing methods, skin lesion images can be greatly enhanced in quality and clarity, leading to increased precision and reliability in following analysis and classification steps for the detection of skin cancer.58 Noise reduction filters, such as the median filter and Gaussian filter, are employed to mitigate salt-and-pepper noise. Edge enhancement filters like Sobel and Laplacian filters accentuate boundaries and rapid intensity changes. Frequency domain filters, like the Fast Fourier Transform, facilitate noise elimination through transformations between spatial and frequency domains.59 Data enhancement methods include contrast enhancement techniques like histogram equalization, color space transformations such as RGB to HSV conversion, and data augmentation through rotations, scaling, and flipping for ML model robustness. Image fusion combines information from different modalities, and super-resolution techniques enhance image resolution. Figure 8, below shows data filtering, enhancement, and preprocessing techniques.60 In the context of DL, normalization, image cropping, and data resampling are used as preprocessing steps to standardize and refine input data for optimal performance in skin cancer diagnosis and analysis.

2.2 Traditional and modern segmentation approaches
The process of segmentation plays a vital role in the examination of skin lesions as it allows for the accurate identification and separation of the specific area affected by the lesion.61 This section aims to elucidate various segmentation methodologies that have been utilized to achieve precise delineation of skin lesions from adjacent healthy skin.62 Thresholding-based Segmentation techniques that rely on thresholding assign a distinct threshold value to delineate the region of interest, specifically the lesion area, by considering the intensity of individual pixels.62 Commonly employed techniques in image processing include simple thresholding methods, such as global thresholding or adaptive thresholding. Below Figure 9 shows the traditional and modern DL segmentation methods. The aforementioned techniques involve the comparison of pixel intensity values with a predetermined threshold to generate a binary mask that effectively distinguishes the lesion from the background.63
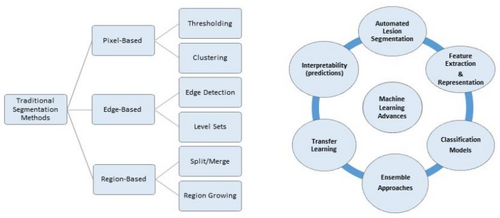
Region-based segmentation methods that are based on regions strive to categorize pixels into cohesive regions by considering their shared attributes, such as color, texture, or spatial proximity.64 Techniques such as region growing, watershed transformation, and graph cuts employ seed points or markers to progressively expand or divide regions according to predetermined criteria.65 The segmentation techniques that are based on edges primarily aim to identify and delineate the boundaries of skin lesions.66 Typically, these methods utilize edge detection algorithms, such as Canny edge detection or gradient-based edge detection, to discern the edges that demarcate the region of the lesion.67 Subsequently, the identified edges are employed to generate a boundary or contour encircling the lesion. Active contour models, commonly referred to as snakes or level sets, are flexible curves or surfaces that undergo evolution in response to image characteristics and imposed limitations.68 The objective of these models is to identify the most optimal contour that accurately represents the boundary of the lesion. Active contour models can accurately capture the irregular shapes and boundaries of skin lesions through the iterative adjustment of the contour.69
As far as DL segmentation, specifically convolutional neural networks (CNNs), has demonstrated significant progress in the field of skin lesion segmentation. The methodologies employed in this study involve the utilization of deep CNN architectures and training datasets to acquire knowledge of the distinctive features and patterns that differentiate the lesion region from the adjacent healthy skin.70 Table 3 below, defines an analysis of DL-based segmentation. The trained models are capable of accurately and reliably segmenting skin lesions. Every segmentation methodology possesses distinct advantages and drawbacks. The selection of the most appropriate methodology is contingent upon various factors, including the attributes of the dataset, available computational resources, and the specific demands of the application.71, 72 Through the utilization of suitable segmentation methodologies, researchers and clinicians can effectively isolate the region of interest, thereby facilitating subsequent analysis and enhancing the overall precision of skin lesion diagnosis and classification.73
| References | Title | Advantage | Limitation | Methodology |
|---|---|---|---|---|
| [74] | Melanoma detection using adversarial training and deep transfer learning | Adversarial training, deep transfer learning | Adversarial examples, data representation | Transfer learning |
| [75] | Convolutional neural network algorithm with parameterized activation function for melanoma classification | Parameterized activation function, feature learning, enhanced discrimination | Optimal parameter selection, data quality, and quantity | Parameterized activation function |
| [76] | Fusing fine-tuned deep features for skin lesion classification | Fine-tuned deep features, enhanced generalization, feature fusion | Optimal hyperparameter tuning, data quality, and quantity | Ensemble deep neural network |
| [77] | Toward automated melanoma detection with deep learning: Data purification and augmentation | Improved discrimination, feature fusion, fine-tuned deep features | Optimal hyperparameter tuning, sensitivity to image variations | Transfer learning |
| [78] | Automated melanoma recognition in dermoscopy images via very deep residual networks | Very deep residual networks, automatic feature learning, high-level abstractions | Computational resources, interpretability | Deep residual network |
| [79] | Combining deep learning and hand-crafted features for skin lesion classification | Hybrid approach, hand-crafted features for interpretable information | Complexity in feature integration, interpretability challenges | Deep neural network |
| [80] | Automatic segmentation of dermoscopy images using saliency combined with adaptive thresholding based on wavelet transform. | Saliency-based segmentation, adaptive thresholding with wavelet transform | Sensitivity to lesion types, optimal parameterization | Adaptive thresholding segmentation based on wavelet transform |
| [81] | Skin lesion classification using hybrid deep neural networks | Hybrid deep neural networks, automatic feature learning, improved generalization | Optimal hyperparameter tuning, dependency on feature relevance | AlexNet; VGG16; ResNet-18 |
| [82] | Classification of dermoscopy patterns using deep convolutional neural networks | Automatic feature learning, spatial hierarchical features, transfer learning | Interpretability challenges, limited to available patterns | Gradient descent algorithm |
| [83] | Automated skin lesion classification using an ensemble of deep neural networks in ISIC 2018: Skin lesion analysis toward melanoma detection challenge | Handling complexity, performance in ISIC 2018 challenge, ensemble learning | Limited to specific challenge dataset | Ensemble deep neural network |
| [84] | Multi-model deep neural network-based features extraction and optimal selection approach for skin lesion classification | Diverse feature extraction, ensemble learning benefits | Computational intensity, optimal hyperparameter tuning | Transfer learning |
| [85] | Soft attention improves skin cancer classification performance | Selective information focus, robustness to image variations | Computational overhead, dependency on the quality of attention maps | Soft attention |
| [86] | Use of neural network-based deep learning techniques for the diagnostics of skin diseases | Automatic feature learning, representation learning | Data quality and quantity, overfitting risk | Transfer learning |
| [87] | Hybrid fully convolutional networks based skin lesion segmentation and melanoma detection using deep feature | End-to-end segmentation and detection, deep feature representation | Dependency on annotated data, optimal hyperparameter tuning | Fully convolutional network |
| [88] | An image-based segmentation recommender using crowdsourcing and transfer learning for skin lesion extraction | Crowdsourcing for annotation, user interaction, and expert involvement | Quality control in crowdsourcing, dependency on crowdsourcing infrastructure | VGG16; ResNet-50; crowd sourcing |
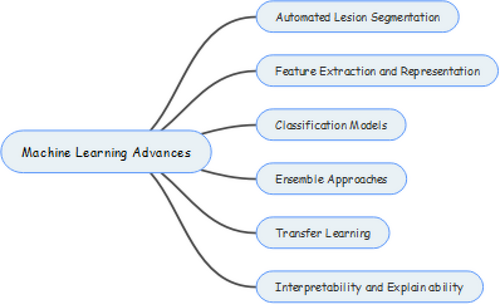
2.3 Advances in ML for skin lesion analysis
In the field of dermatology, machine-learning methods have become important tools for analyzing and making sense of complicated medical data, like images of skin lesions.89 Figure 10 below shows all the advances below. This part talks about the progress that has been made in ML methods for analyzing skin lesions, showing how they could be used to make it easier to find and classify skin cancer.90
2.3.1 Automated lesion segmentation
Using machine-learning algorithms has made great progress in automating the process of separating skin tumors from healthy skin around them.91 By using DL architectures and image processing techniques, these algorithms can correctly find the edges of lesions, which make it easier to analyze and characterize them in detail.
2.3.2 Feature extraction and representation
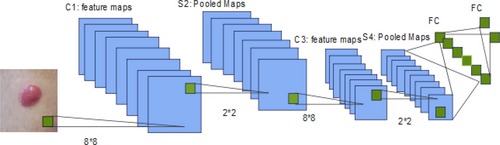
In ML, it is important to extract and describe features. These models are very good at picking out important details from pictures of skin tumors, which make it possible to get a full picture of the visual characteristics that help with diagnosis.92 With the help of CNNs and other methods for feature extraction, important patterns, and distinguishing traits can be found and extracted. A view of the CNN model is shown in Figure 11. In turn, this makes skin lesion labeling even more accurate.
2.3.3 Classification models
In the area of ML, classification models like support vector machines (SVMs), Random Forests (RFs), and deep neural networks have been used a lot to sort skin lesions into different types of cancer. These models learn from large, labeled datasets, which gives them the ability to make predictions based on trends they have learned. So, they can tell the difference between benign and cancerous lesions with a high level of accuracy and sensitivity. ML methods play a big role in making skin cancer detection more accurate, efficient, and easy to use, which improves patient outcomes, an analysis is shown below in Table 4.
| S. no. | Article title | ML classifiers |
|---|---|---|
| 1 | Classification of melanoma from dermoscopic data using machine learning techniques93 | K-Nearest Neighbor (KNN), support vector machine (SVM) |
| 2 | Skin cancer detection using machine learning techniques94 | SVM, KNN, and Naive Bayes |
| 3 | Skin lesion classification of dermoscopic images using machine learning and convolutional neural network95 | SVM |
| 4 | A melanoma skin cancer detection using machine learning technique: support vector machine96 | SVM |
| 5 | A computer-aided diagnosis system for classifying prominent skin lesions using machine learning97 | SVM |
2.3.4 Ensemble approaches
Ensemble methodologies are becoming more and more popular in the area of skin lesion analysis because they combine predictions from many different individual models. By taking advantage of the differences between different models, ensemble methods could improve the accuracy, robustness, and generalization of diagnostic systems.98 This can lead to the creation of systems that work better and last longer. Ensemble approaches in the context of skin cancer involve combining multiple models or classifiers to improve overall performance. This can include methods such as bagging, boosting, or stacking. In bagging, multiple models are trained independently on different subsets of the data, and their predictions are combined through averaging or voting.99 Boosting, on the other hand, focuses on sequentially training models where each subsequent model corrects errors made by the previous ones. Stacking combines the predictions of multiple models using another model, often referred to as a meta-model.100, 101
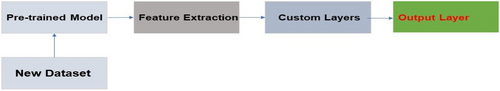
2.3.5 Transfer learning
Transfer learning is a method that lets you use what you've learned in one job or set of data in another. It has shown a lot of promise in the field of skin lesion analysis.102 Skin lesion data can be used to fine-tune models that have already been made, like those that have been trained using large picture datasets like ImageNet.103, 104 This makes it possible to use the learned models and improve classification performance, even when there are only a few labeled samples to work with. Transfer Learning is an ML approach where a model trained on one task is adapted or fine-tuned for a related task.105 In the context of skin cancer image analysis, a pre-trained neural network, such as a CNN, trained on a large dataset for a generic image recognition task, can be fine-tuned on a smaller dataset specific to skin cancer images.106 Transfer learning leverages the knowledge gained from the source task to enhance the performance of the model on the target task with limited labeled data. This approach is particularly valuable when labeled data for the target task is scarce or expensive to obtain.107 The pre-trained model captures general features from the source domain, and the fine-tuning process refines these features for the specific characteristics of skin cancer images.108 Figure 12 below shows transfer learning general structure.
2.3.6 Interpretability and explainability
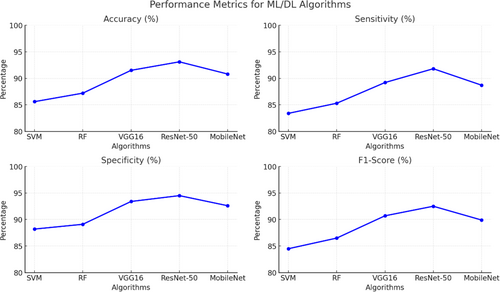
Recent improvements in ML for skin lesion research have put a lot of attention on making things easier to understand and explain. Different methods, such as attention mechanisms, saliency maps, and Gradient-weighted Class Activation Mapping (Grad-CAM), have been made to help us understand how ML models make decisions.109 These methods are meant to help doctors understand and trust the predictions that these models make. Recent improvements in ML methods that can be used to analyze skin lesions have a lot of potential to change how skin cancer is diagnosed and treated.110 Table 5 below shows ML/DL comparative performance. This is done by doing things like automating lesion segmentation, extracting important features, making accurate classification models, using ensemble methods, taking advantage of transfer learning, and making things easier to understand.111 Figure 13. below shows comparative performance metrics of ML and DL algorithms for above same references as in Table 4.
| Algorithm | Accuracy (%) | Sensitivity (%) | Specificity (%) | F1-score (%) |
|---|---|---|---|---|
| Support vector machine (SVM) | 85.6 | 83.4 | 88.2 | 84.5 |
| Random Forest (RF) | 87.2 | 85.3 | 89.1 | 86.5 |
| CNN (VGG16) | 91.5 | 89.2 | 93.4 | 90.7 |
| ResNet-50 | 93.1 | 91.8 | 94.5 | 92.5 |
| MobileNet | 90.8 | 88.7 | 92.6 | 89.9 |
2.3.7 Algorithm analysis
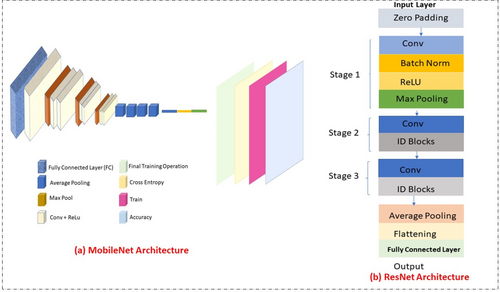
SVM is widely used for skin lesion classification due to its robustness in small, structured datasets.112 It excels in binary classification tasks but struggles with high-dimensional data and scalability. RF employs ensemble learning, which enhances performance by combining multiple decision trees. It is particularly robust to overfitting and handles imbalanced datasets effectively.113 However, it may lack interpretability. CNN models like VGG16 and ResNet-50 outperform traditional ML algorithms by automating feature extraction. ResNet-50, in particular, addresses vanishing gradient issues with residual blocks, making it suitable for diverse lesion datasets. MobileNet lightweight architecture is optimized for real-time diagnosis on mobile devices.114 Its efficiency makes it suitable for resource-constrained environments while maintaining competitive accuracy. Figure 14 below shows the comparison of MobileNet and ResNet architectures.115
2.4 Classification model performance analysis
A comprehensive evaluation of the efficacy of the classification algorithms utilized in the selected scholarly articles. Various performance metrics, such as accuracy, precision, recall, F1 score, and the area under the receiver operating characteristic (ROC) curve were used to evaluate the performance of these algorithms.116, 117 The primary objective of the study was to comprehensively evaluate the accuracy of various classification algorithms in accurately categorizing cases of skin cancer, with a focus on identifying their respective strengths and limitations.118 The performance metrics provided insightful information regarding the algorithms' overall accuracy, their precision in accurately detecting positive cases, their recall in capturing all positive cases, and the F1 score, which accounts for the balance between precision and recall.119, 120 In addition, the area under the ROC curve was analyzed to evaluate the algorithms' ability to distinguish between positive and negative cases. The metric provided significant insights into the sensitivity and specificity performance of the algorithms.121 The primary objective of this review paper was to provide a thorough understanding of the advantages and disadvantages of various classification algorithms for the detection of skin cancer by performing a thorough analysis of performance metrics.122, 123 Figure 15 below shows the performance analysis of different classification algorithms across key metrics.
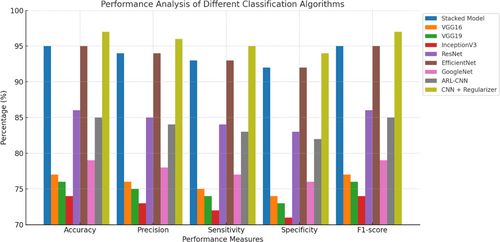
The results of this study have the potential to aid researchers and clinicians in selecting the algorithms best suited for accurate and reliable skin cancer classification. Table 6 above shows the performance analysis of different classification algorithms and their accuracy rate. Using the ISIC dataset,134 researchers were able to accomplish our goal with 0.957% precision. When applied to the ISIC dataset, VGG16, VGG19, and Inception V3 all achieved accuracy rates of 77%, 76%, and 74%, respectively. Over 24 000 cutaneous cancer samples were classified using ResNet, VGG19, and InceptionV3 models in a separate investigation. Among the three models, InceptionV3 had the best results.135 The International Skin Imaging Collaboration (ISIC) 2020 melanoma classification dataset was used alongside EfficientNets and ensemble models to categorize cutaneous lesions. All EfficientNet B6 models plus one EfficientNet B5 model in an ensemble produced the best results (ROC curve of 0.944%).136 The researchers achieved a 91.51% classification accuracy when they used a CNN model to categorize pigmented lesions in the HAM10000 dataset.137
| S. no. | Title | Classification algorithm | Justification of results | Result ACC | Dataset |
|---|---|---|---|---|---|
| 1 | An explainable stacked ensemble of deep learning models for improved melanoma skin cancer detection124 | Stacked model | Its results propose an interpretable stacked ensemble of deep learning models, aiming to enhance the accuracy of melanoma skin cancer detection. | 95% | ISIC dataset |
| 2 | Deep learning architecture using transfer learning for the classification of skin lesions125 | VGG (Visual Geometry Group)16, VGG19, InceptionV3 | Its results by employing a deep learning architecture with transfer learning for the effective classification of skin lesions. | 77%, 76%, 74% | ISIC dataset |
| 3 | Skin cancer disease image classification using deep learning solutions126 | ResNet (Residual Network), InceptionV3, VGG19 | Its results demonstrate the successful classification of skin cancer disease images through the utilization of deep learning solutions. | 75%, 86%, 73% | ISIC dataset |
| 4 | A deep convolutional neural network-based pigmented skin lesion classification application and experts evaluation127 | CNN architecture | Its results present a deep convolutional neural network-based application for classifying pigmented skin lesions, supported by expert evaluations. | 91.5% | HAM-10000 |
| 5 | Integrated design of deep features fusion for localization and classification of skin cancer128 | VGG 16 + AlexNet (a CNN architecture, designed by Alex Krizhevsky) | Its results by proposing an integrated design that fuses deep features to enhance both the localization and classification of skin cancer. | 99% | ISIC 2020 |
| 6 | Skin lesions classification into eight classes for ISIC 2019 using deep convolutional neural network and transfer learning129 | GoogleNet | Its results employ a deep convolutional neural network and transfer learning for the classification of skin lesions into eight classes, particularly for the ISIC 2019 dataset. | 94% | ISIC 2018 |
| 7 | Skin lesion classification using a convolutional neural network with a novel regularizer130 | CNN + Novel Regularizer | Its results by implementing a convolutional neural network with a novel regularize for effective skin lesion classification. | 97% | HAM-10000 |
| 8 | A deep neural network using modified EfficientNet for skin cancer detection in dermoscopic images131 | A deep neural network (DNN) model | The paper justifies its results by comparing the performance metrics of the proposed deep neural network with state-of-the-art models and discussing its generalization capabilities, supported by visualizations and acknowledgment of limitations. | 95.49% | Combined dataset (ISIC 2020, ISIC 2019, ISIC 2018, HAM10000) |
| 9 | Attention residual learning for skin lesion classification132 | Attention residual learning convolutional neural network (ARL-CNN) | Its results introduce attention residual learning for enhanced skin lesion classification. | 85% | ISIC 2018 |
| 10 | Ensemble of convolutional neural networks for dermoscopic images classification133 | GoogleNet, VGG 16, and their ensemble | Its results are by proposing an ensemble of convolutional neural networks to improve the classification of dermoscopic images. | 79%, 80%, 81% | ISIC 2019 |
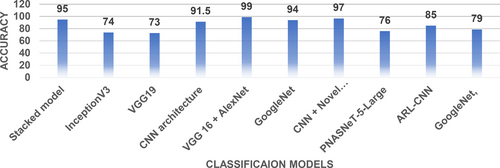
The VGG16 and AlexNet models were gradually combined and improved using principal component analysis (PCA). An astonishing success rate of 99.9% was reached by this method.138 In this study, the HAM-10000 dataset was used using five individual CNN models and four different ensemble models. The purpose of this study was to identify subtypes of cutaneous cancer. Accuracy of 93.20% and 92.83% were achieved using pre-trained ResNetXt101 and ensemble InceptionResNetV2 + ResNetXt101 models, respectively.139 Using the ISIC 2020 dataset, the EfficientNetV2-M model correctly categorized cutaneous lesions 99.23% of the time.140 Hyper-connected convolutional neural network (HcCNN) was created to identify cutaneous lesions. Multimodality testing with 7-point criteria had an accuracy of 74.9%.141 On the ISIC dataset,142 we devised a novel technique using a CNN with a unique regularizer for the binary classification of malignant and benign lesions. There was a 97.49% success rate with this method. For melanoma detection, we used the CNN models InceptionV4, SENet154, InceptionResNetV2, and PNASNeT-5-Large. On the ISIC 2018 dataset, PNASNeT-5-Large achieved a validation result of 0.76. The ARL-CNN model was created to deal with the problem of limited training data by taking intra-class variance and inter-class similarity into account. The accuracy of this method was 85.0% when tested on the ISIC 2017 dataset.143 To solve the problem of cutaneous lesion classification in the HAM-10000 dataset, the MobileNet CNN architecture was created. The first version of the model had an accuracy of 83.93% without any data augmentation procedures.144 Seven classes from the ISIC 2018 dataset were classified using GoogLeNet, VGG, and their ensemble models in a separate study.145 Accuracy of 79.7%, 80%, and 81.5% were attained. Figure 16 defines classification models and their accuracy on skin cancer datasets.
2.4.1 Critical evaluation of ML/DL methods
- Regularization: Techniques like L1 and L2 regularization penalize large weights in the model, encouraging simpler and more generalizable solutions.147
- Dropout: Dropout is a regularization method where a fraction of neurons is randomly ignored during training, reducing the model's reliance on specific pathways.148
- Data Augmentation: Expanding the dataset artificially by applying transformations such as flipping, rotation, and cropping improves the model's ability to generalize.149
- Cross-Validation: K-fold cross-validation is used to assess model performance across multiple subsets of the data, providing a more reliable estimate of its generalization capabilities.152
- Early Stopping: Monitoring the validation loss during training and halting the process when performance plateaus help prevent overfitting.153
- Transfer Learning: Leveraging pre-trained models on large datasets and fine-tuning them on domain-specific data enhances generalization while reducing the need for extensive labeled data.154
3 SKIN CANCER AVAILABLE DATASETS
Since different datasets are used in many studies, it is important to do a full investigation of all skin cancer datasets that are available. When looking at all of the available data, it is important to realize that good samples make up <9% of the total, which has a big effect on the model's recall rate.155 Table 7 shows the number of classes and total number of images available in each dataset. The long tail effect, which is caused by a mismatch in the data, is one of the main things that makes it hard to classify these kinds of datasets. To deal with this problem, methods like transfer learning or learning in groups can be used.156 The methods listed above have shown promise in reducing data imbalance and improving the ability of skin cancer models to classify data. Figure 17 shows the datasets used in reference papers.
| S. no. | Dataset | Description | No of images | Classes |
|---|---|---|---|---|
| 1 | HAM10000 | Dermatoscopic images of pigmented skin lesions. It includes various types of skin lesions, and it is often used for research in skin cancer classification. | 10 015 | 7 |
| 2 | PH2 | The PH2 dataset is a collection of dermoscopic images of benign and malignant melanocytic lesions. | 200 | 3 |
| 3 | ISIC | This dataset is used in challenges and competitions related to skin cancer detection. | 33 126 | 9 |
| 4 | ISIC2016 | These datasets are specific yearly releases from the International Skin Imaging Collaboration (ISIC). | 900 | 9 |
| 5 | ISIC 2017 | Contain dermoscopic images | 2750 | 3 |
| 6 | ISIC2018 | Contain dermoscopic images | 13 600 | 7 |
| 7 | ISIC 2019 | Contain dermoscopic images | 25 331 | 8 |
| 8 | ISIC 2020 | contain dermoscopic images | 33 126 | 2 |
| 9 | Atlas of Dermoscopy | Dermoscopic images are used for educational and research purposes in dermatology. It provides a visual reference for various skin conditions. | 1024 | 7 |
| 10 | Dermofit | Dermofit is a dataset containing high-quality clinical images of common skin diseases. | 1300 | 10 |
| 11 | BCN20000 | BCN20000 is a dataset consisting of 20 000 dermoscopic images used for research in skin cancer classification and detection. | 19 424 | 8 |
| 12 | PAD-UFES-20 | Dermoscopic images are used for research in computer-aided diagnosis of skin lesions, particularly in the context of melanoma detection. | 1641 | 3 |
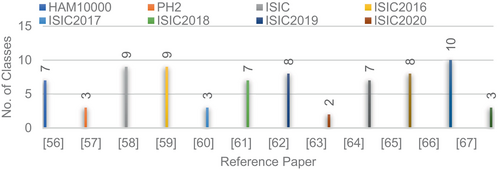
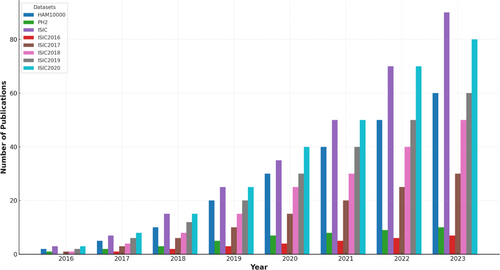
Figure 18 below defines year-wise publications details using various datasets.
3.1 Key datasets and preprocessing techniques
- ISIC Dataset: The International Skin Imaging Collaboration (ISIC) dataset is a widely used benchmark for skin lesion analysis.157 It contains over 25 000 dermoscopic images, categorized into multiple classes, including melanoma, nevus, and seborrheic keratosis. This dataset is instrumental for developing classification models but suffers from significant class imbalance, with melanoma cases being underrepresented.158
- HAM10000 Dataset: The HAM10000 dataset, also known as the Human Against Machine dataset, includes 10 015 dermoscopic images.159 These images span seven classes, such as melanoma, benign keratosis, and basal cell carcinoma.160 Although diverse, the dataset exhibits an imbalance, with benign lesions being overrepresented compared with malignant ones.161
- Normalization: Pixel intensity values were scaled to a standard range (e.g., 0–1) to improve model convergence during training.163
- Data Augmentation: Techniques such as rotation, flipping, zooming, and cropping were applied to increase dataset diversity and reduce overfitting.164
- SMOTE (Synthetic Minority Oversampling Technique): SMOTE was used to generate synthetic samples for underrepresented classes, alleviating the effects of class imbalance.165
3.2 Accuracy of different algorithms
The accuracy of different algorithms is influenced by several factors. First, dataset size plays a crucial role, as larger datasets, such as ISIC and HAM10000, provide sufficient diversity and representation, enabling models like ResNet-50 and VGG16 to achieve higher accuracy. In contrast, smaller datasets often result in overfitting, negatively impacting generalizability.166 Second, preprocessing techniques significantly affect accuracy, with methods like normalization, data augmentation, and oversampling (e.g., SMOTE) improving model performance by reducing noise and addressing class imbalance.167 For instance, data augmentation enhances the accuracy of CNNs by increasing data diversity. Finally, architectural differences among models also influence their accuracy, as different architectures possess varying strengths and weaknesses. ResNet-50, for example, excels due to its deep residual connections, which effectively mitigate vanishing gradient issues. On the other hand, lightweight models like MobileNet prioritizes computational efficiency, resulting in a slight trade-off in accuracy.168 Below Table 8 shows the comparative analysis of algorithms.
| Algorithm | Dataset size impact | Preprocessing techniques impact | Architecture impact |
|---|---|---|---|
| Support vector machine (SVM) | Effective on small datasets | Normalization improves performance | Limited scalability to large datasets |
| RF | Handles medium datasets well | Handles imbalanced data effectively | Robust but computationally intensive |
| CNN (VGG16) | Performs well on large datasets | Data augmentation enhances accuracy | Requires significant computational resources |
| ResNet-50 | Excels on large, diverse datasets | Normalization and augmentation boost performance | Best for deep feature extraction |
| MobileNet | Sufficient for medium datasets | Preprocessing enhances lightweight models | Trades slight accuracy for efficiency |
4 MOST COMMON SKIN CANCER MOBILE APPS
This section discusses about five most commonly used skin cancer mobile applications. These applications are capable of classifying potentially malignant skin lesions as high or low risk for skin cancer by utilizing photographs acquired with a standard smartphone camera.169, 170 It is of the uttermost importance to ensure that the accuracy of these applications exceeds the current standard of care, as doing so will mitigate the potential negative outcomes associated with misclassification.171 If the aforementioned stringent requirements are met, the deployment of mobile skin cancer applications has the potential to provide a workable solution to the problems caused by the large number of patients and complex diagnostic scenarios. These applications are compatible with both Apple and Android smartphones, providing users with convenient options for skin cancer detection and monitoring. The following examples are noteworthy.172
4.1 Miiskin
This application uses high-resolution photography to capture detailed images of vast body regions. Individuals can compare the lesions on their skin over time, allowing them to identify any changes that may be indicative of skin cancer.173
4.2 MoleMapper
An application developed through a collaboration between Oregon Health & Science University, Apple, and Sage Bionetworks, is presently available at no cost to consumers. This technology allows physicians to remotely monitor potentially concerning lesions, thereby reducing the need for frequent in-person consultations.174
4.3 MoleScope
To use the MoleScope application, individuals must purchase a smartphone-compatible accessory. Through the use of the accompanying device, individuals can capture images and submit them to dermatologists for online opinions and evaluations.175
4.4 SkinVision
A council of dermatologists created SkinVision, an application that uses a deep-learning algorithm to analyze photographs of lesions. This application is offered for a fee. In 1 min, the system assesses the level of risk associated with the mole and provides users with valuable information regarding potential skin cancer dangers.176
4.5 UMSkinCheck
The UMSkinCheck application, developed by the University of Michigan Medical School, allows users to perform comprehensive skin cancer examinations and monitor any changes that occur over time, at no cost. The application provides instructions for performing skin examinations, stores initial photographs for comparative analysis, and sends periodic notifications to encourage consistent self-evaluations. Table 9 shows the launch year, cost, and horizon of each mobile app. These applications provide a variety of functionalities and methodologies to aid individuals in monitoring their skin health, providing significant assistance and guidance in the early identification of potential skin cancer risks.
| S. no. | Application | Country | Year | Cost |
|---|---|---|---|---|
| 1 | SkinVision | The Netherlands | 2012 | Free |
| 2 | UMSkinCheck | USA | 2012 | Free |
| 3 | MoleScope | Canadian | 2014 | Paid |
| 4 | Miiskin | UK | 2015 | Paid (Only 30 day free) |
| 5 | MoleMapper | New Zealand | 2019 | Free |
4.6 Application value of ML/DL techniques in clinical settings
The ISIC dataset has been extensively analyzed to demonstrate the practical application of ML and DL techniques for skin cancer detection. Through the use of DL models like ResNet-50 and MobileNet, the analysis highlighted significant improvements in diagnostic accuracy, sensitivity, and specificity. Preprocessing techniques, such as data augmentation and normalization, played a crucial role in enhancing the performance of these models, particularly in clinical-like settings. Similarly, the HAM10000 dataset was leveraged to train and evaluate ML/DL models for classifying diverse skin lesions. This study emphasized the need to address dataset imbalances and successfully applied oversampling techniques, such as SMOTE, to improve the detection of rare lesion types, including melanoma. Furthermore, MobileNet-based models were deployed on mobile devices to showcase the potential of edge AI for real-time diagnostics. This implementation demonstrated the feasibility of using such models in remote areas, providing greater accessibility for early skin cancer detection and diagnosis. Table 10 below shows case studies and clinical application details.
| Case study/example | Dataset used | Techniques applied | Clinical value |
|---|---|---|---|
| ISIC Dataset Analysis | ISIC Dataset | ResNet-50, MobileNet, Data Augmentation | Enhanced diagnostic accuracy and sensitivity |
| HAM10000 Dataset Integration | HAM10000 Dataset | SMOTE, Preprocessing, CNNs | Improved classification of rare lesion types |
| Real-Time Diagnostic Tool | Custom Dataset | MobileNet, Edge AI | Accessible diagnostics for remote areas |
4.7 Challenges in clinical applications of ML/DL
- Data Variability: One of the key challenges in clinical settings is the variability in datasets. Skin lesion datasets often originate from diverse sources and exhibit variations in imaging equipment, resolution, and lighting conditions. These discrepancies can lead to reduced model generalizability when applied to real-world data. To address this, we propose the use of data augmentation techniques and domain adaptation methods to improve model robustness. Additionally, curated datasets with diverse demographic representation should be prioritized.
- Scalability: Scaling ML/DL solutions for widespread clinical use involves computational and infrastructural challenges. For instance, deploying DL models on resource-constrained devices requires optimization techniques such as model quantization, pruning, and lightweight architectures like MobileNet. Furthermore, cloud-based platforms can facilitate scalability by enabling centralized model deployment and real-time inference.
- Clinical Validation: Clinical validation remains a significant barrier to the adoption of ML/DL models. Validation requires extensive testing with diverse and representative patient populations to ensure reliability and accuracy. Collaborations with healthcare institutions and regulatory agencies are essential to develop standardized protocols for model evaluation. Federated learning, where models are trained across multiple institutions without sharing sensitive data, can also accelerate clinical validation.177
To address the challenges in applying ML and DL techniques in healthcare, several solutions have been proposed. Federated learning enables collaborative training of models across institutions without compromising sensitive patient data, thereby addressing privacy concerns and enhancing the diversity of training datasets. Model fine-tuning, which involves adapting pre-trained models to domain-specific datasets, significantly improves performance on clinical data while reducing the need for large volumes of labeled samples.178 Additionally, the establishment of standardized clinical protocols and benchmarks for validation ensures the robustness and reliability of ML/DL models in clinical applications, promoting their effective and trustworthy deployment in real-world scenarios.
5 EMERGING TECHNOLOGIES IN SKIN CANCER DIAGNOSIS
- Quantum Computing: Quantum computing offers the ability to process vast amounts of data in parallel, making it a promising tool for analyzing large and complex skin lesion datasets. Quantum algorithms, such as quantum SVMs and quantum neural networks, have the potential to accelerate diagnosis by reducing computation time. Despite its promise, challenges such as hardware limitations and scalability must be addressed for practical implementation.179
- Federated Learning: Federated learning enables collaborative model training across multiple healthcare institutions without sharing sensitive patient data. This approach addresses privacy concerns while leveraging diverse datasets to improve model robustness and generalizability. In the context of skin cancer diagnosis, federated learning can enhance the diagnostic capabilities of ML/DL models while adhering to strict data protection regulations.180
- Edge AI: Edge AI involves deploying ML/DL models on edge devices, such as smartphones or portable medical devices, for real-time inference. This technology can significantly improve access to diagnostic tools, particularly in remote or resource-constrained areas. With advancements in lightweight architectures like MobileNet and efficient hardware, edge AI is poised to transform early skin cancer detection.181
Quantum computing enhances diagnostic speed by processing data and generating results much faster than traditional methods. Additionally, federated learning improves privacy and collaboration by enabling institutions to work together while ensuring that sensitive data remains secure. Furthermore, edge AI increases accessibility by providing diagnostic tools that function effectively even in areas with limited infrastructure, making advanced technology more widely available.
6 EXISTING RESEARCH ANALYSIS AND CONCLUSION
In this article, we have identified the need for skin cancer diagnosis. We thoroughly overview and perform analysis of state-of-the-art new “feature extraction techniques,” “deep learning,” and “machine learning” base segmentation techniques. All skin cancer dataset is defined, with available classes. Finally, at last, determine which are the most commonly used skin cancer applications around the globe. This survey mainly focuses on recent year publications but some of the previously highly accurate classification models are also included in the above comparison. The objective of this literature review is to conduct a comprehensive investigation and valuation of relevant research studies, procedures, and tactics employed in the investigation of skin lesions. CNNs, due to their strong connotation with computer vision, have established notable efficacy in the fields of image classification and division. This comprehensive review covers a variety of studies that have investigated numerous aspects of skin lesion examination. The above-mentioned studies have surveyed a range of phases including segmentation techniques, feature extraction methods, labeling algorithms, and available datasets. The objective of analyzing the research methodologies utilized in prior studies is to gain a greater thoughtful of the model design, dataset assortment, and evaluation procedures. By the above survey, we find that there remains lacking in some datasets integrity. Datasets also evolve the major factor in methodology designing (feature extraction and segmentation techniques). Undertaking so, it highlights the vital factors that can affect the credibility and constancy of the research findings. A comparative analysis is undertaken to identify commonalities and differences among a range of studies. This includes the assessment and divergence of dissimilar algorithms, techniques, and models used in the examination of skin lesions. By conducting a careful analysis of the resemblances and discrepancies, individuals can get a thorough understanding of the efficacy and stability of diverse methodologies.
6.1 Analysis of previous studies' conclusions
The conclusions derived from prior research investigations were examined to discover the principal innovations and difficulties in the Skin cancer detection process. This survey covered which methodologies are being used, and what type of dataset is being used and this analysis facilitates the identification of the strengths and limitations inherent in these approaches, thus contributing a valuable insight into the progress achieved in the domain of skin lesion analysis.
7 SUGGESTIONS FOR FURTHER STUDY
There are notable challenges associated with accurately diagnosing skin cancer and effectively segmenting skin lesions. Conventional medical methodologies exhibit limited efficiency while relying solely on visual examination introduces subjectivity and consumes significant amounts of time. In recent years, there has been a growing utilization of CAD techniques, to address the aforementioned limitations. This comprehensive analysis examines the potential of ML and DL techniques in the detection and classification of skin cancers while ensuring the absence of any adverse effects on the patient. This comprehensive examination explores various steps involved in skin cancer recognition, including preprocessing, segmentation, feature extraction, and classification. This work, through an inspection of current methodologies, has shed light on the compensations and limitations related to datasets. The conclusions of the survey offer a comprehensive assessment of the prevailing literature on methods for identifying skin cancer. However, there remains an essential for further study and progression in this area. Academics with a concentration on advancing the current level of sorting within sections may reflect a further exploration of old segmentation approaches (such as region-based, cluster-based, and threshold-based methods) as well as feature extraction methods (including color, shape, and texture features). Moreover, it is value observing that CNN has exhibited praiseworthy consequences. However, advancements in quantum computing have the prospective to improve its efficacy by addressing confines related to optimization and generalization. The advancement of skin cancer detection and categorization can be propelled by researchers through the expansion of knowledge derived from this survey. The development of more accurate and efficient systems can be achieved by integrating enhanced categorization methods and exploring emerging technologies such as quantum computing. The aforementioned advancements possess the capacity to augment the identification, assessment, and management of cutaneous malignancies, thereby yielding improved outcomes for individuals and mitigating the consequences of this lethal ailment.
AUTHOR CONTRIBUTIONS
S. S. Z.: methodology, writing the original draft, and conceptualization.M. S. H.: resources, writing original draft, and data curation.W. J.: formal analysis, writing review & editing, and discussion.K. Y.: resources and data curation. All authors contributed to the study conception and design and reviewed and approved the final manuscript.
ACKNOWLEDGMENTS
We wish to extend our heartfelt thanks and genuine appreciation to Prof. Guangmin Sun (Beijing University of Technology) for his support and guidance.
FUNDING INFORMATION
No funding is available for this research.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest to report regarding the present study.
ETHICS STATEMENT
Not applicated.
Open Research
DATA AVAILABILITY STATEMENT
The data utilized in this paper is sourced from benchmark datasets, which can be accessed online and mentioned in references.




