Prostatectomy postoperative urinary incontinence: From origin to treatment
Jing Quan, Jijia Gu, and Ziwei Li contributed equally to this study.
Abstract
Prostate cancer is a common malignant tumor at present, and there are still adverse reactions after radical prostatectomy. As many as 30% of patients complain of urinary incontinence (UI). This review begins with the pathophysiological basis of post-prostatectomy urinary incontinence (PPUI) and describes the maintenance of normal urine control function, anatomical changes, and urodynamic maintenance of PPUI. Then, we talk about the various influencing factors of UI, for example, the differences caused by the basic condition of the patient, the length of the membranous urinary tract, the level of prostate-specific antigen before operation, the method and technique of operation, and so on. Last, we introduce all kinds of treatments of PPUI in detail. It includes noninvasive conservative treatment, traditional surgical treatment, emerging stem cell therapy treatment, and postoperative psychotherapy of PPUI. The present situation of the treatment of UI after prostatectomy is summarized, and the prospect of the technology is put forward.
1 INTRODUCTION
Prostate cancer (PC), a frequently occurring malignant tumor, shows a higher prevalence among middle-aged and elderly men.1 It ranks as the third most prevalent cancer in the male population worldwide,2 solidifying its status as a significant global public health concern. Radical prostatectomy (RP) has emerged as a widely adopted clinical intervention among all the treatments, renowned for its efficacy in early-stage PC management. A spectrum of adverse effects inevitably emerges, encompassing urinary incontinence (UI), erectile dysfunction, postoperative hemorrhage, and urethral anastomotic stricture, among others.1
Post-prostatectomy urinary incontinence (PPUI), constituting the most prevalent post-RP adverse reaction, manifests with an approximate prevalence rate of 60%. The International Urinary Control Association defines PPUI as the involuntary, objective release of urine. This condition significantly impairs patients' postoperative quality of life, exerting a negative influence on daily routines and professional engagements.3, 4 The ramifications extend beyond mere physical discomfort, exacerbating psychological distress, and eroding self-assurance and self-esteem.5, 6
Consequently, the discourse surrounding and the resolution of PPUI have attained escalating significance. This article offers a comprehensive survey of current research and therapeutic approaches concerning PPUI, addressing the exigent need to mitigate its impact.
2 CHANGES IN PATHOPHYSIOLOGICAL BASIS
The bladder neck, urinary tract, and urethral sphincter complex form a normal male outlet of the lower urethra. Micturition is a basic function of human beings. Normal urine control function can achieve the coordination of urine storage and controlled regular release from the bladder. Storage and micturition occur under the control of autonomous will or under the necessity of physiological perception of micturition, and this normal urine control function depends on the comprehensive feedback regulation of neurohumoral fluids. The excretion of urine is controlled by controlling the smooth and striated muscle system of the bladder and urethra.7 Nevertheless, PPUI shows that the patient is unable to control the urine flow according to his own will. RP leads to changes in anatomical structure and nervous system, which affect the physiological basis of normal urination.
2.1 Anatomical changes
The prostate is the physiological component of the male gonad, and it is also a common cancer site in older men. It can enlarge and block the bladder neck. The fundamental purpose of prostatectomy is to remove cancer lesions and obstructive bodies.
Transurethral prostatectomy or open prostatectomy can be performed. The latter can be divided into three types: suprapubic transvesical prostatectomy, retropubic prostatectomy, and perineal prostatectomy. The combined effect of detrusor, proximal intrinsic sphincter, striated sphincter, and urinary tract suspension consisting of pubic urethral ligament helps to control micturition. The most common cause of PPUI is urethral sphincter insufficiency.8 If the proximal intrinsic sphincter, proximal urinary sphincter, and suspension ligament are removed during RP, postoperative incontinence depends on the striated sphincter to a large extent.4
No matter what surgical method is used to remove the prostate, the position and function of the residual anatomical structure of the lower urinary tract, the bladder, the membranous urinary tract, and the urinary sphincter complex will change. The common anatomical changes include the downward displacement of the urinary bladder junction and the proximal membranous urinary tract stump in the pelvis.7 Prostatectomy removes the smooth muscles in the prostate and urethra, and postoperative anastomotic stricture, resulting in increased urinary tract stiffness, decreased urinary tract elasticity, and eventually reduced urinary pressure during pelvic floor muscle contraction7 (Figure 1).
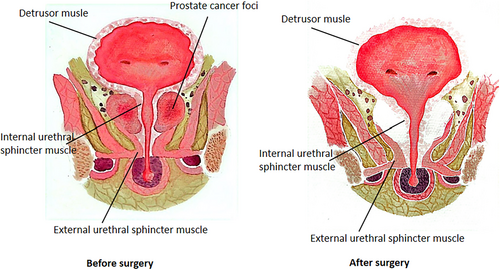
2.2 Changes of neurological function
PPUI after RP may be caused by impaired function of the nerves innervating the pelvic floor or urethral sphincter.9, 10 When normal men urinate, the brain can control the volume of urination. However, the patient's nerves are damaged, the nerves in the bladder are not controlled by the brain, and the urine control function of the bladder or urethra is impaired, which is characterized by involuntary excitation and contraction, urine leakage from the urethral orifice, and then PPUI, which is called urgent PPUI or neurogenic PPUI. The nerves from the pudendal nerve (PN), pelvic plexus autonomic nerve, and dorsal vein complex are related to the external urethral sphincter. Nerve damage caused by RP can affect sphincter function. Patients may also be complicated with urinary retention.2 Metastatic spinal cord compression may also be one of the causes of PPUI, mild autonomic nervous dysfunction may be characterized by urinary retention, but with the deepening of nerve involvement, the symptoms of patients may progress to PPUI.11
3 INFLUENCING FACTORS
PPUI is influenced by a variety of factors, and this section will highlight some of the major common influences.
3.1 Age, body weight, postoperative time, and length of membranous urinary tract
Crucial risk factors contributing to PPUI subsequent to prostatectomy encompass advanced age, elevated body weight (body mass index), limited postoperative duration, and reduced length of the membranous urinary tract (membranous urethral length [MUL]). The propensity for complications intensifies with shorter postoperative time,12 older age,13 diminished MUL, larger prostate volume, and reduced volume and density of striated muscle cells within the striated urethral sphincter.2, 7
The MUL denotes the measurement from the prostate tip to the orifice of the membranous urethra at the penis. MUL encompasses smooth muscle fibers and is encompassed by the striated urethral sphincter throughout its extent. These muscle fiber types are integral in maintaining and enhancing urethral closure functionality through heightened urinary pressure.7 A reduced MUL can influence urethral sphincter inadequacy, while an elongated MUL can gradually augment the restoration of control following RP. Consequently, a strategy involving suburethral suturing and peeling should be explored to maximize the extension of the urinary tract length.
3.2 Exercise-based interventions
Doctors can encourage patients to take comprehensive preoperative exercise interventions, including focused training of striated urethral sphincter, to improve recovery time for fecal and fecal incontinence while reducing additional risks.14 This kind of preoperative intervention is called pre-rehabilitation.
Exercise-based interventions include aerobic exercise, resistance training, and targeted pelvic floor muscle training (PFMT) programs. Organized and continuous exercise training stimulation can improve the physical function of patients, including aerobic exercise ability, muscle strength, and psychosocial health. It can produce beneficial adaptation in individual muscle level as well as cardiovascular, respiratory, musculoskeletal, nervous, metabolic, and endocrine systems, and has a significant positive impact on patients before and after operation.7
The activation and coordinated function of the pelvic floor musculature to generate urethral closure pressures contributes to functional urethral sphincter closure and has been identified as an important determinant of continence status following RP.15 PFMT can effectively improve the activity, coordination, strength, and endurance of pelvic floor muscles.16 It is reported that the hypertrophy of striated muscle and the increase of strength, endurance, and coordination of pelvic floor muscle can magnify urinary tract pressure and effectively restore controllability after RP. The biological basis of initiating PFMT before operation includes increasing neuromuscular reserve and increasing muscle mass, strength, and endurance of the striated muscle of the pelvic floor to compensate for the loss of smooth and striated muscle during RP.16 The 2019 American Urological Association/Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction guidelines recommend starting exercise 3–4 weeks before surgery to achieve neuromuscular adaptation and increase neuromuscular reserves.17
3.3 Surgical methods and skills on the postoperative effect
Advancements in technology and medical devices have ushered in a broader array of surgical approaches for treating PC, extending beyond traditional open surgery. Currently, alongside open retropubic radical prostatectomy (RRP), laparoscopic surgery and robot-assisted laparoscopic surgery have gained widespread adoption.18 Laparoscopic radical prostatectomy (LRP) has emerged as a minimally invasive alternative, affording clinicians enhanced visualization and precise anatomical dissection. This approach facilitates superior surgical accuracy, attaining optimal postoperative control by preserving a longer urethral segment and performing meticulous cystourethral anastomosis. Furthermore, LRP entails reduced intraoperative damage and diminished intraoperative and postoperative bleeding.19, 20
Notably, the introduction of robot-assisted laparoscopic prostatectomy (RALP) has notably curtailed the incidence of bladder neck contracture.4 RALP demonstrates improved early voiding function in comparison to RRP, and it accelerates and enhances postoperative erectile function recovery.21, 22 The utilization of robotic assistance enables surgeons to magnify anatomical structures and mitigate tremor-induced extraneous harm.23 This leads to shorter training periods for acquiring necessary surgical skills compared to traditional laparoscopic prostatectomy.24, 25 In addition, postoperative PFMT shows that laparoscopic and robot-assisted RP outperform laparotomy in enhancing PPUI recovery.23
It is noteworthy that the impact of surgical proficiency on PPUI is on par with the choice of surgical approach. Within the same surgical category, PPUI outcomes vary depending on the surgeon's skill level. Nerve-preserving techniques during RP aid in lowering PPUI incidence and fostering postoperative urinary control recovery. The nerves responsible for urinary control primarily involve the autonomic nerves from the pelvic nerve and somatic nerves from the PN. Retaining nerve bundles has been shown to bolster urine control recuperation after RP.26 Bilateral nerve preservation yields better results than unilateral preservation.27 Techniques such as controlled apical prostatectomy, selective severance of the dorsal vein complex, preserving the pubic prostatic ligament or bladder neck during the procedure, and utilizing bladder-urinary tract anastomosis have been demonstrated to reduce PPUI incidence and enhance postoperative control.28
4 TREATMENT METHOD
In this section, we briefly describe some treatments for PPUI, including conservative treatment, surgical treatment, emerging therapy, and psychotherapy. In Table 1, we can see their advantages, disadvantages, and therapeutic effects more intuitively.
| Treatment method | Advantages | Disadvantages | Outcomes | References | |
|---|---|---|---|---|---|
| Conservative treatment | ES | Faster short-term recovery | Adverse effects (pain, discomfort) | A faster recovery of urinary continence | 44 |
| PFES | Direct and reflex responses of the urethral muscles | Improve incontinence recovery rate | 30 | ||
| EPNS | Excite precisely the PN | Excite PN and simulate PFMT | 30 | ||
| PFMT | Speedup the immediate recovery | Only modest improvements No complete cure |
An improvement in continence in the short term | 45 | |
| BF | First-line treatment | Long-term effectiveness is uncertain | 4, 46 | ||
| Easy to use | |||||
| Few side effects | |||||
| High patient acceptance | |||||
| Pharmacotherapy | Various types of medications | Medication side effects | Reduce detrusor overactivity | 29 | |
| Effective for stress incontinence | |||||
| Surgical treatment | BAS | Little trauma to the retropubic space | A sustained outlet obstruction over time | Displace the urethra into appropriate position Reduce urinary obstruction |
29 |
| RTS | Little compressive action on the urethra | Secondary to a more immobile urethra | 29, 47 | ||
| Few serious complications | |||||
| ARS | Complications (urethral erosions and infections) | 29 | |||
| Relatively safe | |||||
| Postoperative adjustment of the sling tension | |||||
| AUS | Gold standard | Most patients achieve continence | 29 | ||
| High success rates | Relatively high costs | ||||
| Collagen injections | A short-term improvement for patients who are poor surgical risk | More data to be counted | Reduce urine leakage | 19, 48 | |
| More precise and extensive | |||||
| RRP | High success rate Short operation time |
Higher estimated blood loss | Commonly used RP | 25, 49 | |
| Complications (dysfunction) | |||||
| Longer length of hospital stays | |||||
| LRP | Superior surgical accuracy | Small surgical field of view | 24 | ||
| Diminish intraoperative and postoperative bleeding | Difficult partial suture angles | ||||
| RALP | Clearer vision More flexible robotic arm |
Expensive cost | Effective retention urinary control and sexual function | 49-52 | |
| Reduce bladder neck contracture | |||||
| Lesser blood loss | Longer operative time | ||||
| Improve early voiding function | |||||
| Accelerate postoperative erectile function recovery | Lack of large, multicenter, high-quality studies | ||||
| Mitigate tremor-induced harm | |||||
| Shorter training periods | |||||
| Emerging treatment | Regenerative cell injections | Potentially avoid surgical intervention | Ethical concerns and regulatory issues | Facilitate regeneration | 29, 37 |
| High controllability rate | |||||
| BoNT-A injections | An extended duration of action | Require precise injections, high requirements | Temporarily paralyzes bladder muscles by disrupting neurotransmission | 38 | |
| Safe, high success rate | |||||
| Nerve regeneration | Muscle function gains | Not easy to grasp the location and dosage | Enhance functional recovery Reduce neuronal death |
39 | |
| Benefit PN regeneration | |||||
| Er:YAG laser therapy | Promote the collagen remodeling | Require multiple treatments | Improve urethral urine control function | 42 | |
| Improve the compactness and elasticity | Long-term effectiveness is uncertain | ||||
- Abbreviations: ARS, adjustable retropubic sling; AUS, artificial urinary sphincter; BAS, bone-anchored sling; BoNT-A, Intravesical injections of onabotulinum toxin A; BF, biofeedback; EPNS, electrical pudendal nerve stimulation; Er:YAG, erbium-doped yttrium aluminum garnet; ES, electrical stimulation; LRP, laparoscopic radical prostatectomy; PFES, pelvic floor electrical stimulation; PFMT, pelvic floor muscle training; PN, pudendal nerve; PPUI, post-prostatectomy urinary incontinence; RALP, robot-assisted laparoscopic prostatectomy; RP, radical prostatectomy; RRP, retropubic radical prostatectomy; RTS, retrourethral transobturator sling.
4.1 Conservative treatment
Continence status continues to evolve for over a year following prostatectomy. Prior to exploring more invasive interventions, it is recommended to initiate conservative treatments. Notable among these conservative options are behavioral therapies and PFMT, accompanied by the potential use of biofeedback (BF), electrical stimulation (ES), and pharmacotherapy.29
Specifically, a BF-guided program can be employed for accurate PF muscle contraction, either alone or in conjunction with other conservative approaches like PFMT and ES. The administration methods of ES show considerable variability. Another noninvasive option is functional pelvic floor electrical stimulation (PFES), which artificially activates the PN and its branches, inducing direct and reflex reactions in urethral and periurethral striated muscles.8 PFMT enhances pelvic floor muscle strength, yet the demanding exercise regimen often leads to low long-term patient compliance. BF aids patients in correctly contracting pelvic floor muscles, and combining BF-assisted PFMT with ES can yield improved therapeutic effects.30
A crucial initial step in conservative management of PPUI involves behavioral and lifestyle adjustments, such as fluid restriction, limiting caffeine and alcohol, quitting smoking, timed voiding, or double voiding, while avoiding bladder irritants.29 In addition, electrical pudendal nerve stimulation (EPNS) has been proposed as a novel conservative physiotherapy for patients with PPUI. EPNS effectively targets PN stimulation to treat stress urinary incontinence (SUI) and urgency incontinence by contracting the pelvic floor muscles.30
Antimuscarinic drugs, phosphodiesterase inhibitors, and alpha-adrenergic agonists have emerged as potential PPUI therapies. Tadalafil, a phosphodiesterase type 5 inhibitor, has demonstrated efficacy in improving UI. Antimuscarinic drugs attempt to mitigate detrusor overactivity contributing to PPUI. Duloxetine, a serotonin and norepinephrine reuptake inhibitor, has shown promise for stress incontinence due to its impact on the urethral sphincter. The beta-3 agonist mirabegron also showed an effect for overactive bladder symptoms. Although pharmacotherapy offers mild benefits and complements other approaches, it is not a definitive solution for PPUI. Surgical management becomes the next option if conservative strategies prove ineffective.29
4.2 Surgical treatment
In contemporary practice, surgical interventions for PPUI encompass four principal methods: bulking agents, male synthetic slings, compressive balloon systems (also known as ProACTTM), and the artificial urinary sphincter (AUS), which stands as the current gold standard.31 It is crucial to distinguish between bladder instability, prevalent in the early postoperative period, and intrinsic sphincter dysfunction, responsible for persistent stress incontinence. The latter does not respond to physiotherapy and anticholinergic medication. Although AUS implantation has revolutionized the prognosis for stress incontinence after RP, it is accompanied by notable AUS-related morbidity and a substantial device revision rate.29
Despite these challenges, AUS remains the preferred surgical choice for stress incontinence due to its consistent excellent success rates, even among patients with severe urgency incontinence or detrusor hypocontractility. Variants such as postradiotherapy AUS and AUS with Tandem or Transcorporal Cuffs also exhibit positive therapeutic outcomes.29
Retrograde injection of substances like autologous fat, Teflon, silicone, and collagen into the external sphincter region has shown satisfactory short-term success rates. Collagen, particularly, has been a focus of injection therapy studies. Antegrade injections via a suprapubic approach tend to yield better results than retrograde injections. Percutaneous antegrade collagen injection has been explored for RP-induced incontinence, reporting a 75% continence rate at 6 months and 37.5% at 12 months of follow-up. The suprapubic approach enables more precise and extensive injection around the bladder neck compared to the transurethral approach.19
Regarding male slings, the bone-anchored sling (BAS) boasts success rates of 40%–88%, linked to a mesh infection rate of 2%–12%. The BAS involves compressing the urethra with a silicone-coated polypropylene mesh anchored to the bony pelvis, eliminating the need for access to the scarred retropubic space. The retrourethral transobturator sling (RTS) demonstrates a success rate of 76%–91%. This method passes ‘outside-in’ through the obturator foramen, utilizing polypropylene mesh sutured to the ventral surface of the bulbar urethra. Tensioning induces cranial displacement of the urethra without causing urodynamic obstruction. The adjustable retropubic sling (ARS) achieves a success rate of 72%–79%. However, erosion (3%–13%) and infection (3%–11%) may necessitate explantation. The ARS comprises silicone foam bolster, ribbed silicone struts, and adjustable tensioning washers. It is positioned using a trocar that traverses the perineal membrane, retropubic space, and abdominal fascia.32
Compared with RRP and LRP, RALP has less bleeding, less trauma, and lower blood transfusion rate, but the operation time is longer and the cost is higher.33 The operation time of RRP was the shortest, but the intraoperative blood loss and the use of analgesics were significantly higher than those of the other two methods.34 The fecal and UI and erectile dysfunction of RALP decreased significantly, and the overall percentage of positive margin was also lower. However, in addition to surgical methods, patient factors and the surgical experience of surgeons may also affect perioperative outcomes.35
4.3 Emerging treatment
In recent years, emerging therapies have shown potential in addressing PPUI. Sphincteric damage stands as a major PPUI cause. Injecting various regenerative cell types into the urinary sphincter aims to facilitate regeneration. For instance, transurethral ultrasound-guided injection of fibroblasts and myoblasts into 63 men, at least a year after prostatectomy, yielded a 65% complete continence rate with no deterioration after 1 year. Another study involving adipose-derived stem cell (ADSC) injection into the rhabdosphincter of 11 patients with SUI post-prostate surgery showed significant improvement in 8 of 11 men, with one achieving total continence. Although further research is necessary, preliminary data indicate the promise of regenerative stem cell injections in avoiding surgical intervention by revitalizing the smooth muscle and extracellular matrix of the external urinary sphincter.29
Stem cell therapy holds promise for various urological conditions, with practical limitations on the use of controversial embryonic stem cells due to ethical concerns and regulatory issues.36 Autologous adult stem cells, like bone marrow stromal cells, offer a desirable alternative. Muscle-derived stem cells (MDSCs) and ADSCs are easier to obtain in larger quantities. Stem-cell therapy, especially MDSCs and ADSCs, presents a promising avenue for SUI treatment.37
Intravesical injections of onabotulinum toxin A (BoNT-A) have been explored. BoNT-A, derived from Clostridium botulinum bacteria, temporarily paralyzes bladder muscles by disrupting neurotransmission. Its subtype BoNT-A has an extended duration of action, around 6 months for idiopathic overactive bladder patients.38
Nerve regeneration is also under scrutiny. Administering brain-derived neurotrophic factor (BDNF) to nerve transection sites enhances functional recovery and reduces neuronal death. Various BDNF treatment methods have shown improved cholinergic motoneuron activity. Continuous local administration to injury sites yielded successful results in nerve recovery. BDNF therapy has demonstrated efficacy in cavernous nerve injury models of erectile dysfunction and increased sympathetic pelvic ganglion cell sprouting.39
In recent years, non-ablative transurethral erbium-doped yttrium aluminum garnet (Er:YAG) laser therapy has been widely used in the clinical treatment of mild and moderate SUI.40, 41 Er: YAG laser can cause collagen denaturation in the deep layer of tissue relying on special SMOOTH noninvasive heat conduction technology. So as to promote the collagen remodeling of urethral mucosa and keep the superficial local tissue intact, improve the compactness and elasticity of the whole urethral wall tissue, and achieve the purpose of treating SUI.42 Through repeated treatment, cumulative laser effect to improve urethral urine control function.
4.4 Psychotherapy
PPUI may not pose a threat to patients' lives, but it will greatly affect their quality of life. The damage caused by PPUI affects the patient's physiological balance, leading to water restriction, lack of sleep, sexual dysfunction, skin problems, and ulcers. This discomfort lowers the overall quality of life and further exacerbates anxiety and depression, which leads to social, psychological, and physical pain. In addition, PPUI brings great financial pressure and psychological burden to patients and their families, instilling a sense of shame and loss.43
Therefore, medical staff should pay attention to the psychological problems of patients after operation. When necessary, appropriate psychological nursing measures should be taken, including cognitive behavioral therapy, psychodynamic therapy, supportive therapy, etc. It can effectively alleviate the negative emotions of patients and improve their self-confidence and compliance. So that patients can maintain an optimistic attitude during the follow-up recovery period. It has a positive influence on the prevention of complications and the treatment after the occurrence of complications.
5 CONCLUSION
A notable portion of patients continue to experience UI after undergoing RP. Despite the limitations of conservative treatment, including drugs, alternative interventions, and surgical techniques, the artificial urinary sphincter (AUS) remains the prevailing gold standard. Although emerging therapies have demonstrated success rates nearing 90%, the data on their application specifically within the post-RP population are relatively sparse. Nevertheless, there is optimism that advancements in technology could potentially lead to a decrease in the rate of UI after RP.
AUTHOR CONTRIBUTIONS
Conceptualization: Jing Quan. Methodology: Weizhang Xu. Software: Xuan Sun and Qiuyang Chen. Validation: Jing Quan, Jijia Gu, and Ziwei Li. Formal analysis: Jing Quan. Investigation: Weizhang Xu. Resources: Weizhang Xu. Data curation: Xuan Sun. Writing—original draft preparation: Jijia Gu and Ziwei Li. Writing—review and editing: Jing Quan. Visualization: Qiuyang Chen. Supervision: Qiuyang Chen. Project administration: Shanhong Li. Funding acquisition: Shanhong Li. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This research was funded by Jiangsu University Philosophy and Social Science Research, 2021SJA0307; special fund project for health technology development in Nanjing, GBX22320; Nanjing Medical University Education Research Project 2021LX041 and thanks for Cai Hongzhou's guidance and coordination.
CONFLICT OF INTEREST STATEMENT
The author declares that there is no conflict of interest.
ETHICS STATEMENT
This review article did not require ethical approval because it did not involve any primary data collection or analysis.




