Which inflammatory marker might be the best indicator for sacroiliitis?
Abstract
This study aimed to investigate the potential of inflammatory markers, including platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and C-reactive protein-to-lymphocyte ratio (CLR), in identifying sacroiliitis. Present retrospective study was conducted at the Abant Izzet Baysal University Hospital, including patients diagnosed with sacroiliitis between August 2020 and March 2023. Control subjects with normal sacroiliac joints were also included. Sacroiliitis patients were further categorized into active and chronic sacroiliitis groups. Demographic data and laboratory characteristics, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and various blood parameters, were recorded. Inflammatory markers were calculated, including PLR, NLR, LMR, and CLR. Statistical analyses were performed to compare the study groups and evaluate the diagnostic performance of these markers. A total of 226 subjects, including 132 sacroiliitis patients and 94 control subjects, were included in the study. Serum CRP levels were significantly higher in sacroiliitis patients compared to the control group. NLR, PLR, and CLR values were elevated in sacroiliitis patients, while LMR was decreased. There were significant correlations between these markers and established inflammatory markers. Receiver operating characteristic (ROC) analysis demonstrated moderate diagnostic performance for NLR, PLR, LMR and CLR in detecting sacroiliitis. Inflammatory markers, specifically NLR, PLR, LMR and CLR, showed significant differences between sacroiliitis patients and the control group. In addition PLR is useful in distinguishing active and chronic sacroiliitis. These markers, in conjunction with established inflammatory markers, may serve as supportive diagnostic tools for sacroiliitis.
1 INTRODUCTION
Sacroiliitis is an inflammation of one or both sacroiliac joint (SIJ). SIJs are typically affected in the majority of cases of axial spondyloarthropathy, with sacroiliitis being the initial presentation. Sacroiliitis can cause varying degrees of inflammatory back pain, which is a characteristic symptom.1 The established classification criteria rely on a combination of clinical symptoms and radiographic findings, but radiographs are often normal at the onset of symptoms, causing a delay in diagnosis.2 Various criteria, such as the modified New York criteria,3 Amor criteria,4 and European Spondyloarthropathy Study Group criteria,5 have been developed to aid diagnosis. Consequently, the ASAS has developed new classification guidelines for axial spondyloarthropathies that involve the use of MRI to facilitate early detection.6 Magnetic resonance imaging (MRI) can detect early signs of inflammation in the SIJ, such as bone marrow edema, synovitis, and enthesitis, as well as later structural changes including subchondral sclerosis, erosion, backfill/fat metaplasia, and ankyloses. Thus, MRI is a valuable tool for detecting both early and late changes in the SIJ.7, 8
It has been reported that inflammatory markers such as platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and C-reactive protein-to-lymphocyte ratio (CLR) are associated with inflammatory diseases. These markers can be measured through a blood test and provide clues about the presence or severity of inflammation. It has been found that PLR and NLR have a high predictive value in rheumatic diseases with predominantly neutrophilic inflammation, such as Behçet's disease and familial Mediterranean fever, and high PLR along with high platelet count may be potentially useful in diagnosing certain systemic vasculitis, particularly giant cell arteritis.9 On the other hand, it has been reported that high LMR ratios are associated with inflammatory processes such as appendicitis and atherosclerosis.10, 11 Studies in the literature have reported an increase in the NLR ratio in COVID-19 infection and cardiovascular diseases.12, 13 Similarly, it has been reported that a high initial CLR ratio detected in COVID-19 patients is a predictor of poor prognosis.14 Rouhin Sen and colleagues have reported a relationship between NLR and PLR in predicting radiographic sacroiliitis and active disease in axial spondyloarthritis (SpA), which could assist in the management of SpA when evaluated in conjunction with clinical findings.15
However, to our knowledge, these inflammatory markers have not been collectively evaluated in sacroiliitis. Therefore, our aim was to investigate inflammatory markers (PLR, NLR, LMR, CLR) in patients with and without sacroiliitis, and also to evaluate the differences in these markers between active and chronic sacroiliitis patients.
2 METHODS
Present retrospective study was conducted in the Radiology Department of the Abant Izzet Baysal University Hospital after obtaining ethical approval (Abant Izzet Baysal University Ethics committee, date: May 9, 2023, and approval no: 2023/155). Patients with sacroiliitis who visited our institution between August 2020 and March 2023 were enrolled in this study. Sacroiliitis were due to either ankylosing spondylitis or brucellosis in this study. MRI study of the participants were obtained from the patients at the time of diagnosis, before receiving treatment. Control subjects were volunteers whom radiological imaging studies revealed normal SIJ. We further grouped sacroiliitis patients according to the lesions characteristics either as active or chronic sacroiliitis. Patients with active infection (other than brucellosis), inflammatory conditions (other than ankylosing spondylitis), cancer, recent surgery or trauma, and pregnancy were excluded.
Age, gender, laboratory characteristics, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), aspartate and alanine transaminases (AST and ALT), ferritin, serum albumin, blood urea, creatinine, leukocyte count (WBC), hemoglobin (Hb), hematocrit (Htc), neutrophil count (Neu), lymphocyte count (Lym), monocyte count (Mono), and platelet count (Plt) were recorded from patients' files and institutional database. PLR was calculated by simple division of platelet to lymphocyte count. NLR was calculated with simple division of neutrophil to lymphocyte count. LMR was calculated lymphocyte to monocyte count. Similarly, CLR was calculated with division of CRP by to lymphocyte count. Characteristics and laboratory data of the study groups were compared.
2.1 Statistical analyses
Statistical software (SPSS 18 for Windows, IBM Co, Chicago, IL) was used for statistical analyses. Kolmogorov Smirnov test was applied to the study variables for normality analysis. Variables that fit into normal distribution were conducted with independent samples t test and expressed as means and standard deviations. Other variables that not fit into normal distribution were expressed as median (min–max) and compared with Mann–Whitney U test. Categorical variables were compared between study groups with chi-square test and given as numbers and percentages. Correlation between study variables were analyzed with Pearson's correlation test. Specificity and sensitivity of study parameters in detecting sacroiliitis were analyzed with ROC analysis test. It is considered as significant when the p value was lower than 5%.
3 RESULTS
Study population was consisted of 226 subjects; 132 in sacroiliitis and 94 in control group. Mean ages of the sacroiliitis and control groups were 40.9 ± 12.1 years and 40.6 ± 12.2 years, respectively (p = .85); 92 (69%) of sacroiliitis group and 72 (76%) of control group were women. Gender of the study and control groups was not statistically different (p = .25). Table 1 shows the demographic characteristics of the study population.
| Sacroiliitis | Control | p | ||
|---|---|---|---|---|
| Gender n (%) | Women | 92 (69%) | 72 (76%) | .25 |
| Men | 40 (31%) | 22 (24%) | .25 | |
| Age (years) | 40.9 ± 12.1 | 40.6 ± 12.2 | .85 | |
There were no statistical differences between study and control groups in terms of ESR (p = .51), AST (p = .76), ALT (p = .45), ferritin (p = .58), albumin (p = .07), urea (p = .17), creatinine (p = .5), WBC (p = .92), Hb (p = .99), Htc (p = .52), Plt (p = .39), Neu (p = .58), Lym (p = .75), Mono (p = .75), red cell distrubution width (RDW) (p = .91), platelet distrubution width (PDW) (p = .49), and mean platelet volume (MPV) (p = .16) levels.
Serum CRP (p = .004) levels of the study and control groups were significantly different. Serum NLR was significantly elevated in sacroiliitis group (2.28 [0.96–8.03]) compared to that in control group (1.92 [0.37–4.99]), (p < .001). Similarly, median PLR of the sacroiliitis group (144.44 [33.93–421.78]) was significantly higher than that of the control group (124.44 [51.52–342.27]) (p = .006).
Median LMR of the control group (3.87 [1.35–8.96]) was significantly higher than that of the sacroiliitis group (3.19 [0.88–14.83]) (p = .002). Table 2 summarizes the data of study and control groups.
| Sacroiliitis | Control | p | |
|---|---|---|---|
| Median (min–max) | |||
| ESR (mm/h) | 17 (2–122) | 17 (2–76) | .51 |
| CRP (mg/L) | 2.1 (0.1–139) | 1.15 (0.1–17.7) | .04 |
| AST (U/L) | 18 (8–38) | 17 (10–50) | .76 |
| ALT (U/L) | 17 (6–61) | 15 (6–68) | .45 |
| Ferritin | 29.75 (3.59–277) | 25.24 (1.64–263) | .58 |
| Albumin (mg/L) | 47 (24–56) | 46 (37–54) | .07 |
| Urea (mg/dL) | 28 (12–53) | 25,5 (11–60) | .17 |
| Creatinine (mg/dL) | 0.75 (0.30–1.30) | 0.75 (0.47–1.06) | .5 |
| WBC (/mm3) | 6970 (3180–13 600) | 6900 (3810–13 930) | .92 |
| Hb (gr/dL) | 13.4 (8.7–17.4) | 13.5 (9–16.3) | .99 |
| Htc (%) | 40.2 (12.9–52.6) | 40.6 (28.2–48.7) | .52 |
| Plt (/mm3) | 282 000 (90 000–629 000) | 267 000 (68 000–604 000) | .39 |
| Neu (/mm3) | 4.04 (1.15–10) | 4.24 (1.83–10.47) | .58 |
| Lym (/mm3) | 1.88 (0.92–5.1) | 1.97 (0.86–3.83) | .75 |
| Mono (/mm3) | 0.59 (0.24–1.6) | 0.6 (0.15–1.28) | .82 |
| RDW (%) | 13 (11.3–24.6) | 12.9 (11.8–24.6) | .91 |
| PDW (%) | 11.8 (8.2–20) | 11.8 (7.8–16.8) | .49 |
| MPV (fL) | 10.3 (6.9–13.5) | 10.4 (8.2–12.4) | .16 |
| NLR (%) | 2.28 (0.96–8.03) | 1.92 (0.37–4.99) | <.001 |
| PLR (%) | 144.44 (33.93–421.78) | 124.44 (51.52–342.27) | .006 |
| LMR (%) | 3.19 (0.88–14.83) | 3.87 (1.35–8.96) | .002 |
| CLR (%) | 1.04 (0.04–96.96) | 0.60 (0.03–8.43) | .006 |
- Note: Bold values are significant p values.
- Abbreviations: ALT, alanine transaminases; AST, aspartate transaminases; CLR, C-reactive protein-to-lymphocyte ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; Htc, hematocrit; LMR, lymphocyte-to-monocyte ratio; Lym, lymphocyte count; Mono, monocyte count; Neu, neutrophil count; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; Plt, platelet count.
In correlation analysis, NLR was positively and significantly correlated with serum CRP levels (r = .25, p < .001). PLR was positively and significantly correlated with serum CRP (r = .22, p = .001) and ESR (r = .20, p = .002) levels. LMR was positively and significantly correlated with serum CRP (r = .16, p = .02) and ESR (r = .16, p = .02) levels. CLR was positively and significantly correlated with serum and ESR (r = .29, p < .001).
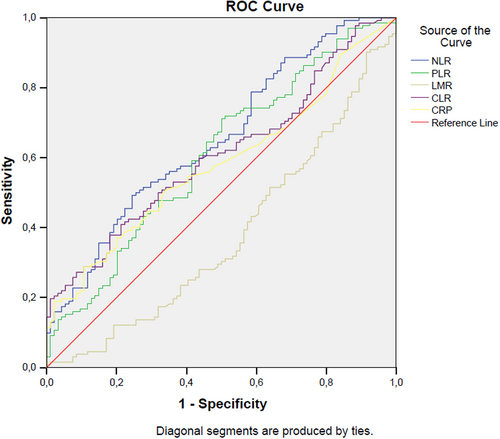
In ROC analysis, a NLR level higher than 1.93 has 64% sensitivity and 51% specificity in detecting sacroiliitis (AUC: 0.65, p < .001, 95% CI: 0.58–0.73). A PLR level higher than 123.68 has 71% sensitivity and 50% specificity in detecting sacroiliitis (AUC: 0.61, p = .006, 95% CI: 0.53–0.68). A LMR level lower than 3.36 has 60% sensitivity and 57% specificity in detecting sacroiliitis (AUC: 0.62, p = .002, 95% CI: 0.55–0.70). A CLR level higher than 0.58 has 62% sensitivity and 49% specificity in detecting sacroiliitis (AUC: 0.61, p = .006, 95% CI: 0.54–0.68) (Figure 1).
In subgroup analysis of sacroiliitis patients according to presence of active lesions, we found that none of the laboratory data nor age was different among patients with active and chronic lesions but PLR. PLR of the subjects with active and chronic lesions were 151.3 (33.9–421.8)% and 128.2 (41.7–292.6)%, respectively (p = .03).
In Binary logistic regression analysis, NLR was found to be an independent risk factor for presence of sacroiliitis when adjusted with serum albumin, gender, serum creatinine, disease activity, and ESR. A unit increase in NLR levels were increased the odds of sacroiliitis by 1.68 times (p = .046, OR: 1.68, 95% CI: 1.1–2.8).
4 DISCUSSION
Based on our study results, we found that the NLR, PLR, LMR, and CLR values were significantly different between sacroiliitis patients and the control group. NLR, PLR, and CLR were elevated, while LMR was decreased in sacroiliitis patients. These ratios were found to be correlated with established inflammatory markers, such as CRP and ESR, as well. ROC analysis showed that NLR, PLR, LMR, and CLR may be useful in detecting sacroiliitis, although the diagnostic performance of these ratios was moderate. Therefore, these ratios may serve as supportive diagnostic tools in clinical practice for patients suspected of having sacroiliitis.
Increased serum levels of various inflammatory markers such as CRP and ESR have been reported in sacroiliitis. Poddubnyy et al. reported that an elevated level of CRP is a strong positive predictor of sacroiliitis progression.16 Similarly, there are studies reporting the association of high serum CRP levels with MRI findings and as a predictor of chronic sacroiliitis.17 Accordingly, we found increased CRP levels in sacroiliitis compared to the control group.
NLR has been extensively studied and proven to be a highly sensitive marker for identifying infection, inflammation, and sepsis. Clinical investigations have consistently demonstrated the sensitivity of NLR in diagnosing and stratifying systemic infections, sepsis, bacteremia, and its strong predictive and prognostic value.18 Similarly, it has been reported that NLR and PLR ratios increase in inflammatory diseases such as rheumatoid arthritis.19 NLR was considered as a diagnostic marker in other inflammatory conditions, too. These include inflammatory bowel disease,20 diabetes mellitus,21 gastrointestinal conditions,22 thyroiditis,23 and SARS Cov2 infection.24 In addition, elevated PLR is a characteristic feature of liver fibrosis,25 thyroid conditions,26 gastrointestinal diseases,22 thyroiditis,27 cancer,28 Type 2 diabetes mellitus,29 irritable bowel disease,30 and Covid-19 infection.31 NLR has been introduced as an independent risk factor for cancer.32 Cancer is also associated with some degree of inflammation.33 Given that sacroiliitis is characterized by an augmented inflammatory load, similar to those conditions, we observed heightened NLR and PLR values in individuals with sacroiliitis in comparison to the control group. Accordingly, in this work, we found that NLR was an independent risk factor for sacroiliitis.
CLR, another established inflammatory predictor, has been extensively investigated across various medical conditions. These consist of infectious ailments like SARS-CoV-2 infection,34 infections at the surgical site,35 and periprosthetic joint infection.36 Additionally, high CLR levels have been associated with various types of cancer such as lung cancer,37 nasopharyngeal carcinoma,38 gastric cancer,39 and inflammatory bowel disease.40 CRP-derived marker, CLR, has been introduced as a marker of inflammation in various diseases including thyroiditis,41 hepatitis,42 and Covid-19 infection.43 Just like sacroiliitis, these conditions are characterized by inflammation. Consequently, we observed increased CLR levels in individuals with sacroiliitis compared to the control group. Furthermore, our study demonstrated that the level of CLR is more useful than serum CRP level in sacroiliitis patients. The results of our study indicated that among the inflammatory markers, PLR exhibited the highest sensitivity in sacroiliitis. Moreover, PLR was the only marker that associated active sacroiliitis.
LMR has also been demonstrated as a new systemic inflammatory indicator in various diseases, including active infections, solid organ malignancies, hematological malignancies, and rheumatic diseases. A decrease in LMR can result from lymphopenia and/or monocytosis.44 For instance, in cases of influenza H3N2, respiratory syncytial virus, and human rhinovirus infections, symptomatic patients have shown a decrease in LMR compared to asymptomatic patients.45 Furthermore, it has been reported that patients with Clostridium difficile infection have lower LMR values compared to healthy controls, while their NLR values are higher.46 On the other hand, there are studies indicating that a low LMR is associated with poor survival in patients with different types of nonhematologic solid tumors.47 Additionally, both NLR and LMR have been identified as tumor microenvironment biomarkers that can serve as prognostic factors not only in solid tumors but also in hematological malignancies.48 Moreover, Huang et al. have reported that the monocyte-to-lymphocyte ratio (MLR) is a reliable, cost-effective, and potentially new parameter for assessing disease severity in axial SpA.49 Proportion of monocyte and lymphocyte counts can be expressed as either LMR or MLR. MLR increases in inflammatory conditions such as malignancy,50 diabetic nephropathy,51 functional bowel conditions,30 frailty,52 gastrointestinal conditions22 and Covid-19 infection.31 Accordingly, we found decreased LMR levels in sacroiliitis, which also refers to an elevation in MLR.
Possible limitations of the current study could involve its retrospective design, the fact that it was conducted in a single center, and the relatively small size of the study cohort. However, to the best of our knowledge, our study is the first to collectively investigate inflammatory markers such as NLR, PLR, LMR, and CLR in sacroiliitis.
In conclusion, our study provides valuable insights into the role of inflammatory markers in sacroiliitis. We observed significant differences in NLR, PLR, LMR, and CLR values between sacroiliitis patients and the control group, suggesting their potential utility in the diagnosis of sacroiliitis. Future research with larger cohorts and prospective designs is warranted to validate our findings and explore the clinical implications of these inflammatory markers in sacroiliitis management.
AUTHOR CONTRIBUTIONS
All works releated to this study was performed by MEK & ZC equally.
FUNDING INFORMATION
This work has not been funded by any organization.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies
Melike Elif Kalfaoglu, MD, Specialist in Radiology, Bolu Abant İzzet Baysal University, İzzet Baysal Training and Research Hospital, Bolu/Turkey. [email protected]; orcid.org/0000-0003-1678-763X
Zeliha Cosgun, MD, Specialist in Radiology, Bolu Abant İzzet Baysal University, İzzet Baysal Training and Research Hospital, Bolu/Turkey. [email protected]; orcid.org/0000-0003-1996-1568




