Non-Isothermal Decomposition Kinetics, Heat Capacity, and Thermal Safety of 2-Nitroimino-5-Nitro-Hexahydro-1,3,5-Triazine (NNHT)
Abstract
The kinetic equation describing the thermal decomposition reaction of NNHT obtained by TG-DTG data, integral isoconversional non-linear method and integral method of treating TG-DTG curves is 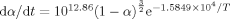 . The specific heat capacity (Cp) of NNHT was determined with the continuous Cp mode of the microcalorimeter. The equation of Cp (T) was obtained. The standard molar heat capacity of NNHT was 218.41 J mol−1 K−1 at 298.15 K. With the help of the onset temperature (Te) and maximum peak temperature (Tp) from the non-isothermal DTG curves of NNHT at different heating rates (β), the apparent activation energy (EK and EO), and the pre-exponential constant (AK) of the thermal decomposition reaction obtained by Kissinger’s method and Ozawa’s method, Cp obtained by microcalorimetry, density (ρ) and thermal conductivity (λ), the decomposition heat (Qd, taking half-explosion heat), Zhang-Hu-Xie-Li’s formula, Smith’s equation, Friedman’s formula, Bruckman-Guillet’s formula, and Wang-Du’s formulas, the values (Te0 and Tp0) of Te and Tp corresponding to β→0, thermal explosion temperature (Tbe and Tbp), adiabatic time-to-explosion (tTIad), 50 % drop height (H50) of impact sensitivity, critical temperature of hot-spot initiation (Tcr), thermal sensitivity probability density function [S(T)] versus temperature (T) relation curves for spheroidic NNHT with radius of 1 m surrounded with ambient temperature of 300 K, peak temperature corresponding to the maximum value of S(T) versus T relation curve (
. The specific heat capacity (Cp) of NNHT was determined with the continuous Cp mode of the microcalorimeter. The equation of Cp (T) was obtained. The standard molar heat capacity of NNHT was 218.41 J mol−1 K−1 at 298.15 K. With the help of the onset temperature (Te) and maximum peak temperature (Tp) from the non-isothermal DTG curves of NNHT at different heating rates (β), the apparent activation energy (EK and EO), and the pre-exponential constant (AK) of the thermal decomposition reaction obtained by Kissinger’s method and Ozawa’s method, Cp obtained by microcalorimetry, density (ρ) and thermal conductivity (λ), the decomposition heat (Qd, taking half-explosion heat), Zhang-Hu-Xie-Li’s formula, Smith’s equation, Friedman’s formula, Bruckman-Guillet’s formula, and Wang-Du’s formulas, the values (Te0 and Tp0) of Te and Tp corresponding to β→0, thermal explosion temperature (Tbe and Tbp), adiabatic time-to-explosion (tTIad), 50 % drop height (H50) of impact sensitivity, critical temperature of hot-spot initiation (Tcr), thermal sensitivity probability density function [S(T)] versus temperature (T) relation curves for spheroidic NNHT with radius of 1 m surrounded with ambient temperature of 300 K, peak temperature corresponding to the maximum value of S(T) versus T relation curve (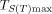 ), safety degree (SD), and critical ambient temperature(Tacr) of thermal explosion of NNHT are calculated. The following results of evaluating the thermal safety of NNHT are obtained: TSADT=Te0=453.34 K, TSADT=Tp0=454.86 K, Tbe=462.68 K, Tbp=467.22 K, tTIad=1.03 s, H50=17.69 cm, Tα=461.4 K. SD=72.74 %, PTE=27.26 %, and Tacr=321.96 K.
), safety degree (SD), and critical ambient temperature(Tacr) of thermal explosion of NNHT are calculated. The following results of evaluating the thermal safety of NNHT are obtained: TSADT=Te0=453.34 K, TSADT=Tp0=454.86 K, Tbe=462.68 K, Tbp=467.22 K, tTIad=1.03 s, H50=17.69 cm, Tα=461.4 K. SD=72.74 %, PTE=27.26 %, and Tacr=321.96 K.




