Urinary collagen peptides: Source of markers for bone metabolic processes in kidney transplant recipients
Abstract
Introduction: Kidney transplant recipients (KTRs) are at an increased risk of fractures. Total urinary hydroxyproline excretion served as marker for bone resorption (BR) but was replaced by β-CrossLaps (CTX), a C-terminal collagen α-1(I) chain (COL1A1) telopeptide. We investigated the low-molecular-weight urinary proteome for peptides associated with changes in bone metabolism after kidney transplantation. Methods: Clinical and laboratory data including serum levels of CTX in 96 KTR from two nephrology centers were correlated with signal intensities of urinary peptides identified by capillary electrophoresis mass spectrometry. Results: Eighty-two urinary peptides were significantly correlated with serum CTX levels. COL1A1 was the predominant peptide source. Oral bisphosphonates were administered for decreased bone density in an independent group of 11 KTR and their effect was evaluated on the aforementioned peptides. Study of the peptides cleavage sites revealed a signature of Cathepsin K and MMP9. Seventeen of these peptides were significantly associated with bisphosphonate treatment, all showing a marked reduction in their excretion levels compared to baseline. Discussion: This study provides strong evidence for the presence of collagen peptides in the urine of KTR that are associated with BR and that are sensitive to bisphosphonate treatment. Their assessment might become a valuable tool to monitor bone status in KTR.
Abbreviations
-
- KTR
-
- Kidney Transplant Recipients
-
- BR
-
- Bone Resorption
-
- CTX
-
- β-CrossLaps
-
- COL1A1
-
- C-terminal collagen α-1(I) chain
-
- MMP9
-
- Matrix Metalloproteinase 9
1 INTRODUCTION
Kidney transplant recipients (KTRs) are at an increased risk of bone fractures [1-4]. In the first year after renal transplantation, there is, on average, a decrease in bone density ranging from 7% to 9%. This decrease is partially attributed to an increase in bone resorption compared to bone formation [5]. This excessive resorption mainly affects patients who mostly have a long-standing history of chronic kidney disease (CKD) with its associated negative impact on bone metabolism termed CKD mineral and bone disorder (CKD-MBD). KTR therefore keep a high risk level of fractures in the 5 years following transplantation [6]. Although many factors that favor the persistence of increased bone resorption, such as treatment with corticosteroids, hyperparathyroidism, hyper/hypophosphatemia, hypomagnesemia, and vitamin D deficiency in the post-transplant context were identified [7], the pathophysiological and molecular mechanisms are not well understood.
Dual-energy X-ray absorptiometry is commonly used for the study of bone density but cannot reliably predict individual fracture risk in KTR [8]. Bone biopsies are rarely performed due to their invasive nature. Due to these reasons non–invasive monitoring with serological markers of bone formation and resorption is commonly used in KTR and includes osteocalcin, bone alkaline phosphatase, and amino- and carboxy-terminal collagen-I crosslinks (NTX and CTX respectively). Especially in KTR blood levels of these markers is not only a function of bone metabolism but is also affected by high variability in kidney function [9]. Still, many patients with rapid decline of bone mass are missed. Hence, there is a clear need for diagnostic markers with increased specificity for KTR-associated rapid bone loss to prevent bone fractures, for example by the administration of bisphosphonates.
In the 1960s–1980s, a vast number of medical studies established an association between urinary collagen peptides and bone resorption (BR) in both humans and animal models [10-18]. Essentially, these studies showed that there are peptides containing hydroxyproline (HyP) in the urine, which are in part derived from bone and are associated with BR. At the time of the discovery of these peptides, however, their amino acid sequences could not be determined. In line with this, total urinary HyP (including free and peptide-bound HyP) was used as a marker of BR for several decades. Later, this approach was questioned based on the assumption that urinary HyP peptides from other sources including food intake and skin may interfere and reduce the diagnostic performance [19]. Advances in tandem mass spectrometry (MS) have recently enabled better characterization of these peptides including information on their amino acid sequence [20]. More recently, MS has substantially improved analysis of urinary proteins and peptides. Studies have shown the wide variety of low molecular weight peptides in human urine, even in healthy subjects [20-24]. Urine in particular displays a large variety of collagen fragments, especially COL1A1. The detailed analysis of the amino acid sequences of these fragments reveals the richness in sequences containing hydroxyproline [25]. Hundreds of different fragments of this type have been identified, using both capillary electrophoresis (CE) and liquid chromatography as high-resolution separation techniques prior to MS (see for instance the supplemental files of reference [23]). Evidence that these COL1A1 peptides are not only of intra-renal origin is derived from a study where the low molecular weight proteome of blood and urine was compared using CE coupled to MS (CE-MS) [26]. In that study a significant overlap of collagen-derived peptides was detectable in blood and urine with their levels correlating to each other.
In the current study, we hypothesized that urinary collagen peptides which can now be precisely identified using MS may serve as a source for new diagnostic markers for bone remodeling and response to therapy. For this purpose, we studied the low molecular weight urinary proteome of individuals from two cohorts of renal transplant patients and searched for peptides whose urinary levels after normalization to internal housekeeping peptides correlated with the concentration of the established bone metabolism markers CTX, osteocalcin, and bone alkaline phosphatase. The selected peptides were then characterized in terms of their amino acid sequences, linkage to bone resorption protease activities, and the sensitivity of their urine levels to bisphosphonate therapy.
2 METHODS
2.1 Patients and samples
The 96 KTR patients enrolled in this study are graft recipients in the first-year post-transplantation from two clinical centers in France. The patient cohort from the University Hospital of Strasbourg is divided into two subgroups. The first subgroup consists of 27 patients with various degrees of BK virus-related post-kidney transplantation complications, from whom samples were taken at a single time point after kidney transplantation. The second subgroup from this center comprises 11 KTR patients affected by bone loss due to pre-transplant osteopenia or osteoporosis with samples taken at two different time points: before the initiation and after several months of treatment with bisphosphonates. A third KTR study cohort, composed of 58 patients, is derived from Necker Hospital in Paris, representing a cross-sectional group of patients with (n = 29, eight with antibody-mediated rejections (ABMR), five with T cell-mediated rejections (TCMR), 16 with interstitial fibrosis and tubular atrophy (IFTA)) and without (n = 29) transplantation-related complications.
Bone metabolism is routinely monitored at quarterly intervals during the first post-transplantation year and then once each subsequent year. Monitoring is performed using serum CTX and BAP at the University of Strasbourg, and serum CTX and osteocalcin at Necker Hospital in Paris. Urine samples collected within a time range of less than 15 days from the time of bone metabolism marker assessment were selected from the biobanks of the two hospital centers to perform proteomic analysis by CE-MS. Clinical and demographic characteristics for the KTR patients from the University Hospital of Strasbourg and Necker Hospital in Paris are provided in Table 1.
| Parameters | KTR cohort Strasbourg | KTR cohortParis | KTR StrasbourgBP treatment group | p-values for group differences |
|---|---|---|---|---|
| Number of patients (n) | 27 | 58 | 11 | |
| Sex (M/F) | 14/13 | 34/24 | 6/5 | 0.56 |
| Age (years) | 54.3 (41.1–63.5) | 54.8 (41.8–61.8) | 57.7 (54.9–71.7) | 0.92 |
| Height (cm) | 169 (161–174) | 170 (160.8–176) | 170 (163.8–178.8) | 0.62 |
| Weight (kg) | 69 (60.5–72) | 73 (62–83.9) | 87.7 (73.2–92.5) | 0.33 |
| Body mass index (kg/m2) | 23.53 (21.53–28.78) | 25.5 (22.76–28.46) | 30.51 (25.5–32.99) | 0.45 |
| Body surface area (m2. Mosteller) | 1.76 (1.67–1.88) | 1.86 (1.67–2) | 2.03 (1.83–2.15) | 0.36 |
| Serum creatinine (μmol/L) | 136.2 (110.6–165.1) | 128 (115.5–148.5) | 141.5 (99.5–156.8) | 0.73 |
| eGFR (mL/min/1.73m2; MDRD) | 45 (34–60.5) | 43.98 (37.8–57.51) | 48 (42–53) | 0.72 |
| Serum Beta-CrossLaps (μg/L) | 0.81 (0.37–1.22) | 0.6 (0.35–0.94) | 0.42 (0.07–0.74) | 0.13 |
| Serum bone alkaline phosphatase (μg/L) | 16.9 (10.7–28.5) | NA | 15 (10.55–20.2) | NA |
| Serum parathyroid hormone (pg/mL) | 122.5 (74.7–172.5) | 74.5 (58.3–139.5) | 146.4 (109.3–193.4) | 0.05 |
| Plasma vitamin D (25-OH). μg/L | 30.3 (26.5–37.1) | 30 (25–36) | 36.5 (30.5–40.9) | 0.34 |
| Plasma vitamin D (1.25-OH). μg/L | 66.3 (45.6–75.4) | 71 (47.5–84) | 61.6 (43.4–80.3) | 0.34 |
| Plasma osteocalcin (μg/l) | NA | 28 (17–51) | NA | NA |
| Kidney donor age | 56 (38.5–68.5) | 55 (47.5–65) | 68 (58–71.3) | 0.63 |
| Kidney donor height (cm) | 171 (159–175) | 170 (162–175) | 167 (159.3–179.3) | 0.50 |
| Kidney donor weight (kg) | 72 (60.5–85) | 71 (62–79) | 79.5 (69–101.9) | 0.57 |
| Start of bisphosphonate treatment after transplantation (days) | NA | NA | 185 (165.8–744.8) | NA |
| Delay post transplantation | 99 (93–105.5) | 362 (97–372) | 294 (196.5–372) | |
| Cyclosporine (%) | 29.6 | 13.8 | 9.1 | 0.08 |
| Everolimus (%) | 7.4 | 5.2 | 9.1 | 0.68 |
| Tacrolimus (%) | 66.7 | 84.5 | 90.9 | 0.06 |
| IMPDH inhibitor (%) | 88.9 | 79.3 | 90.9 | 0.28 |
| Azathioprine (%) | 0 | 5.2 | 0 | |
| Sirolimus (%) | 3.7 | 1.7 | 0 | |
| Corticosteroid (%) | 81.5 | 94.8 | 68.2 | 0.50 |
| Eculizumab (%) | 0 | 1.7 | 9.1 |
- Note: Values are given as median and interquartile range in square brackets unless stated otherwise. In the bisphosphonate (BP) study group, treatments were assessed for each of the 22 time points of sample procurement.
- Abbreviations: eGFR, estimated glomerular filtration rate; IMPDH, Inosine-5'-monophosphate dehydrogenase; IQR, interquartile range; NA: not available.
All patients provided informed consent. The study was approved by the institutional review board of Strasbourg University Hospital under the reference CPP-EST DC-2013-1990 for patients from Strasbourg. For patients from the Necker Hospital the study was approved by the Ethics Committee of Ile-de-France XI (#13016).
Demographic and clinical data was collected from each KTR and their respective kidney allograft donor and included age, sex, height, weight, body surface area (using the Mosteller formula [27]), body mass index (kg/m2).
Spot urine samples were transferred to urine monovettes and immediately frozen without additives at − 20°C. Shipment to the central laboratory performing CE-MS (Mosaiques Diagnostics, Hannover, Germany) was carried out on dry ice to ensure that the samples were thawed only once before CE-MS analysis.
2.2 Immunoassays and laboratory tests
Laboratory markers from the KTR included serum creatinine, estimated glomerular filtration rate (eGFR) using the MDRD formula [28], serum intact PTH (1-84), serum 25(OH) vitamin D, and 1,25(OH)2 vitamin D as determined by the use of standard clinical laboratory methods. For patients from Strasbourg serum CTX-I and BAP were analyzed using the Automated Chemiluminescence Immunoassay (CLIA) (Crosslap, Ostase) on an iSYS automate (IDS-iSYS Multi–Discipline Automated System). In patients from Paris CTX and osteocalcin were analyzed by the Elecsys β-CrossLaps and the N-MID Osteocalcin serum assays respectively (Roche Diagnostics) on the Cobas e411 analyzer.
2.3 Urine sample preparation
Sample preparation for proteomic analysis of urine samples by CE-MS was performed as described elsewhere [23]. Briefly, urine was prepared by 1:2dilution in 2 M urea, 10 mM NH4OH, and 0.02% SDS (pH 10.5). This was followed by centrifugation at 1500 × g through Centrisart centrifugal ultrafiltration units of 20 kDa molecular weight cut-off (Sartorius, Germany) for isolation of the low molecular weight fraction of urine and for clearance of all high molecular weight components including cells, cellular debris and proteins > 20 kDa. A 1.1 mL filtrate was collected and desalted by gravity flow over Sephadex G-25 resin using PD10 columns (GE Healthcare, Sweden) and ultrapure water to remove urea and salts that interfere with CE-MS analysis. Samples were then lyophilized, resuspended in HPLC-grade water to a total peptide concentration of 0.8 μg/μL and a 100 nLaliquot were injected into the CE-MS under constant flow and pressure conditions at a pH of 2.2 to ensure that all peptides are positively charged.
2.4 CE-MS analysis and proteomic data processing
CE-MS sample acquisition was performed using a P/ACE MDQ capillary electrophoresis system (Beckman Coulter, Fullerton, USA) on-line coupled to a Micro-TOF MS (Bruker Daltonic, Bremen, Germany). Prerequisite for coupling of the CE with the MS was that the ESI sprayer (Agilent Technologies, Palo Alto, USA) was grounded, the ion spray interface potential set between −4.0 and −4.5 kV and contact-close-relays were used to control MS data acquisition automatically by the CE. Spectra were accumulated every 3 s over a m/z range of 350–3000.
For raw proteomic data processing, the in-house software MosaiquesVisu [29] was used to deconvolute mass spectral ion peaks representing identical molecules at different charge into single masses. MosaiquesVisu uses both isotopic distribution as well as conjugated masses for charge-state determination of peptides with charge states > 1 and signal-to-noise ratios > 4 observed in a minimum of three consecutive spectra. The software generates a list of peptides in the sample characterized by molecular mass, CE-migration time and ion signal intensity, which were subsequently normalized by global and local linear regression using internal standard peptides with FT-ICR MS-determined exact molecular mass ensuring inter-comparability of CE migration times and molecular masses across measurements. In order to compensate for dilution effects due to different hydration status of the urine samples, the calibration method for peptide signal intensities established by Jantos-Siwy et al. [30] was used. This method is based on the relative quantification of 29 internal collagen peptides of high abundance in human urine. In subsequent studies with large numbers of patient samples and covering a broad range of disease etiologies [31-34], this method has been demonstrated to be better suited than urinary creatinine specifically for comparative analysis of CE-MS peptide profiles. This remains true, even if some of the peptides used for calibration are affected by group differences. The normalized peptide profiles were deposited, matched, and annotated in a Microsoft SQL database, allowing further analysis and comparison of multiple samples. Peptides were considered identical within different samples, when mass deviation was lower than 50 ppm for peptides less than 2000 Da or 75 ppm for larger peptides. After calibration, deviation of migration time was controlled to be < 0.45 min.
2.5 Peptide sequence analysis
For amino acid sequence identification, CTX-associated urinary peptides derived from the CE-MS spectra, based on their MS-detected molecular mass and characteristic migration time in the CE, were searched against Mosaiques’ in-house human urinary peptide database containing sequences from both LC- and CE-MS/MS using a Dionex Ultimate 3000 RSLS nano flow system (Dionex, Camberly, UK) for LC- and a Beckman CE/Orbitrap Q Exactive plus combination for CE-MS/MS (Thermo Scientific, Waltham, MA) [35].
2.6 In silico protease mapping
In silico protease maps were generated using Proteasix (available online at http://proteasix.org/), which allows retrieving protease/cleavage sites (CS) associations based on the MEROPS (https://www.ebi.ac.uk/merops/) and the SWISS-PROT database (http://www.uniprot.org/) [36]. N- and C-terminal cleavage sites were retrieved for the sequenced BR-associated peptides, based on an alignment to the respective substrate sequence information. The association of proteases with their cleavage sites is based on octapeptide consensus sequences. A list of 6000 random CS sequences was used as reference to determine the specificity of prediction against false-positive associations. Only proteases/CS associations which were previously observed or predicted with high/medium confidence level and having at least two cleavage site associations were considered.
2.7 Statistical analysis and definition of peptide markers
Statistical analyses were carried out using the statistical programming language R and the statistical software MedCalc version 12.7.5.0 (MedCalc Software; Mariakerke, Belgium).
Signal intensities of each of the 3555 annotated peptides were correlated with serological laboratory markers using Spearman's rank correlation for univariate analysis. To reduce the risk of false discovery, the patient cohorts from Strasbourg and Paris were studied independently from each other. Statistical adjustment of p-values for multiple testing was performed by the method of Benjamini and Hochberg [37] and by taking into account the zero-inflated distribution of the peptide's intensity values [38].
For the selection of age- and gender-matched normal individuals, as well as age-, gender-, and eGFR-matched CKD patients as reference groups to compare signal intensities of selected peptides, the R-script “MatchIt” [39] was used, which conducts nearest neighbor matching with logistic regression to estimate the propensity score. These urinary peptide reference profiles were from normal individuals and CKD patients of previously described study populations recorded by CE-MS under identical operating conditions and instrument settings [40-42].
3 RESULTS
3.1 Urinary peptides with association to CTX, BAP, and osteocalcin
In a first step, association of urinary peptides associated with bone metabolism in the setting of kidney transplantation was investigated. For this purpose, we analyzed 85 KTR from Strasbourg and Paris for their individual peptide profiles by CE-MS. The 27 patients from Strasbourg are a highly selective group of KTR patients affected by BK-virus infection, whereas the 58 patients from Paris represent a cross-sectional group of whom 29 are with (8 ABMR, 5 TCMR, 16 IFTA) and 29 are without transplantation-related complications Demographic and clinical data for the two patient groups from Strasbourg and Paris are presented in Table 1. As indicated by this table, both cohorts are comparable to the specific presented clinical and demographic characteristics. For most of the patients, samples were taken within the first 2 years after transplantation. The only differences were higher prevalence of steroid treatment in the Paris group and serum PTH being higher in the Strasbourg group.
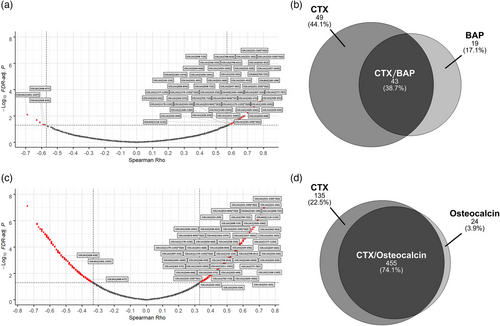
Applying a minimum frequency threshold of 30%, 3555 urinary peptides were selected and evaluated in the 85 kidney transplantation patients. The 30% lower limit was selected as this was determined in previous studies using CE-MS [43-45] to be an adequate data foundation and optimal tradeoff to obtain statistically meaningful results in multivariate analysis and to compensate for biological variability between individuals in respect to protein turnover and proteolytic environment, for example, distinct protease profiles and enzyme activities. In previous biomarker studies, the use of 30% threshold enabled establishment of biomarker patterns for different disease conditions [43-45]. The signal intensities of the 3555 annotated peptides in each individual peptide profile were then correlated to the serum levels of the bone metabolism laboratory markers CTX, osteocalcin, and bone alkaline phosphatase (BAP) by Spearman rank sum analysis. As shown in the volcano plot in Figure 1A, in the case of the Strasbourg cohort of 27 patients this resulted in the identification of 92 peptides with significant correlation (FDR < 0.05) to CTX of which 87 (95%) showed positive correlation with Spearman's rho coefficients in the range of 0.58–0.82 and only five (5%) showed inverse correlation with Spearman rho coefficients in the range of −0.59 to −0.69. As shown in the Venn diagram of Figure 1B, 43 (47%) of these 92 peptides were also significantly correlated with serum levels of BAP as second bone metabolism marker. Of note, all 43 peptides were positively correlated to both CTX and BAP. In the case of the Paris group of 58 patients, 590 urinary peptides are significantly associated with CTX. A volcano plot of Spearman's rho values for urinary peptides of the Paris group is presented in Figure 1C. From these, 387 (66%) showed a positive correlation (range of Spearman rho values: 0.34–0.75), whereas 203 (34%) were found inversely regulated (range of Spearman rho values: −0.33 to −0.74). In the case of the Paris group, osteocalcin was available as a second bone marker. As shown in the Venn diagram in Figure 1D, an overlap of 455 (77%) peptides was observed showing a significant correlation in the same direction to both CTX and osteocalcin. Out of these, 293 demonstrated positive and 162 inverse correlations to both bone metabolism markers.
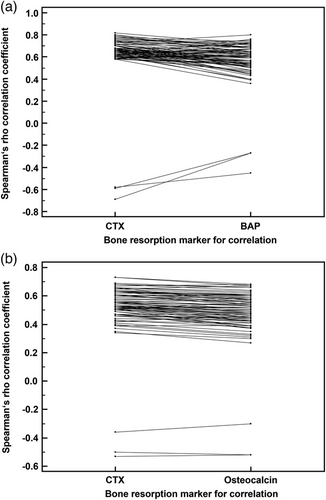
Eighty-two out of the pool of BR-associated urinary peptide candidates were found to be significantly correlated with CTX in both the Strasbourg and Paris patient groups, which represents 89% (82 out of 92) of the total number of CTX-correlated peptides in the Strasbourg group. The 82 peptides are presented in Table S2 by their CE-MS characteristics and group-specific signal distributions and are also labelled with their respective peptide IDs in the volcano plots of Figure 1A for the patients from Strasbourg and in Figure 1C for the patients from Paris. As presented in Figure 2A, congruence between the Spearman Rho correlation factors of CTX and BAP was observed for the Strasbourg cohort. The same applies to the Spearman Rho correlation factors of CTX and osteocalcin in the Paris cohort (Figure 2B). Together this indicates that the 82 selected peptides are not only correlated to CTX, but also to other bone metabolism markers irrespective if these are resorption or formation markers. Conversely, none of these peptides were correlated with serum PTH and creatinine levels, as well as with the estimated glomerular filtration rate, neither in the Strasbourg cohort nor in the Paris cohort.
3.2 Identification of urinary peptides related to bone metabolism
From these 82 CTX-associated urinary peptides, 59 (72%) could be resolved by their amino acid sequence. As evident from Table 1, most of the CTX-associated peptides, namely 46, originate from alpha-1 type I collagen (COL1A1). From the remaining 13 sequenced peptides, nine were derived from other collagen chains (COL1A2, n = 1; COL3A1, n = 2; COL4A1, n = 1; COL4A2, n = 1; COL4A5, n = 2; COL7A1, n = 1; COL8A2, n = 1) and four from the non-collagen proteins cadherin-1 (CADH1), α−1-antitrypsin (A1AT), EMI domain-containing protein 1 (EMID1), and gelsolin (GSN). All COL1A1 peptides show a positive correlation with bone metabolism markers, whereas the COL3A1 peptides represent two out of three peptides that are inversely correlated (with the third not resolved by sequence).
Alignment of the 46 COL1A1peptide fragments to the linear sequence of COL1A1, as shown in Figure 3, indicates that the peptides originate from certain specific regions of the full–length protein. This observation may be interpreted as a sign of regions of increased susceptibility to proteolytic cleavage. To investigate this hypothesis, a search was carried out for observed and in silico predicted endoproteinase-specific cleavage sites in the immediate vicinity of these hot spots. Octapeptide sites matching with the consensus recognition sites of the proteases Cathepsin K (CTSK) and metalloproteinase 9 (MMP9) were identified by in silico-prediction using the software tool Proteasix, as indicated in Figure 3.

3.3 Identification of the CTX-associated marker peptides in plasma
From the 59 out of the 82 urinary bone remodeling markers for which sequence information is available, 36 were also detectable in human plasma [26]. as presented in Table S2. Except for the Gelsolin peptide 605-WVGTGASEAEKTGAQEL-621, all of these peptides are derived from collagen chains and here especially from COL1A1 (n = 30). In contrast, when comparing all 3648 peptide sequences in our urinary sequence database with the 3035 peptide sequences identified so far in plasma samples, an overlap of only 143 peptides were detected which corresponds to 3.9% agreement (143 out of 3648). This limited overlap between peptides in human urine and plasma was reported previously by Magalhães et al. [26] and Parker et al. [46]. Comparing this proportion to the 61% overlap (36 out of 59) in case of the bone remodeling markers, this amounts to a 15-fold enrichment for the bone-specific clearance process that was highly significant (p < 0.000001) in Fisher's exact test.
3.4 Effect of bisphosphonate therapy on the CTX-associated urinary peptides
In order to evaluate the impact of therapeutic interventions against bone loss on the 82 CTX-associated urinary peptides, the peptides abundance before and after bisphosphonate treatment was determined in a separate group of 11 KTR recruited from the University Hospital of Strasbourg. For bisphosphonate therapy, all but one patient received 70 mg oral alendronic acid once a week, whereas the remaining patient received 35 mg oral risedronate once a week. From the set of 82 peptides, 17 showed a significant change after bisphosphonate treatment, consistently a decrease, indicating bisphosphonate treatment adherence and possibly reduced bone resorption following treatment (Table 2).
| KTR Strasbourg Bisphosphonate (BP) treatment group (N = 11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before BP therapy | After BP therapy | ||||||||
| Peptide IDa | Molecular mass (Dalton) | CE-migration time (minutes) |
Protein symbol [AAb] |
Protein sequencec |
Mean Amp (SD) | Freq | Mean Amp (SD) | Freq | Two-tailed probability pd |
| 4088 | 1143.514 | 36.93 | COL1A1[142-154] | GPpGPpGPpGPpG | 1866 (3303) | 82 | 262 (841) | 18 | 0.0039 |
| 5261 | 1200.538 | 24.50 | COL1A1[709-722] | KGDAGApGApGSQG | 16304 (7925) | 100 | 4988 (4170) | 91 | 0.001 |
| 5574 | 1216.537 | 24.61 | — | — | 2814 (1655) | 91 | 593 (985) | 45 | 0.0049 |
| 7405 | 1321.595 | 28.25 | COL1A1[798-811] | ApGDRGEpGPpGPA | 21806 (8966) | 100 | 6010 (6070) | 82 | 0.001 |
| 10508 | 1523.733 | 39.95 | COL1A1[1177-1193] | VGPpGPpGPpGPpGPPS | 23763 (11942) | 100 | 8063 (7816) | 82 | 0.001 |
| 13155 | 1716.770 | 28.30 | COL8A2[182-199] | GpPGFQGEPGPQGEPGPP | 844 (1041) | 91 | 70 (186) | 18 | 0.0068 |
| 13675 | 1750.786 | 23.88 | COL1A1[221-239] | GPpGPpGKNGDDGEAGKpG | 2802 (1900) | 100 | 363 (598) | 36 | 0.001 |
| 13886 | 1765.806 | 30.96 | COL1A1[650-668] | GPpGEAGKpGEQGVpGDLG | 3040 (1614) | 91 | 906 (1353) | 55 | 0.002 |
| 14554 | 1825.788 | 31.81 | COL1A1[698-719] | GANGApGNDGAKGDAGApGApG | 1044 (571) | 91 | 198 (383) | 27 | 0.0059 |
| 17098 | 2070.914 | 25.45 | COL1A1[431-453] | GNSGEpGApGSKGDTGAKGEPGp | 1386 (899) | 91 | 152 (284) | 55 | 0.002 |
| 17164 | 2076.955 | 21.79 | COL1A1[221-242] | GPpGPpGKNGDDGEAGKpGRpG | 2793 (2347) | 91 | 171 (348) | 36 | 0.002 |
| 18340 | 2194.973 | 20.16 | COL1A2[932-952] | NDGPpGRDGQpGHKGERGYpG | 631 (772) | 73 | 79 (146) | 27 | 0.037 |
| 19328 | 2276.015 | 27.19 | COL1A1[819-844]*O(2) | ADGQPGAKGEpGDAGAKGDAGPpGPA | 7267 (5483) | 100 | 1057 (2591) | 64 | 0.001 |
| 19514 | 2292.022 | 27.22 | COL1A1[819-844]*O(3) | ADGQpGAKGEpGDAGAKGDAGPpGPA | 8890 (5213) | 100 | 1546 (3044) | 82 | 0.001 |
| 20067 | 2339.008 | 33.97 | COL1A1[698-725] | GANGApGNDGAKGDAGApGApGSQGApG | 2352 (1259) | 100 | 458 (733) | 45 | 0.001 |
| 22989 | 2644.219 | 21.18 | COL1A1[224-250] | GPpGKNGDDGEAGKpGRpGERGPpGPQ | 808 (820) | 73 | 162 (453) | 27 | 0.0078 |
| 25349 | 2912.174 | 25.48 | — | — | 1119 (589) | 100 | 106 (239) | 27 | 0.001 |
- All 17 peptides, of which 15 are identified by their amino acid sequence and are derived from collagen chains, demonstrate reduced abundance after BP therapy. p-values were calculated based on pairwise comparison using the Wilcoxon signed rank test.
- Abbreviations: AA, amino acid; BP, bisphosphonate; Freq, frequency of occurrence (%); KTR, kidney transplant recipient; Mean Amp, mean signal amplitude (ion counts); N, number of patients; SD, standard deviation.
- a Peptide identification number.
- b First and last amino acid positions within the protein's linear sequence, according to UniProt Knowledge Base numbering. In cases where peptides only differ in the extent of their proline hydroxylation, the number of hydroxyprolines in the peptide's sequence is also indicated, for example, ‘*O(2)’ means two hydroxyprolines.
- c Lower case p indicates hydroxyproline.
- d acc. Wilcoxon signed rank test.
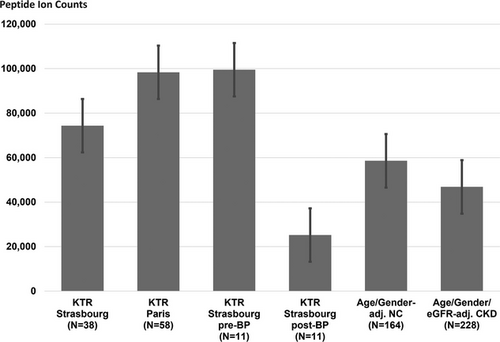
We compared the excretion levels of the 17 bisphosphonate-sensitive peptides of our KTR patient groups with those found in age- and gender-matched hindividuals, as well as age-, gender-, and eGFR-matched chronic kidney disease patients. Since we were unable to match normal individuals for eGFR aswell, we included CKD patients into this comparative analysis in order to investigate the impact of eGFR on the peptide's excretion levels. As presented in Figure 4, overall excretion of these 17 peptides is significantly higher in KTR patients not treated with bisphosphonate compared to normal individuals but also CKD patients. After bisphosphonate therapy the regulation direction is reversed, now being significantly lower in bisphosphonate treated KTR patients compared to both the normal individuals and CKD patient reference groups.
3.5 Involvement of CTX-associated urinary peptides in CKD
To investigate if the 82 CTX-associated urinary peptides are also associated with CKD, these peptides were compared with the peptides (N = 273) contained in the CKD273 urinary classifier, which was developed on a large set of CKD patients and disease-matched controls using the same CE-MS peptide annotation database [23]. By this comparison it was found that 17 of the bone metabolism–related markers are also included in the CKD273 model. The expected chance overlap was calculated to be 2.3%, since 82 differentially regulated peptides were identified in a data space of 3555 urinary peptides. Correlating this to the observed overlap of 6.2% (17 out of 273) yielded a significant difference compared to the random condition (p = 0.001, Fisher's exact test).
4 DISCUSSION
Chronic kidney disease (CKD) is associated with mineral bone disease, a group of conditions including varying degrees of osteodystrophy, osteomalacia, adynamic bone, and abnormal bone mineralization [47, 48]. While several metabolic disorders improve after renal transplantation, impaired bone status can persist or may even worsen [49]. The mechanisms of this post-transplantation impairment are numerous and complex. The persistence of hyperparathyroidism and the initiation of anti-rejection treatment, mostly including corticosteroids, are important causes. Bone dynamics are here marked by an imbalance between the activity of bone resorption and bone formation causing a reduction in bone mineral density (BMD). The reduction in BMD predominates in the first year after transplantation [50] and contributes to a threefold increase in fracture risk [51].
Markers of bone remodeling are used to monitor patients at risk of osteoporosis and include markers of bone formation and bone resorption and both categories are further subdivided into non-collagen derived and collagen-derived markers. The integration of these markers into clinical dashboards has been adopted by numerous transplantation centers including our own, to enhance patient monitoring. [52, 53]. Serum levels of the C-terminal crosslinked telopeptide of collagen I (CTX), a resorption marker, have been shown to be associated with fracture risk in numerous studies [54-57].
In the present study we screened the urinary peptidome of KTRs to identify peptides correlated with CTX. While slowly progressing bone mineral density changes in the general population including those at risk for osteoporosis are best described by BMD measurements, CTX better reflects the highly dynamic process of bone remodeling as observed in kidney transplant recipients during the first year of transplantation. Kidney transplantation occurs after years of gradual status deterioration due to dialysis, leading to substantial changes in parathyroid hormone status. There is also a sudden improvement in renal function folowing transplantation, which is further enhanced by the administration of steroids as part of post–transplantation care. These factors mmay negatively impact the correlation between bone–derived peptide markers in urine and bone mineral density measurements. In consequence, this leads to dynamic changes in peptide clearance rates without having immediate effect on bone mineral density. Since the aim of this study was to identify sensitive markers for risk stratification and therapy monitoring, specific focus was laid on the highly dynamic process of bone remodeling rather than on slower progressing bone mineral density changes.
Using conservative statistics, we detected a set of 82 peptides meeting the criterion of demonstrating significant association with CTX in two independent groups of KTRs. Most of these peptides are fragments of COL1A1, the most abundant protein in bone [58]. The data indicate that CTX is not the only COL1A1 fragment that is released into the circulation as a result of bone metabolism. Whether they are freely filtered by the glomerulus, actively secreted by the tubule, or released into urine by exosome transport remains to be elucidated. However, one conceivable scenario would be that these peptides are freely filtered and, as a result of proline hydroxylation, are excluded from tubular reabsorption. Our results provide insight into the previously observed increase in urinary hydroxyproline in mineral bone disease. This increase is likely associated with an increase in urinary collagen peptides due to heightened collagen turnover in bone. The vast majority of urinary collagen peptides carries proline hydroxylation, which is generally not observed in peptides originating from any other protein. As a result of increase in collagen peptides, total urinary hydroxyproline is also increased.
The 82 peptides being correlated with the bone resorption marker CTX were also correlated with osteocalcin and BAP, reported to be bone formation markers [59, 60]. Although not reaching statistical significance, a positive correlation with a Spearman rho value of 0.335 (p = 0.09) between CTX and BAP was found in the Strasbourg cohort and of 0.297 (p = 0.07) between CTX and osteocalcin in the Paris cohort of KTR patients. The finding that patients with a high bone turnover have high values of both markers for bone formation and markers for bone resorption was reported earlier for late postmenopausal women during osteoporosis [61], elderly men with type-2 diabetes mellitus [62] and patients with systemic lupus erythematosus [63]. Treatment in kidney transplant recipients with bisphosphonates was reported to decrease both resorption and formation serum markers, with decrease being more pronounced in case of the former [64]. This was also observed in our bisphosphonate study cohort, where bisphosphonate treatment resulted in a 2-fold decrease in BAP and a 5.5-fold decrease in CTX levels.
While all the COL1A1 peptides were positively correlated to CTX, two out of the three peptides showing negative correlation to CTX were fragments of COL3A1. Since COL3A1 serves as intrinsic growth stimulus in osteoblasts leading to its tight regulation on the RNA level [65], this negative correlation to CTX might be attributed to reduced expression of COL3A1 by osteoblasts during the process of increased bone resorption.
Aside from collagen-derived peptides, there were only four peptides derived from non-collagenous proteins. These are cadherin-1 (CADH1), α−1-antitrypsin (A1AT), gelsolin (GSN), and EMI domain-containing protein 1 (EMID1). CADH1 and A1AT are both connected in the literature to osteoporosis. CADH1 elicits osteoblast differentiation by its ability to modulate the activity of Smurf1, a ubiquitin ligase in the bone morphogenetic protein (BMP) pathway [66]. Akbar et al. [67] found that A1AT it is able to reduce bone loss in an ovariectomized mouse model by inhibiting osteoclast-associated mineral resorption. GSN is involved in the podosome assembly, a process used by several cell types including osteoclasts for matrix attachment and cytoskeletal reshaping [68]. EMID1 has been shown to be an osteoblast marker in bone-forming metastases of prostate cancer [69]. The peptide itself is derived from the collagen-like domain of EMID1 and has striking sequence similarities with other collagen peptides of our set, including 3 GPP amino acid sequence repeats.
By alignment of the identified COL1A1 peptides to the linear sequence of the full-length protein, we found that most of the CTX-associated COL1A1 peptides originate from certain specific regions, which points towards the involvement of endoproteinases. Therefore, we searched for putative proteases in the MEROPS proteolytic database and discovered that the most frequent cleavage sites for the CTX-associated urinary peptides in the COL1A1 linear sequence match to the consensus sequence of Cathepsin K (CTSK) and MMP9. Involvement of CTSK is further supported by the finding that for the gelsolin-derived peptide 605-WVGTGASEAEKTGAQEL-621, as one non-COL1A1 CTX-associated urinary peptide we identified in our study, cleavage by CTSK between leucin in position 604 and tryptophane in position 605 is reported by Vizovišek et al.[70]. Cathepsin K is a cysteine protease which is known to play a pivotal role in osteoclast-mediated bone resorption [71]. It degrades collagen I in the organic matrix of bone [72] and has become a drug target in the treatment of osteoporosis [73]. COL1A1 is also a known substrate of MMP9 a protease required for bone repair following fractures and involved in bone resorption in combination with other matrix metalloproteinases [74, 75]. resorption. The connection of the identified urinary peptides to CSTK and MMP9 collectively provides further evidence that the selected peptides were generated through osteoclast–mediated bone resorption.
We tested the effect of bisphosphonates on the urinary peptides of interest. Bisphosphonates are stable analogues of inorganic pyrophosphate that exhibit strong affinity for the bone mineral matrix and inhibit the functioning of osteoclasts [76]. This process results in an overall reduction of bone turnover and prevents bone loss, thereby reducing the incidence of osteoporotic fractures. Because we had strong arguments in favor of an osseous origin of the urinary CTX-associated peptides, we tried to replicate the results of a seminal experiment from 1971 showing the effect of bisphosphonates on urinary output of HyP-containing peptides [77]. In that study sodium etidronate was administered orally to four patients with Paget's disease. As a result, previously elevated serum levels of alkaline phosphatase and both plasma and urinary HyP decreased by more than half after 3 months of treatment. Urinary HyP was present in the form of peptides whose sequence remained unknown. These results suggested that the reduction in bone resorption following treatment with bisphosphonates was accompanied by a decrease in collagen fragments (possibly derived from resorption) in the blood and urine. According to a similar study scheme, we compared the urinary levels of the 82 CTX-associated peptides before and after treatment with oral bisphosphonates administered during several months to KTRs at risk of fractures. As we expected, the abundance of 14 HyP-containing type I collagen fragments (and three peptides of unknown sequence) significantly and sharply declined (on average by 82%) following bisphosphonate treatment.
Urine is known to be enriched with collagen peptides [78]. In blood other proteins and peptide fragments thereof are more prominent such as albumin, fibrinogen, α−1-antitrypsin, apolipoproteins, complement factors [79]. It is striking that 36 of the 59 sequenced urinary bone markers could also be identified in the human plasma. Compared to the generally low overlap in peptides in plasma and urine of less than 5% as was determined in this study, but also by others [26, 46], the identified urinary peptides associated with bone remodeling in the KTR study groups are significantly enriched providing strong evidence for their transport in blood to the kidneys after their release from bone tissue. This appears to reflect a highly dynamic process that is particularly sensitive to certain disease states and treatment modalities. This sensitivity is evident from the substantial difference in the number of identified urinary peptide markers correlated with CTX blood levels between different study groups. For example, in the more general KTR group from Paris, 590 CTX–correlated peptides were identified, compared to only 92 in the highly selective BKVN group from Strasbourg. Additional studies on clearly defined KTR patient groups in respect to their disease states and treatment regimens are required to answer this question in more clarity.
Our study has limitations. CTX was selected as reference for the peptide associations since it is currently the most specific marker to monitor response to bisphosphonate treatment [80]. Bisphosphonates are well known to reduce the risk of bone fractures in KTR patients and are effective to reduce CTX levels. In our bisphosphonate treatment cohort on average a 5.5-fold reduction in CTX serum levels was observed. Comparative biomarker studies must be conducted on selected KTR cohorts with clearly defined osteoprotective post–transplantation treatment regimens to test the potential added value of some of the identified peptides over CTX, especially when integrated into a peptide marker model. These studies should use the percent change in bone mineral density during long–term follow–up as a surrogate endpoint for the risk of fractures. Moreover, the association between CTX and urinary peptides was different in the two KTR cohorts, with a far greater number of peptides correlating with bone data in the Paris cohort than in the Strasbourg cohort (see volcano plots, Figure 1A and 1C), partly due to cohort size and subsequent statistical power differences. This reduced the number of peptides in our peptide set but suggests that there could be far more peptides associated with BR than we report. Moreover, the bisphosphonate study was small with only 11 patients. However, the decrease in the urinary levels of 17 peptides was so dramatic following bisphosphonate treatment that it partly overcame the problem of this limited statistical power. Here too, we hypothesize that more bisphosphonate-sensitive peptides can be identified by increasing the number of study subjects.
Our observations combined with established knowledge on bone remodeling suggest that peptides whose urinary abundance strongly correlates with serum CTX levels originate through bisphosphonate-sensitive bone mechanisms. This hypothesis is in agreement with results from older studies using isotopes to trace the fate of collagen metabolism and showing that HyP-containing peptides from bone are released into the blood stream following bone resorption and finally end up in urine [13, 81–83]. The use of these peptides as biomarkers could become complementary to that of existing bone remodeling markers in this KTR population.
Finally, our study focuses on KTRs but whether the results we observed in this population can be extrapolated to other populations remains to be determined in future studies. The finding that a significant number of the bone remodeling peptides identified in this study are also constituents of the CKD273 classifier, which consists of 273 urinary peptides used to evaluate CKD progression, strongly indicates that these peptides are involved in CKD as well. Multiple underlying molecular processes at the different stages during CKD progression are integrated into the CKD273 classifier [84]. One of these might be chronic kidney disease-mineral and bone disorder (CKD-MBD), which must however be further investigated in studies specifically targeting CKD patients with clinical signs of osteoporosis.
CONFLICT OF INTEREST STATEMENT
Agnieszka Latosinska and Jochen Metzger are employed by Mosaiques Diagnostics. Harald Mischak is the co-founder and co-owner of Mosaiques Diagnostics. All other authors have nothing to disclose. The results presented in this paper have not been published previously in whole or part.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, DM. The data are not publicly available due to their containing information that could compromise the privacy of research participants.




