Identification of Malonylation, Succinylation, and Glutarylation in Serum Proteins of Acute Myocardial Infarction Patients
Abstract
Purpose
To identify protein malonylation, succinylation, and glutarylation in human and rat serum.
Experimental design
Immunoprecipitation coupled with MS/MS is employed to compare the relative abundance of malonylation, succinylation, and glutarylation of serum protein in acute myocardial infarction human and rat.
Results
One hundred thirty and 48 unique malonylated, succinylated, or glutarylated peptides are found in human and rat serum, respectively. Succinylation is the most predominant modification. The most modified protein is albumin. Abundance of serum protein succinylation and glutarylation is significantly (p < 0.05) lower in the peripheral serum of ST-segment elevation myocardial infarction patients compared with healthy volunteers, which is also observed in acute myocardial infarction rats.
Conclusions and clinical relevance
Malonylation, succinylation, and glutarylation widely exist in mammalian serum proteins, and may reveal novel mechanism of acute myocardial infarction.
1 Introduction
Protein posttranslational modifications (PTMs) play important roles in various biological processes such as metabolism,1 DNA damage response,2 insulin signaling,3 immune response,4 and aging.5 Acylation are new types of recently discovered PTMs that use metabolic intermediates as group donors.6 For example, acetylation, which is the most studied and well-known acylation, takes acetyl-CoA as group donor in the reaction.7-9 Malonylation, succinylation, and glutarylation use malonyl-CoA, succinyl-CoA, and glutaryl-CoA as substrate in their reaction.10 Although thousands of lysine sites with a variety of acylation have been identified, their biological roles are recently identified. Acetylation occurring on histones can relax chromatin and promote gene expression, while acetylation on metabolic enzymes regulate flux of metabolism.7-9 Malonylation, succinylation, and glutarylation were also found in histones11 and metabolic enzymes.12-15 Although their roles in histone remain unclear, several group have demonstrate their roles in metabolism regulation.10 Most of the acylation sites were identified on mammalian cell lines, liver tissues, and non-mammalian species. Acylation such as malonylation, succinylation, and glutarylation on other mammalian tissues like serum were seldom studied.
Serum biomarkers are important for diagnosis and prognosis prediction of many diseases. Substances in serum that can be used as biomarkers include proteins, lipids, and other small molecules. PTMs on serum proteins have been used as biomarkers of diseases. For example, glycated hemoglobin is one of the useful biomarkers for diagnosis of type 2 diabetes. In addition, serum proteins suffer from various types of PTMs, such as nitrosylation, cysterinylation, guanylation, acetylation, and methylation.16 The biological role of these PTMs remains to be understood. In the present study, we focus on identifying three different types of PTMs on serum proteins. We found >100 malonylated, succinylated, and glutarylated lysine sites on >20 human serum proteins. Analysis of these acylation lysine sites should contribute to the serum biology.
2 Results
2.1 Identification of Malonylation, Succinylation, and Glutarylation of Human Serum
To identify malonylation, succinylation, and glutarylation on human serum proteins, we collected serum samples from healthy volunteers and atherosclerotic patients. Three types of serum samples were collected. The first sample was from periphery blood of healthy volunteers with normal coronary angiography (Ctl group). The second sample was from aspiration blood of ST-segment elevation myocardial infarction (STEMI aspiration group). The third sample was from periphery blood of STEMI. Digestion of serum proteins, immunoprecipitation of modified peptides, and identification by LC–MS/MS were similar to our previous procedure (Figure S1A, Supporting Information).17 With this approach, 130 unique peptides corresponding to 21 proteins in human serum (Table S2, Supporting Information) and 48 unique peptides corresponding to 14 proteins in rat serum (Table S3, Supporting Information) were identified (Table S1, Supporting Information). We only considered reviewed proteins collected in UniProt database when aligning peptides to proteins. Interestingly, we found that succinylation was the most abundant modification in human serum. Twenty proteins have been found succinylated in at least one lysine site. There were one malonylated and four glutarylated proteins identified in human serum (Table S2, Supporting Information). The results indicate that at least some of human serum proteins can be malonylated, succinylated, and glutarylation.
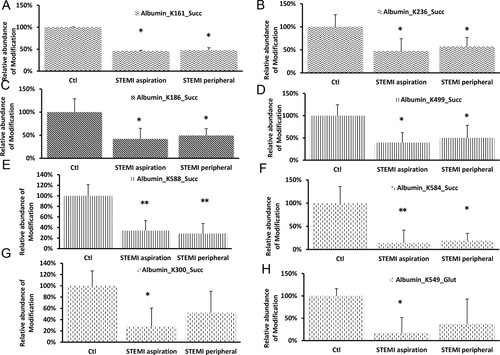
To compare the intensity of these modifications among different samples, we extracted ion chromatograms from the raw data of our LC–MS/MS results. We calculated the area under the peak associated with modified peptides. We found that the intensity of most of the identified peptides was below 107, which suggested a low abundant of modification of the peptides (Table S1, Supporting Information). Several lysine sites on albumin protein (top items in Table S1, Supporting Information) are modified with high intensity. Abundance of modifications such as succinylation of K161, K186, K236, K300, K499, K584, and K588 on human albumin and glutarylation of K549 on albumin was significantly (p < 0.05) lower in the peripheral serum of STEMI patients comparing with control patients (Figure 1).
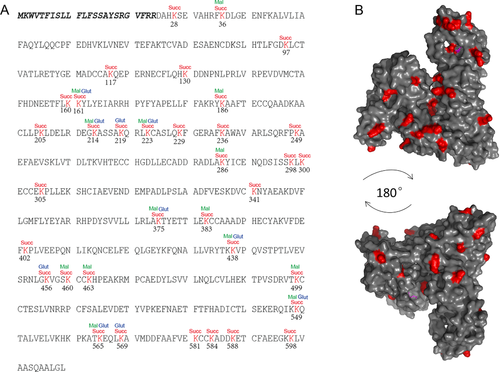
2.2 Albumin is Heavily Malonylated, Succinylated, and Glutarylated
For all the three types of acylation, albumin was heavily modified. There were 35 succinylated, 14 malonylated, and ten glutarylated lysine sites on albumin (Figure 2A). Analysis of the modified lysine sites on albumin protein suggested that some sites were prone to be modified. Malonylation and glutarylation occur on lysine sites that can also be succinylated. Some lysine sites can be modified by all the three types of modification. These sites are lysine 161, 214, 223, 375, 438, 549, and 565. These sites happen to be highly intensive modified sites based on the quantification results. It has been reported that acylation (including malonylation, succinylation, and glutarylation) can occur spontaneously under particular conditions.18 The occurrence of acylation is determined by factors such as exposure of the lysine on proteins, local amino acid sequence of the lysine, and microenvironment of the lysine sites. To understand why some of the lysine sites on albumin were prone to be modified, we analyzed the position of the modified lysine on 3D structure of albumin (PDB:1BJ5). Most of the modified lysine were on surface of the albumin protein (Figure 2B). No particular region was found to be preferred for modification. Altogether, human albumin is susceptible to malonylation, succinylation, and glutarylation on exposed lysine sites.
Clinical Relevance
In the present study, we simultaneously detected serum protein malonylation, succinylation, and glutarylation in samples from STEMI patients and myocardial infarction rats; the results are highly consistent. There are reports that malonylation, succinylation, and glutarylation have potential linkage with cardiovascular disease such as coronary heart disease and heart failure, which can affect energy metabolism as a result. There is a possibility that serum protein malonylation, succinylation, and glutarylation modification may reveal novel mechanism of acute myocardial infarction.
2.3 Identification of Malonylation, Succinylation, and Glutarylation in Serum from Rat
To confirm the existence of these modifications in mammalian serum, we determined malonylation, succinylation, and glutarylation in the serum from acute myocardial infarction (AMI) as well as sham surgery (Ctl) rats. Using the same methods, we identified 48 peptides on 14 proteins with at least one of the three types of modifications (Tables S2 and S1, Supporting Information). Similarly, albumin is the highly intensive and most frequently modified protein. Modifications on proteins in immunoglobulin families were also identified. The results suggest that malonylation, succinylation, and glutarylation might be conservative in mammalian serum.
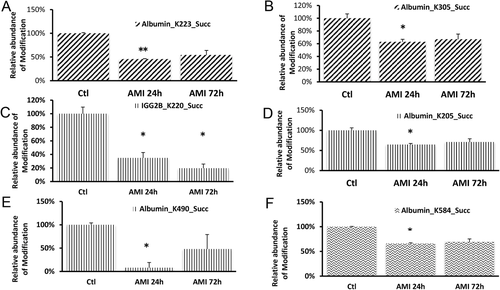
Quantification analysis of modified peptides showed that succinylation of several lysine sites on albumin decreased significantly after 24 and 72 h induction of myocardial infarction (top items of the list in Table S1, Supporting Information). Abundance of modifications such as succinylation of K205, K305, K223, K490, and K584 on rat albumin protein and succinylation of K220 on rat IGG2B protein was significantly (p < 0.05) lower in the peripheral serum of AMI rats (24 h after operation) comparing with sham surgery rats (Figure 3).
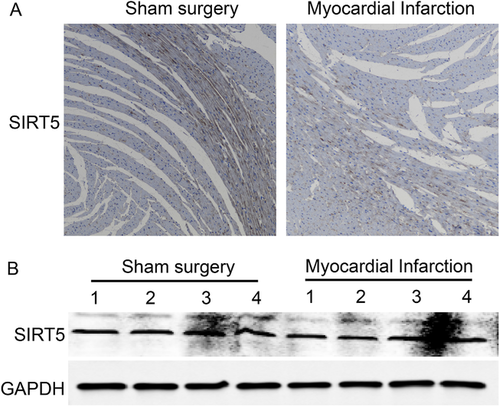
SIRT5 was found to be the sole enzyme for all three types of modification.10 We detected expression of SIRT5 in heart tissues 24 h after myocardial infarction. The level of SIRT5 was comparable before and after myocardial infarction as shown by immunohistochemistry (Figure 3A) and Western blotting (Figure 3B). Interestingly, high level of SIRT5 was frequently observed in the middle of myocardium. Myocardial infarction might not affect expression of SIRT5 in rat heart.
3 Discussion
In the present study, we discovered that some serum proteins could be malonylated, succinylated, and glutarylated in both human and rat samples. Albumin is the most abundant protein in blood. It constitutes about half of the serum protein. Other proteins identified to be modified in our study are also high abundant serum proteins, such as Ig family protein, serotransferrin, and α-2-macroglobulin. Since there was no abundant serum protein depletion step in our experimental procedure, it was reasonable to identify the modification of those proteins on priority. Although there are only 21 proteins identified to be malonylated, succinylated, or glutarylated in human serum, it could not be excluded that other low abundant serum proteins also suffer from the same type of modification. Signals from low abundant serum proteins can be overwhelmed by that of high abundant protein when detected together by the MS. Serum is the typical sample with the 20 most abundant proteins accounting for over 90% of the total protein quality. Under this situation, identification of modification on low abundant serum protein remains of great challenge.
We only identified malonylation, succinylation, and glutarylation in serum. It should be kept in mind that serum proteins are susceptible to various types of modification. Where the modifications occur? For malonylation, succinylation, and glutarylation, they are speculated to happen in liver, because there are high concentrations of malonyl-, succinyl-, and glutaryl-CoA, which are essential materials for modification in liver. It is also deduced that the modified form of a protein only constitute a small fraction of that protein in serum, especially for malonylation, succinylation, and glutarylation. Most of the time, they are spontaneously modified in liver and secreted into blood. Our results showed the level of malonylation, succinylation, and glutarylation of serum proteins was reduced in acute myocardial infarction, but SIRT5 level was unchanged after acute myocardial infarction. As there are reports that SIRT5 is abundant and actively modifies malonylation, succinylation, and glutarylation in liver,19 and liver metabolism could be modified during acute myocardial infarction,20 it is possible that the reduction of serum protein malonylation, succinylation, and glutarylation happens in liver.
We simultaneously detected the three types of modifications in samples from STEMI patients and myocardial infarction rats; the results were highly consistent. There are reports that malonylation, succinylation, and glutarylation have potential linkage with cardiovascular disease such as coronary heart disease and heart failure, which can affect energy metabolism as a result.14, 21, 22 There is a possibility that serum protein malonylation, succinylation, and glutarylation modification may reveal novel mechanism of acute myocardial infarction.
4 Experimental Section
4.1 Study Population
This is a mono-center retrospective case-control study. The study subjects were selected from the Department of Cardiology, Beijing Tsinghua Changgung Hospital (Beijing, China). In total, 12 patients diagnosed with STEMI between September 1, 2017 and December 1, 2018 were recruited as AMI group (STEMI). Twelve gender- and age-matched healthy subjects with no past medical history and normal coronary angiography were enrolled as control group (Ctl). STEMI was diagnosed and treated according to the 2004 American College of Cardiology/American Heart Association guidelines.23 All patients arrived at hospital and underwent primary PCI within 12 h after the onset of symptoms, and the thrombolysis in myocardial infarction (TIMI) flow grade was ≥2 subsequent to PCI. Exclusion criteria included patients with an age of >80 years, cardiogenic shock at admission, TIMI flow grade <2 following PCI, previous history of myocardial infarction, significant valvular heart disease, peripheral vascular disease, chronic heart failure, chronic inflammatory diseases, significant kidney or hepatic diseases, cancer, and administration of glycoprotein IIb/IIIa inhibitors. The present study was approved by the ethics review boards of Beijing Tsinghua Changgung Hospital (Beijing, China). All patients provided written informed consent for participation in the study.
4.2 Blood Collection and Preparation
STEMI patients were treated with a loading dose of aspirin (300 mg; Bayer AG, Leverkusen, Germany) and clopidogrel (600 mg; Sanofi, Paris, France) at admission, and a bolus of 100 IU kg–1 heparin prior to emergency PCI. The PCI procedure was performed according to ACC/AHA/SCAI guidelines.24 During PCI, blood was collected from radial artery (peripheral blood) before the procedure and from the culprit coronary lesion with an aspiration catheter (aspiration blood) as previously described.25 Ctl group was treated with a loading dose of aspirin (300 mg; Bayer AG, Leverkusen, Germany) and clopidogrel (300 mg; Sanofi, Paris, France) at admission, and a bolus of 100 IU kg–1 heparin prior to coronary angiography. Peripheral blood was collected immediately prior to angiography through radial artery. Blood samples were drawn by venipuncture into vacationer tubes containing heparin lithium. Plasma was isolated from whole blood by centrifugation, with three samples pooled from STEMI and Ctl groups and was kept in –80 °C until experiment.
4.3 Animal Experiments
A total of 24 male Wistar rats (weight, 220–240 g) were purchased from Vital River Co, Ltd (Beijing, China) and kept under standard animal room conditions (temperature 21 ± 1 °C; humidity 55–60%) with food and water continuously available, which was in accordance with the guidelines of the Animal Management Regulations of the People's Republic of China (revised 2017). All efforts were made to minimize animal suffering. Induction of rat myocardial infarction was induced by coronary artery ligation for 2 h. Midline sternotomy was performed, followed by pericardiotomy. The left anterior descending artery, close to its origin and ≈3 mm away from the left coronary ostium, was ligated with 7-0 Prolene (Ethicon, Inc, Somerville, NJ), and a slipknot was tied to establish reversible coronary artery occlusion, as described previously. In total, 12 rats received myocardial infarction surgery (AMI) and six rats received sham surgery (Ctl).
Peripheral blood was collected from six AMI rats after 24 h of surgery and from six AMI rats after 72 h of surgery, and afterward, they were sacrificed. For Ctl rats, peripheral blood was collected 24 and 72 h after surgery and the rats were sacrificed after 24 h. Plasma was isolated from whole blood by centrifugation, and three samples each from AMI and Ctl groups were pooled and kept in –80 °C until experiment. Rat hearts from AMI and Ctl groups were sectioned. Morphological characters were analyzed via hematoxylin and eosin staining. Sirt5 expression was detected by immunohistochemical staining with anti–Sirt5 antibody (catalog number 15122-1-AP, Rosemont, IL, USA). Slides were visualized under a bright field microscope (Leica DM2500, Tokyo, Japan), and pictures of the entire slice were taken with identical exposure settings for all sections and analyzed using image analysis software (ImageJ, National Institutes of Health, Bethesda, MD, USA).
4.4 Serum Digestion and Affinity Enrichment of Malonylated, Succinylated, and Glutarylated Peptides
For protein digestion, 100 µL serum was resolved in 8 m urea with 50 mm Tris-HCl, pH 8.0. The samples were then reduced by 10 mm dithiothreitol (DTT), alkylated by 10 mm iodoacetamide (45 min at RT in the dark), and digested with sequencing-grade modified trypsin (Promega) with a substrate ratio of 1:50 (w/w) overnight at 37 °C. After digestion, the samples were desalted and eluted with acetonitrile. The eluted peptides were dried and resolubilized in NETN buffer (50 mm Tris–Cl, pH 8.0, 0.5% NP-40, 100 mm NaCl, and 1 mm EDTA). Anti-malonyllysine, anti-succinyllysine, and anti-glutaryllysine antibody-conjugated resins were mixed and added into the digested peptides mixtures. The tubes were then incubated at 4 °C for 12 h with gentle shaking. Then the samples were centrifuged at the speed of 2000 × g for 2 min. The supernatant was discarded. The beads were washed two times with NETN buffer, once with ETN buffer (50 mm Tris–Cl, pH 8.0, 100 mm NaCl, and 1 mm EDTA), and once with purified water. The bound peptides were eluted by 0.1% trifluoroacetic acid (TFA) and dried using the SpeedVac. The dried peptides were dissolved in 0.1% FA and desalted with C18 ZipTips.Serum protein digestion and immunoprecipitation.
4.5 MS Identification
LC–MS/MS analyses were performed as previously described.12 Additional criteria used here were: the cysteine carbamidomethylation were specified as fixed modifications. The methionine oxidation and lysine malonylation (+86.0004 Da), lysine succinylation (+100.0160 Da), or lysine glutarylation (+114.0317) were chosen as variable modifications.
4.6 Statistical Analysis
Descriptive data are presented as the mean ± SD for continuous variables. The clinical and laboratory data were analyzed with an independent t-test or one-way analysis of variance for continuous variables. Significance was assumed at a two-sided p-value < 0.05. Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Acknowledgements
B.Z. and Y.D. contributed equally to this work. This project was supported by Grant 81970299(to B. Zhou), 31671175, 31771257 from the National Natural Science Foundation of China, Grant 2017YFA0205501 from the National Key R&D Program of China, and Grant XDA12030207 from the Strategic Priority Research Programs (Category A) of the Chinese Academy of Sciences.
Conflict of Interest
The authors declare no conflict of interest.