Effects of methotrexate and salazosulfapyridine on protein profiles of exosomes derived from a human synovial sarcoma cell line of SW982
Abstract
Purpose
To elucidate effects of salazosulfapyridine (SASP) and methotrexate (MTX), major anti-rheumatic drugs, on exosomes derived from SW982 of a human synovial sarcoma cell line.
Experimental design
SW982 was treated with SASP and/or MTX under interleukin-1β (IL-1β)-treated or nontreated conditions. Exosomes were isolated from the culture media, and exosomal proteome was analyzed by 2D-DIGE. Protein spots whose intensity was significantly altered by the above treatments were identified by MS.
Results
Two hundred ninety-four protein spots were detected in the exosome preparations by 2D-DIGE. Compared to the nontreated cells, SASP-, MTX-, and (SASP + MTX)-treated cells displayed 8, 10, and 21 exosomal protein spots with more than ±2.0-fold intensity differences (p < 0.05), respectively. Similarly, the IL-1β-treated cells displayed 58 exosomal protein spots with more than ±1.5-fold intensity differences (p < 0.05). In about half of the 58 spots, the IL-1β-induced intensity changes were suppressed by simultaneous addition of SASP and/or MTX. Most of the identified proteins were immunity- or anti-oxidation-related proteins.
Conclusions and clinical relevance
The SASP and/or MTX treatments altered the protein profiles of exosomes and suppressed the effects of IL-1β on the exosomal proteome. Exosomes may play roles in the actions of these anti-rheumatic drugs.
Abbreviations
-
- DMARD
-
- disease-modifying antirheumatic drug
-
- ESCRT
-
- endosomal sorting complex required for transport
-
- IL-1

- IL-1
-
- interleukin-1β
-
- MMP
-
- metalloproteinase
-
- MTX
-
- methotrexate
-
- MVE
-
- multivesicular endosome
-
- RA
-
- rheumatoid arthritis
-
- SASP
-
- salazosulfapyridine
-
- SP
-
- sulfapyridine
-
- TLR
-
- Toll-like receptor
1 Introduction
Clinical Relevance
Exosomes are membranous extracellular vesicles with diameters of 30–100 nm, which have recently attracted much attention for their various immunomodulatory functions. Since rheumatoid arthritis (RA), characterized by systemic synovial inflammation, is associated with various immunological disorders, exosomes are presumed to be related to the pathophysiology and treatment of RA. However, roles of exosomes in the pathophysiology and treatment of RA have been poorly understood. This study showed that salazosulfapyridine (SASP) and methotrexate (MTX) of representative anti-rheumatic drugs greatly affected protein profiles of exosomes derived from SW982 of a synovial sarcoma cell line. Our findings would help understanding of roles of exosomes in the pathogenesis and treatment of RA.
Exosomes are membranous extracellular vesicles with diameters of 30–100 nm 1. They are initially formed as intracellular vesicles of multivesicular endosomes (MVEs) and then are secreted from cells by fusion of MVEs with plasma membrane 2. Functionally, exosomes have been reported to play roles in various immune reactions 1, 3, 4, intercellular communication 5, and transfer of RNAs and proteins 6.
Rheumatoid arthritis (RA) is a systemic autoimmune disorder characterized by synovial inflammation and hyperplasia. Chronic inflammation in multiple joints sometimes leads to severe joint destruction. Methotrexate (MTX) and salazosulfapyridine (SASP) are oral disease-modifying anti-rheumatic drugs (DMARDs) used widely in the world. MTX, a folate antagonist, is one of the most effective DMARDs. MTX prevents de novo pyrimidine and purine syntheses, and consequently inhibits proliferation of lymphocytes 7. Further, MTX suppresses synthesis of synovial metalloproteinases (MMPs) 8. SASP, consisting of sulfapyridine (SP) and 5-aminosalicylic acid, is also commonly used in the treatment of RA. Action mechanisms of SASP include inhibition of dendritic cell maturation 9, suppression of IL-2 production by T cells 10, and inhibition of synovial cell proliferation 11. Clinically, MTX and SASP are often used in combination, since the combination therapy was found more effective than a mono-therapy with MTX or SASP 12.
Since RA is associated with various immunological disorders and exosomes are involved in various immunoreactions, exosomes are presumed to play roles in the pathophysiology and treatment of RA. For example, exosomes derived from synovial fibroblasts were reported to contain a membrane form of tumor necrosis factor-α 13. It was also reported that exosomes from interleukin-1β (IL-1β)-stimulated synovial fibroblasts upregulated MMP-13 expression in chondrocytes 14. As for effects of DMARDs on exosomes, no report has been available to our knowledge, although many studies have focused on effects of DMARDs on synovial cells themselves.
Based on these backgrounds, we here tried to analyze effects of MTX and SASP on the protein profiles of exosomes derived from a synovial sarcoma cell line of SW982 under IL-1β-stimulated or nonstimulated conditions by 2D-DIGE and subsequent MS analysis. SW982 has been frequently used as a substitute of synovial cells in studies on arthritis, although synovial sarcoma including SW982 is classified as a miscellaneous tumor of uncertain histological origin 15-17. We found that the treatments with SASP and/or MTX altered the protein profiles of exosomes and that the treatments with SASP, MTX, and in particular, the combination of SASP and MTX suppressed a part of the protein profile changes induced by IL-1β. Our data would promote understanding of roles of exosomes in the pathogenesis and treatments of RA.
2 Materials and methods
2.1 Reagent preparation, cell culture, and stimulation with drugs
A synovial sarcoma cell line of SW982 was obtained from American Type Culture Collection (Manassas, Va., USA, Cell number HTB-93). SW982 cells were cultured and stimulated as described in the Supporting Information. Preparation of SASP, MTX, and IL-1β is also described in the Supporting Information. The cells were treated with SASP, MTX, SASP + MTX, IL-1β, IL-1β + SASP, IL-1β + MTX, and IL-1β + SASP + MTX in triplicate for 48 h. Nontreated cells were used as a negative control. Then supernatants of the culture were collected for preparation of exosomes. On the other hand, the cells were washed in PBS and proteins were extracted into a cell lysis buffer (30 mM Tris-HCl pH 8.0, 7 M Urea, 2 M Thiourea, 4% CHAPS). The resulting cell lysates were used for cellular protein analyses.
2.2 Preparation of cellular and exosomal proteins, transmission electron microscopic analysis, and Western blotting for exosome markers
For preparation of exosomal proteins, exosomes were isolated from the collected supernatants using ExoQuick-TC® (System Bioscience, Mountain View, CA). Briefly, the supernatants were centrifuged at 3000 × g for 15 min at 4℃ to remove cell debris. Then, the resulting supernatants were subjected to 0.22-μm vacuum filters to remove impurities such as apoptotic bodies, and then an appropriate volume of ExoQuick-TC was added, according to the manufacturer's instructions. The Exoquick-TC/supernatant mixtures were refrigerated at 4℃ overnight, and then were centrifuged at 1500 × g for 30 min at 4℃. The pellets consisting of exosomes were collected and dissolved in the cell lysis buffer. The protein concentrations of the resulting exosome lysates were determined by the Bradford method. In some cases, a part of the exosome pellets was resuspended in PBS for transmission electron microscopic analysis. Preparation of the cellular proteins, transmission electron microscopic analysis, and Western blotting for exosome markers are described in the Supporting Information.
2.3 2D-DIGE analysis and protein identification
The exosome lysates and the parent cell lysates were separated by 2D-DIGE, respectively, as described previously 18. The procedures were briefly described in the Supporting Information. Protein identification is also described in the Supporting Information.
2.4 Statistical analysis
Statistical significance was calculated by Student's t-test. A p value of less than 0.05 was considered to be statistically significant.
3 Results
3.1 Isolation of exosomes from culture supernatants of SW982 cells
We stimulated SW982 cells with above drugs and then isolated exosomes from the culture supernatants. As a result, almost all the isolated extracellular vesicles showed diameters of 30–100 nm in transmission electron microscopic analysis, as shown in Fig. 1A. The observed sizes were compatible with the definition of the exosome 1. Furthermore, representative exosomal markers of Alix and HSP70 were detected in lysates of the isolated exosomes by Western blotting, as shown in Fig. 1B. These results indicated the successful isolation of exosomes. In addition, we confirmed the cell viability in these culture conditions was nearly 100% in our preliminary experiments (data not shown).
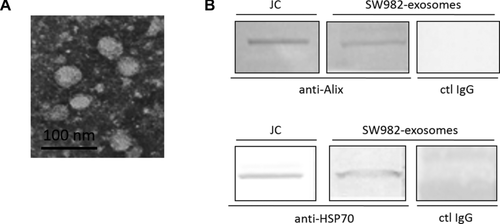
A transmission electron micrograph of the isolated exosomes and detection of exosome markers by Western blotting in the isolated exosomes. (A) A part of the exosomes isolated using Exoquick-TC was subjected to transmission electron microscopic analysis. A representative result is shown. (B) Lysates from the isolated SW982 exosomes were separated by SDS-PAGE and transferred onto nylon membranes. Then Alix and HSP70, representative exosome markers, were detected by their specific antibodies. Whole cell lysates from Jurkat cells were used as a positive control. JC: Jurkat cells, ctl IgG: control IgG.
3.2 Effects of SASP, MTX, and SASP + MTX on the exosomal protein profile
We then extracted proteins from the isolated exosome samples for 2D-DIGE to compare protein profiles among the four groups of SASP-treated, MTX-treated, (SASP + MTX)-treated, and nontreated as a negative control. As a result, the extracted exosomal proteins were separated to make 294 protein spots in total on the gels, as shown in Fig. 2A. Then intensity of the separated protein spots, adjusted by that of the internal control, was compared among the groups. The number of protein spots whose intensity was significantly altered by these drugs is summarized in Table 1. In the SASP-, MTX-, and (SASP + MTX)-treated group, 58, 44, and 42 out of the 294 protein spots showed significantly altered intensity compared to the nontreated group, respectively (p < 0.05) (Table 1). These data demonstrated that the SASP and/or MTX treatments altered the protein profiles of exosomes considerably. Representative spots whose intensity was significantly altered by the drug stimulation are shown in Supporting Information Fig. 1.

2D-DIGE images showing protein profiles of exosomes derived from SW982 cells. (A) SW982 cells were treated with 100 μg/mL SASP alone, 1 μg/mL MTX alone, 100 μg/mL SASP + 1 μg/mL MTX, and with media alone as a negative control. Then exosomes were isolated from the cell culture supernatants. Extracted exosomal proteins were separated by 2D-DIGE. (B) SW982 cells were treated with 100 μg/mL SASP, 1 μg/mL MTX, and 100 μg/mL SASP + 1 μg/mL MTX with the simultaneous addition of 2 ng/mL IL-1β. Then exosomal proteins were analyzed similarly as (A).
| Drug | Number of protein spots with altered intensity (p < 0.05) | Difference (x-fold) | Number of spots |
|---|---|---|---|
| SASP | 58 | 2.0<x | 2 |
| 1.5<x | 8 | ||
| 1.0<x | 16 | ||
| x<1.0 | 42 | ||
| x<1/1.5 | 24 | ||
| x<1/2.0 | 6 | ||
| MTX | 44 | 2.0<x | 8 |
| 1.5<x | 25 | ||
| 1.0<x | 31 | ||
| x<1.0 | 13 | ||
| x<1/1.5 | 11 | ||
| x<1/2.0 | 2 | ||
| SASP + MTX | 42 | 2.0<x | 20 |
| 1.5<x | 28 | ||
| 1.0<x | 33 | ||
| x<1.0 | 9 | ||
| x<1/1.5 | 8 | ||
| x<1/2.0 | 1 |
- In total, 294 protein spots were detected in the exosome preparation. Numbers of the protein spots intensity of which was significantly altered by the drugs are shown (p < 0.05).
Of note, the 58 protein spots affected by SASP and the 44 spots affected by MTX shared only ten spots (Fig. 3A), indicating different pharmacological mechanisms of the two drugs. Again, focusing on the 21 protein spots that changed their intensity more than 2.0-fold or less than 1/2.0-fold (p < 0.05), 17 spots (81.0%) showed significant intensity changes only by the SASP + MTX treatment, not by the SASP treatment alone or by the MTX treatment alone (Fig. 3B). The result indicates additive or synergistic effects of SASP and MTX to form the exosomal protein profile change.

Venn diagrams showing shared protein spots intensity of which was significantly altered by SASP, MTX, and/or IL-1β. (A) Classification of all the exosomal protein spots whose intensity was significantly altered by the SASP and/or MTX treatments. (B) Classification of exosomal protein spots whose intensity was significantly altered more than 2.0-fold or less than 1/2.0-fold by the SASP and/or MTX treatments. (C) The spots whose IL-1β-induced alterations were significantly suppressed by simultaneous addition of SASP and/or MTX.
3.3 Effects of SASP, MTX, and SASP + MTX on the IL-1β-induced changes of the exosomal protein profiles
Next, we investigated whether IL-1β affected the protein profiles of exosomes and if affected, whether SASP and MTX suppressed the effects of IL-1β. For this aim, we treated SW982 cells with SASP, MTX, or SASP + MTX under the IL-1β-stimulated condition and then compared exosomal protein profiles among these groups by 2D-DIGE similarly as above (Fig. 2B). As a result, out of the 294 detected protein spots, 62 spots (21.1%) changed their intensity by the IL-1β treatment (p < 0.05). Fifty-eight out of the 62 spots showed more than 1.5-fold or less than 1/1.5-fold changes (Table 2).
| IL-1β-/nontreated | (IL-1β + SASP and/or MTX)- /IL-1β-treated | |||||
|---|---|---|---|---|---|---|
| Number of spots (p < 0.05, Difference >1.5 or 1/1.5) | Difference | SASP | MTX | SASP + MTX | ||
| 58 | Upregulated | 16 | Upregulated (p < 0.05) | 1 | 1 | 0 |
| Not significant (p ≧ 0.05) | 15 | 15 | 16 | |||
| Downregulated (p < 0.05) | 0 | 0 | 0 | |||
| Downregulated | 42 | Upregulated (p < 0.05) | 4 | 6 | 16 | |
| Not significant (p ≧ 0.05) | 38 | 36 | 26 | |||
| Downregulated (p < 0.05) | 0 | 0 | 0 | |||
Focusing on the 58 protein spots, we then examined whether the SASP and/or MTX treatments suppressed the effects of IL-1β. As a result, the addition of SASP, MTX, SASP + MTX suppressed the IL-1β-induced changes (p < 0.05) in 4 (6.9%), 6 (10.3%), and 16 (27.6%) out of the 58 spots, respectively (Table 2, Fig. 3C, Supporting Information Fig. 2). A representative image of spot ID 464 is shown in Supporting Information Fig. 1.
Of note, the addition of SASP, MTX, and SASP + MTX enhanced the IL-1β-induced changes with significance only in 1 (1.7%), 1 (1.7%), and none (0%) out of the 58 spots, respectively (Table 2). Thus, we concluded that IL-1β affected the exosomal protein profiles greatly and that SASP, MTX, and SASP + MTX, in particular, SASP + MTX suppressed a considerable part of the effects of IL-1β.
3.4 Comparison of protein profile change between exosomes and their parent cells
It would be of interest whether the changes of exosomal protein profiles are simple reflections of those of the parent cells treated by the drugs. To investigate this point, we treated SW982 cells with SASP + MTX, which greatly affected the exosomal protein profiles. Then we detected cellular protein spots with significant intensity changes using 2D-DIGE, similarly as the analysis of exosomal proteins. As a result, we detected 994 protein spots in total (Supporting Information Fig. 3), out of which ten spots changed their intensity more than 1.5-fold or less than 1/1.5-fold (p < 0.05) (Supporting Information Table 1A). Importantly, seven out of the ten spots were not detected in the 2D panels of the exosomal proteins. The intensity of the remaining three spots, which showed 1.77-, 1.86-, and 2.08-fold increases by the SASP + MTX treatment in the whole cell extract, did not show any increase in the exosomal extracts (0.82-, 0.99-, and 0.92-fold change by the SASP + MTX treatment, respectively). On the other hand, in the 2D panels of the exosomal proteins, we detected 36 protein spots whose intensity was changed by SASP + MTX more than 1.5-fold or less than 1/1.5-fold (p < 0.05) (Table 1, Supporting Information Table 1B). Intensity of 32 out of the 36 exosomal spots did not show actual changes (0.92∼1.20-fold) in the 2D panels of SW982 cellular proteins (Supporting Information Table 1B). Three (ID 673, 134, 40) out of the remaining four spots were not detected in the panel from the cell bodies, and remaining one (ID131) spot showed increased intensity in the panels of the cellular proteins in contrast with the decreased intensity in the panels of the exosomal proteins. Taking these data together, we concluded that the protein profile changes of exosomes by the SASP + MTX treatment were distinct from those of their parent cells.
3.5 Identification of exosomal proteins affected by SASP, MTX, and SASP + MTX under the conditions with/without IL-1β-treatment
We tried to identify the 35 exosomal protein spots which showed more than 2.0-fold or less than 1/2.0-fold intensity changes (p < 0.05) by the treatment with SASP and/or MTX as shown in Fig. 3B and Supporting Information Fig. 4. By MS analysis, we identified 17 out of the 35 protein spots as listed in Supporting Information Table 2. They were classified into three groups from the standpoint of biological function: immune system-related proteins of Ras-related protein Rab-7b and GTP-binding nuclear protein Ran; oxidative stress-related proteins of cytoglobin, NAD(P)H dehydrogenase 1, and glyceraldehyde-3-phosphate dehydrogenase; and others. The two immune system-related proteins were upregulated by the MTX treatment and more greatly by the SASP + MTX treatment. The three oxidative stress-related proteins were upregulated by the MTX treatment and more greatly by the SASP + MTX treatment.
Similarly, we tried to identify 21 exosomal protein spots, which showed more than 1.5-fold or less than 1/1.5-fold intensity changes (p < 0.05) by the treatment with IL-1β and the changes were suppressed by the simultaneous addition of SASP and/or MTX as shown in Fig. 3C and Supporting Information Figs. 2 and 4. By MS analysis, we identified 14 out of the 21 protein spots as listed in Supporting Information Table 3. They were also classified into three groups: immune system-related proteins of GTP-binding nuclear protein Ran and SH2 domain containing protein 1B; oxidative stress-related proteins of Clusterin, Thioredoxin-interacting protein, NAD(P)H dehydrogenase 1, and glyceraldehyde-3-phosphate dehydrogenase; and others. The two immune system-related proteins were downregulated by IL-1β treatment and the IL-1β-induced decreases were canceled by MTX or SASP + MTX treatment. The four oxidative stress-related proteins were downregulated by IL-1β treatment and the IL-1β-induced decreases were canceled by SASP or SASP + MTX treatment.
4 Discussion
In this study, we analyzed effects of SASP, MTX, and/or IL-1β on protein profiles of exosomes derived from a human synovial sarcoma cell line of SW982. Our findings are summarized as follows: (1) The SASP and/or MTX treatments altered the protein profiles of exosomes considerably. (2) IL-1β affected the exosome protein profiles greatly and SASP and/or MTX, in particular, SASP + MTX suppressed a considerable part of the effects of IL-1β. (3) In exosomes, the number of detected protein spots by 2D-DIGE was only about one-thirds of that in the cell bodies and protein spots greatly affected by the SASP + MTX treatment were different between the cell bodies and exosomes. (4) The proteins affected by SASP and/or MTX under the conditions with/without IL-1β were mostly those related to the immune system and oxidative stress.
On the first point, this study demonstrated for the first time that anti-rheumatic drugs affected the protein profiles of exosomes, to our knowledge (Table 1). The main body of the affected proteins was different between the SASP- and MTX-treated groups, which would reflect different pharmacological functions of the two drugs. Interestingly, a considerable number of protein spots were affected only by the combination of SASP and MTX. This indicates the additive and/or synergistic effects between SASP and MTX. This is in accordance with the actual clinical use of the two drugs in combination and the fact that the combination therapy is more effective than mono-therapy with only one of the two 12.
On the second point, IL-1β is known to stimulate synovium to produce inflammatory cytokines such as IL-6 and enzymes like cyclooxygenase-2 and MMPs in RA 19. Exosomes from IL-1β-stimulated synovial fibroblasts were reported to upregulate MMP-13 expression in chondrocytes 14. Thereby, IL-1β was expected to affect exosomal proteins. Here, we demonstrated that IL-1β greatly altered the protein profile of exosomes. Furthermore, a considerable part of the IL-1β-induced changes of the exosomal protein profiles was suppressed by the simultaneous treatments with SASP, MTX, and in particular, SASP + MTX (Table 2). Previous reports have revealed that SASP and MTX inhibit the IL-1β-induced inflammation 8, 11. The suppression of the IL-1β-induced changes of exosomal proteins by SASP and/or MTX would be resulted from the blockage of the IL-1β signal pathway in the cell bodies.
On the third point, the changes of exosomal proteins by the SASP + MTX treatment were distinct from those of the cell bodies, in other words, the exosomal protein profiles were not a reflection of whole cell protein profiles. It has been reported that endosomal sorting complex required for transport (ESCRT) machinery are essential for both the formation of MVEs and the sorting of proteins into MVEs 20. A part of the protein-sorting process by ESCRT machinery occurs in an ATP-dependent manner 20, 21. MTX, which increases extracellular adenosine levels by inhibition of AMP deaminase and adenosine deaminase 7, 8, could influence ATP metabolism and affect the sorting process by ESCRT machinery. This point needs to be investigated in the future.
On the last point, we identified several functionally notable exosomal proteins altered by the treatment of SW982 cells with SASP, MTX, and/or IL-1β. Generally, exosomes released to the extracellular environment from parent cells have been reported to affect target cells by several mechanisms such as cellular binding via receptor-ligand interactions, fusing with target cell membrane or internalization by target cells by endocytosis 2. Since exosomes have an ability to transfer specific proteins to target cells to deliver signals 22, functions of exosomes in target cells would be affected by change of exosomal protein profiles.
The identified proteins in this study were classified into three functional groups: immune system-related, oxidative stress-related, and others (Supporting Information Tables 2 and 3). The three immune system-related proteins suppress immune reaction by monocytes, T cells or NK cells 23-26, and the five oxidative stress-related proteins have protective functions from oxidative stress 27-30 or inhibit anti-oxidative molecule 31.
One example of the immune system-related proteins, Ras-related protein Rab-7b is one of Rab proteins that are small guanosine triphosphatases. Rab-7b is localized to late endosomes, lysosomes, and Trans Golgi Network, directing the transport between these compartments 32. It has been reported that Rab-7b promotes degradation of Toll-like receptor (TLR) 4 and TLR9 and downregulates TLR4- and TLR9-mediated inflammatory responses in macrophages 23, 24. It has been also reported that exosomes are taken up more effectively into macrophages than into nonphagocytic cells 33. Together with our result that exosomal Rab-7b was increased by the SASP + MTX treatment, intake of the increased exosomal Rab-7b may provide anti-inflammatory effects in macrophages.
One example of the oxidative stress-related proteins, Cytoglobin, whose expression is elevated in response to oxidant exposure, exerts a protective influence against oxidative stress 27. Several reports have shown that both SASP and MTX induce ROS, which inhibit cell proliferation and induce apoptosis 7, 34. In our study, Cytoglobin in exosomes were increased by the treatment with SASP and MTX. Thereby, exosomes secreted from the SASP + MTX-treated cells may have protective functions from oxidative stress.
Lastly, differences between synovial sarcoma and synovial cells should be noted. The name of “synovial sarcoma” was originally given because it occurs primarily in the vicinity of large joints and resembles developing synovium 35, 36. However, later, significant differences between synovial sarcoma and synovial cells have been evidenced 17, 36, 37. Furthermore, synovial sarcoma has been found to arise in organs without synovial structure 36. Thereby, synovial sarcoma is now classified as a miscellaneous tumor of uncertain histological origin, and is thought to arise from undifferentiated mesenchymal cells 15, 16. On the other hand, SW982 has been frequently used as a substitute of synovial cells in studies on arthritis like RA, probably because it is extremely difficult to establish cell lines from primary synovial cells. Since SW982 and primary synovial cells have been reported to share similar physiological and immunological properties such as cytokine expression and signaling profiles 38, 39, SW982 would still be a useful cell line in studies on arthritis. Considering these circumstances, it should be investigated whether our findings from the experiments using SW982 can be applied to primary synovial cells in the future.
In conclusion, we have demonstrated that SASP and MTX changed exosomal protein profiles and that the drugs suppressed the IL-1β induced change of exosomal protein profiles. Our data implies possible roles of exosomes in the actions of SASP and MTX.
Acknowledgments
We would like to thank Professor M. Takagi and Mr. Y. Natsuki for their help in transmission electron microscopic analysis, Dr. S. Tatsunami for his help in statistical analysis and Ms. M. Katano for her excellent technical assistance.
The authors have declared no conflict of interest.




