Trauma-associated human neutrophil alterations revealed by comparative proteomics profiling
Colour Online: See the article online to view Figs. 1 and 2 in colour.
Abstract
Purpose
Polymorphonuclear neutrophils (PMNs) play an important role in mediating the innate immune response after severe traumatic injury; however, the cellular proteome response to traumatic condition is still largely unknown.
Experimental design
We applied 2D-LC-MS/MS-based shotgun proteomics to perform comparative proteome profiling of human PMNs from severe trauma patients and healthy controls.
Results
A total of 197 out of ∼2500 proteins (being identified with at least two peptides) were observed with significant abundance changes following the injury. The proteomics data were further compared with transcriptomics data for the same genes obtained from an independent patient cohort. The comparison showed that the protein abundance changes for the majority of proteins were consistent with the mRNA abundance changes in terms of directions of changes. Moreover, increased protein secretion was suggested as one of the mechanisms contributing to the observed discrepancy between protein and mRNA abundance changes. Functional analyses of the altered proteins showed that many of these proteins were involved in immune response, protein biosynthesis, protein transport, NRF2-mediated oxidative stress response, the ubiquitin-proteasome system, and apoptosis pathways.
Conclusions and clinical relevance
Our data suggest increased neutrophil activation and inhibited neutrophil apoptosis in response to trauma. The study not only reveals an overall picture of functional neutrophil response to trauma at the proteome level, but also provides a rich proteomics data resource of trauma-associated changes in the neutrophil that will be valuable for further studies of the functions of individual proteins in PMNs.
Abbreviations
-
- MODS
-
- multiple organ dysfunction syndrome
-
- PMN
-
- polymorphonuclear neutrophils
-
- SCX
-
- strong cation exchange
1 Introduction
Polymorphonuclear neutrophils (PMNs) are the most abundant white blood cells that play an important role in innate immune response by providing the first line of defense against microbial threats 1-3. Extensive investigations have been made to elucidate the biological function of PMNs in immune response. The most accepted hypothesis is that PMNs are activated by “damage-associated molecular patterns” 4, such as proinflammatory cytokines, followed by the migrating to infected/injured tissues by chemotaxis, mediating the inflammatory functions through adhesion, rolling, firm adhesion, and transendothelial migration 1, 5, 6. After entering the target tissue, PMNs mediate secondary tissue damage by activation of the NADPH oxidase enzyme complex 7, resulting in a burst of oxygen consumption, generation of excessive ROS, and release of ROS and toxic enzymes 8. The release of chemokines, cytokines, complex antibiotic arsenal, and granule enzymes plays as alarm signals that activate antigen-presenting cells 9. PMNs have been recognized as life-saving decision makers that coach dendritic cells, monocytes, and lymphocytes, and help the organism to decide whether to initiate and maintain an immune response 10.
The activity of PMNs is directly linked to the immune response induced by trauma. As one of the leading causes of human death, trauma is usually associated with over-activation of innate immune responses followed by a subsequent immune suppression, which leads to enhanced susceptibility to infection, sepsis, and multiple organ dysfunction syndrome (MODS) 5, 6, 11. Following extensive basic science research, the roles of various inflammatory signal molecules, the innate immune systems, and its pattern recognition receptors have been recognized for initiating systemic inflammatory response syndrome following traumatic injury 11-13; however, the complex underlying mechanisms for PMN response to trauma, systemic inflammatory response syndrome, and MODS development after trauma are still poorly understood.
Given the importance of PMNs, there has been an increasing interest in applying discovery-oriented genomics and proteomics approaches with the aims of elucidating the underlying signaling pathways of the complex human diseases 14-16. For example, several studies that reported the application of genome-wide expression analyses to circulating blood leukocytes and tissue samples derived from trauma patients have gained insights into the pathways that underlie systemic inflammation in humans 14, 17. Several studies have reported the profiling of the PMN proteome or subproteome by either 2DE or LC-MS-based approaches 18-22, allowing a large number of PMN proteins to be identified. In this study, we performed a comprehensive analysis of the trauma-associated alterations of the neutrophil proteome by comparing the PMNs isolated from the blood samples collected from five severe trauma patients around 4–7 days post injury, when a peak modified Marshall score 23 was observed, and five healthy controls applying 2D-LC-MS/MS. The results revealed 197 proteins showing significant changes in protein abundances following injury, including proteins associated with immune response pathways and functional categories relevant to neutrophil activation and cell survival such as translation initiation factor (EIF) 2 signaling, aminoacyl-tRNA biosynthesis, NRF2-mediated oxidative stress response, the ubiquitin-proteome system, and apoptosis.
2 Materials and methods
2.1 Human neutrophil samples
Blood samples from five controls and five severe trauma patients were collected for the global proteomics analysis. All patients selected had no clinical evidence of infection and were of the same age group, gender, and ethnicity, and a brief summary of patient demographics is provided in Supporting Information Table 1. All blood samples for the proteomics study were collected at peak modified Marshall score 23 (i.e., 4–7 days). Blood was collected with BD Vacutainer brand tubes with EDTA as anticoagulant (Becton Dickinson, NJ, USA). Neutrophils were isolated from individual subjects immediately following blood collection by a ficol-dextran method as previously described 24, 25. Cell pellets were washed with ice-cold PBS and transferred to 1.5 mL Fisherbrand low-retention microcentrifuge tubes (Fisher Scientific) and were frozen at –80°C. Approximately 5–10 million cells were obtained for each subject. The purity of neutrophils was greater than 95% as determined by fluorescence-activated cell sorter light scatter patterns.
Blood samples from an independent cohort of ten controls and 101 severe trauma patients were obtained for the microarray analysis. A brief summary of patient demographics is provided in Supporting Information Table 2. All blood samples were collected into EDTA Vacutainer collection tubes (Becton Dickinson) and run on the microfluidic device to generate mRNA and cell lysate samples for genomics and proteomics studies as described by Kotz et al. 26. A portion of the samples (ten controls and eight trauma subjects) was used for targeted validation for selected candidates identified from the global profiling. All experimental procedures were approved by the Institutional Review Boards of the University of Rochester (Rochester, NY), Pacific Northwest National Laboratory (Richland, WA), Massachusetts General Hospital (Boston, MA), and University of Florida College of Medicine (Gainesville, FL) in accordance with federal regulations.
2.2 Protein digestion and fractionation
PMN cell pellets isolated from individual subjects were lysed in 50% 2,2,2-trifluoroethanol by sonication for 30 s in ice water. Protein concentrations were measured by the BCA assay (Pierce, Rockford, IL, USA) and ∼200–300 μg protein was recovered for each sample. All protein samples were digested with a 2,2,2-trifluoroethanol-based protocol 27. Digested peptide samples were pooled into two trauma pools and two control pools, respectively. Approximately 300 μg total peptides were generated for each pooled sample for subsequent strong cation exchange (SCX) fractionation. Each pooled peptide sample was fractionated into 25 fractions similarly as previously described 28.
2.3 LC-MS/MS analysis
Each of the 25 SCX fractions was further analyzed using a fully automated custom-built capillary HPLC system coupled on-line with an LTQ ion trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA) using an in-house manufactured electro spray ionization interface. The RP capillary column was slurry packed using 3 μm Jupiter C18 particles (Phenomenex, Torrance, CA, USA) in a 75 μm (id) × 65 cm fused silica capillary (Polymicro Technologies, Phoenix, AZ, USA). The mobile phases consisted of A (0.2% acetic acid and 0.05% TFA in water) and B (0.1% TFA in 90% ACN). An exponential gradient was employed during the separation, which started with 100% A gradually increased to 60% B over the course of 100 min. The instrument was operated in data-dependent mode with an m/z range of 400–2000. The ten most abundant ions from the MS analysis were selected for MS/MS analysis using a normalized collision energy setting of 35%. A dynamic exclusion of 1 min was used to avoid repetitive analysis of the same abundant precursor ion. The heated capillary was maintained at 200°C, and the ESI voltage was held at 2.2 kV.
2.4 Proteomics data analysis
LC-MS/MS spectra were analyzed by the SEQUEST algorithm against the human International Protein Index database with a total of 51 252 protein entries (Version 3.19) with the decoy database searching option for assessing false discovery rate (FDR) 29. The search parameters used were 3 Da precursor ion mass tolerance, 1 Da fragment ion mass tolerance, and a maximum of three missed tryptic cleavages. Filtering criteria similar to those previously reported 30 were applied to limit the FDR at the unique peptide level to <1%. Identified proteins were grouped to a nonredundant protein groups using ProteinProphet software 31 and only one protein International Protein Index reference number was used to represent each protein group. Only those proteins or protein groups with two or more unique peptide identifications were considered to be confident protein identifications.
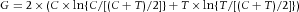
2.5 Microarray analysis
RNA extraction followed a modified commercial protocol (QIAGEN RNeasy Plus) yielding purified total RNA that was analyzed on an Agilent Bioanalyzer 2100 system. cDNA was synthesized with the Ovation Biotin RNA Amplification and Labeling System (NuGEN Technologies) from 20 ng of total RNA as starting material. The labeled cDNA was hybridized onto a custom-designed, 6.9 million–feature Affymetrix human exon-junction array as described by Xu et al. 36. MAExpress 37 was applied to perform quantile normalization of the raw expression data and then EDGE 38 was used to perform time series analysis.
2.6 Targeted verification using SRM
Targeted verification of selected proteins was performed by using SRM with a subset of the same cohort (ten healthy controls and eight trauma patients with blood samples collected at both 4-day and 1-week post injury) that was used for the microarray analyses. Five proteins along two housekeeping proteins identified from shotgun proteomics were selected for targeted quantification (see Supporting Information Table 6). The peptides and SRM transitions was selected and screened as previously described 39. Ten final tryptic peptides of the seven proteins (one or two peptides per protein) were selected for label-free SRM quantification based on their unambiguous detection. At least six transitions of each peptide were monitored for confident identification and accurate peak assignment. The predicted collision energies from Skyline were used for all peptides.
All LC-SRM experiments were performed on a Waters nanoACQUITY UPLC system (Waters Corporation, Milford, MA, USA) directly coupled to a Waters Xevo TQS instrument (Waters Corporation). Peptide separations were performed at a mobile-phase flow rate of 400 nL/min using a BEH (ethylene bridged hybrid) 1.7 μm C18 column (100 μm id × 10 cm, Waters Corporation). The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B). Four microliters of sample (0.25 μg/ μL) were injected for each analysis using a binary gradient of 10–15% B in 3.5 min, 15–25% B in 21 min, 25–38.5% B in 11 min, 38.5–95% B in 1 min, and 95% B for 8 min with a total of ∼44.5 min. The inlet capillary of the mass spectrometer was maintained at 110°C with an ESI voltage of 2.6 kV.
Datasets were analyzed by Skyline software (Version 1.4.0) 40. The peak areas were calculated without any smoothing and the best transition of each peptide was used for relative quantification. In order to eliminate any variations from the amount of sample injections, actin B (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GADPH) were monitored as the internal reference for normalization. Relative abundances for each protein were calculated as the ratio against the average area of the reference peptides from these two proteins. All subsequent data analyses were performed in DAnTE 41, a statistical tool for quantitative analysis.
3 Results
3.1 Human neutrophil proteome coverage
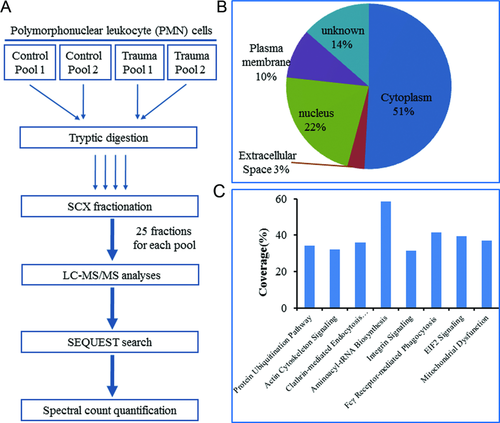
Five trauma patients and five healthy subjects matched by age, sex, and ethnicity were selected for comparative proteomics profiling of enriched PMNs in order to identify proteins with differential abundances in response to traumatic injury. The experimental workflow of comparative profiling using 2D-LC-MS/MS is illustrated in Fig. 1A. Both trauma and control samples were combined into two pools with three subjects in one pool and two subjects in the other pool. All four pooled samples were individually digested by trypsin and fractionated into 25 fractions per pool by SCX and each fraction was analyzed by LC-MS/MS. On average, ∼1 571 000 spectra were acquired and ∼98 000 spectra were confidently identified as peptides. The extensive profiling resulted in confident identification of a total ∼22 880 unique peptides with <1% FDR from all samples, which corresponded to a total of 2536 proteins identified by at least two unique peptides. Gene ontology analysis of the 2536 proteins revealed protein identifications from all major cellular compartments (Fig. 1B). Good coverage of major cell signaling pathways was also observed within this dataset (Fig. 1C). All peptides and proteins identified were listed in Supporting Information Tables 3 and 4.
3.2 Protein abundance alterations in response to trauma
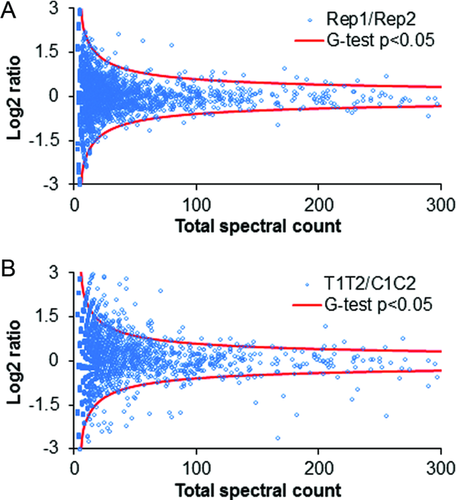
In order to identify protein abundance changes in response to trauma, spectral count 32, 42, 43 as the number of MS/MS spectra identifying a given protein was used as a semiquantitative measure. A G-test was further used to determine the statistical significance. As shown in Fig. 2A, only a few outliers were identified after the significance analysis by G-test (p <0.05, G-value >3.84, df = 1, see Supporting Information Table 5) in the comparison between replicate datasets. However, a relatively large number of proteins passed the significance test in the comparison between trauma datasets and control datasets (Fig. 2B). Furthermore, consistent changes in each pair of comparisons between control and trauma conditions (i.e., control pool 1 vs. trauma pool 1, control pool 1 vs. trauma pool 2, control pool 2 vs. trauma pool 1, control pool 2 vs. trauma pool 2) with a minimum of 40% difference were required for final significant proteins. A total of 197 proteins passed all the filters. Among them, 144 proteins were up-regulated and 53 proteins were down-regulated following trauma.
Five proteins, growth-inhibiting protein 12 (LTF), serine/threonine-protein kinase TAO3 (TAOK3), fatty acid synthase (FASN), eukaryotic initiation factor 4A-1 (EIF4A1), and caspase-1 (CASP1), were selected for validation by targeted SRM-based quantification using a cohort of ten controls and eight trauma subjects with blood collected at two time points. As shown in Fig. 3, the relative abundance changes of four proteins in response to injury were statistically significant. The observed directions of changes are in good agreement with the spectral count data from pooled samples. One of the proteins, caspase-1 (CASP1), was not confidently detected by 1D LC-SRM, presumably due to its low abundance.

3.3 Comparison between protein abundance and gene expression changes
The observed protein abundance changes were further compared to gene expression data from an independent cohort of patients (101 trauma patients and ten controls) where genome-wide expression analyses were performed on enriched PMNs from controls and trauma patients at time points ranging from half day to 28 days post injury. A subset of 174 out of 197 protein candidates from proteomics study was observed with significant changes in their gene expression levels following trauma (FDR < 0.01). We selected the gene expression changes observed for the 4- and 7-day time points post injury for comparing to protein abundance changes for the set of 197 proteins since the samples for proteomics were collected at similar time periods following injury. As shown in Fig. 4A, the directions of abundance changes (up or down) for ∼67% proteins are consistent with those observed changes in mRNA abundance levels.
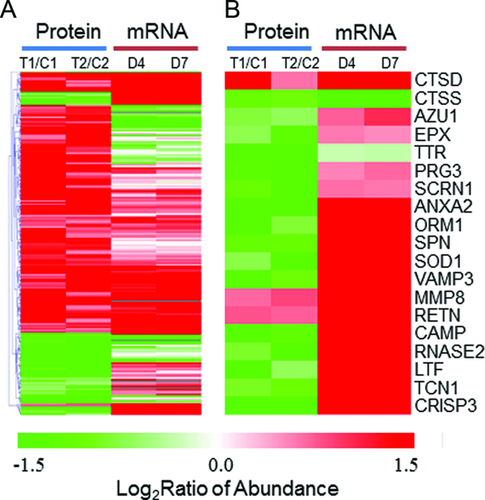
Considering that PMNs are known to secrete multiple antimicrobial products 10, we next examined the concordance between protein abundance and gene expression changes for genes that were known to produce secretory proteins. Figure 4B shows the patterns of protein abundance and gene expression changes for 19 proteins that were reported as potential secretory proteins 19, 22, 44. A number of microbicidal products are known to secrete from PMN granules through a process called degranulation during inflammation 45, which includes leukosialin (gene symbol:SPN), cathepsins (CTSD, CTSS), antibacterial protein Fall-39 (CAMP), growth-inhibiting protein 12 (LTF), and azurocidin (AZU1), all of which play critical roles in bacterial clearance 46, 47. As shown, most of these genes were observed to have a significant increase in their mRNA levels following injury; however, the abundances for most proteins were decreased significantly in the trauma conditions. The observation with significant decreased intracellular protein abundances supports that neutrophils have increased secretion to the extracellular milieu following degranulation 45 in response to the inflammatory conditions. Therefore, these data illustrate the value of an integrated genomics and proteomics data to gain insights into the dynamics of individual gene products by revealing increased protein secretion under trauma conditions.
3.4 Functional analyses of trauma-responsive proteins
The functions and associated pathways of trauma-associated proteins were analyzed based on the Ingenuity Pathway Analysis knowledge base, gene ontology information, and relevant literature. A number of functional categories were revealed to be associated with this set of trauma-responsive proteins, including several known immune response associated pathways, protein biosynthesis, protein transport, NRF2-mediated oxidative stress response, the ubiquitin-proteasome pathway, and apoptosis (see Supporting Information Table 5 for full listed functional annotations).
3.4.1 Known immune response associated pathways
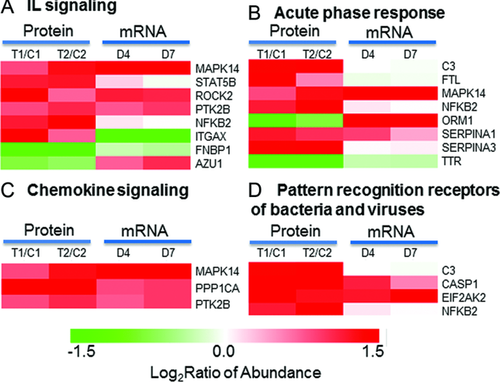
Significant up-regulations were observed for proteins involved in several known immune response associated pathways, as shown in Fig. 5. Proinflammatory cytokines such as interleukins (IL), pattern recognition receptors, and acute-phase response proteins were previously reported to be associated with early activation of PMNs 48 and trauma injury 11-13, 49, 50. Chemokine signaling pathway is associated with the migration of PMNs to target tissues. All these pathways appear to be significantly up-regulated in response to trauma (Fig. 5). The discrepancy of protein abundance data and mRNA data for azurocidin 1 (AZU1) and orosomucoid 1 (ORM1) is potentially related to the increased protein secretion as described previously. The up-regulation of complement component 3 (C3) and integrin alpha X (ITGAX) suggest an increased potential for the adhesion of PMNs to endothelial cells and accumulation of immune cells at the injured tissue 51-53.
3.4.2 Protein biosynthesis
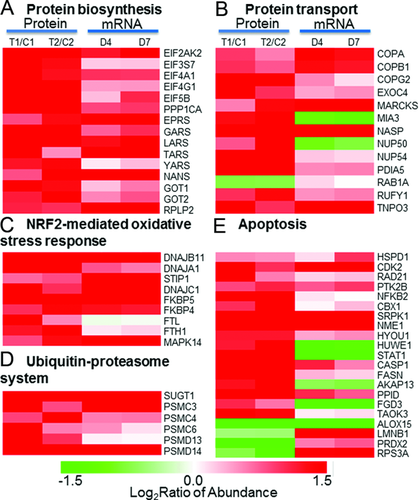
Figure 6A shows significant up-regulation in both protein abundances and gene expression for a set of proteins that are known to be involved in pathways associated with protein biosynthesis. Most of these proteins are associated with eukaryotic EIF2 signaling and aminoacyl-tRNA biosynthesis pathways. EIF2 signaling and aminoacyl-tRNA biosynthesis pathways play essential roles in the initiation and translational phases of protein biosynthesis, respectively. The concordant up-regulation in both mRNA and protein abundance levels for multiple EIFs, tRNA synthases, as well as 40S and 60S ribosomal proteins provides solid evidence that protein biosynthesis is activated in PMNs under trauma conditions. These genomics and proteomics observations of increased protein synthesis are supportive for the observed increased protein secretion (Fig. 4B) and it is also in good agreement with previous reports on activated PMNs 54, 55.
3.4.3 Protein transport
Increased protein abundances and mRNA abundances were observed for the majority proteins and genes that were known to be involved in the regulation of protein transport (Fig. 6B). These proteins include several coatomer subunits, exocyst complex component, nuclear pore complex proteins, small GTPases, and several other proteins. Coatomer is known as a large protein complex that coats membrane-bound transport vesicles and potentially plays a role in forward transport from the ER to the Golgi apparatus and through the Golgi apparatus 56, 57. The observed up-regulation of three coatomer subunits (COPA, COPB, and COPG), and exocyst complex component 4 suggests enhanced intracellular vesicle trafficking under trauma conditions.
3.4.4 NRF2-mediated oxidative stress response
Besides the activation of protein synthesis and transport, we also observed trauma-induced up-regulation in protein and mRNA abundances for most of the proteins associated with the NRF2-mediated oxidative stress response pathway (Fig. 6C), suggesting activation in the oxidative stress response. NRF2-mediated oxidative stress response pathway is essential for cellular defense response to oxidative stress by regulating a battery of detoxifying enzymes and antioxidant enzymes. NRF2 was previously reported to be associated with oxidative regulation of lipopolysaccharide-induced innate immune response in PMNs and the activation of NRF2-mediated oxidative response pathway led to a protective role from the lipopolysaccharide-induced inflammatory response and mortality 58. Moreover, it has been recently demonstrated that Cullin-3 directly interacts with oxidative stress sensor keap1 to regulate NRF2 turnover 59. A number of chaperone and stress response proteins known to be activated by NRF2 were also observed with increased protein abundances in response to injury. These chaperone and stress response proteins include HSP group (DNAJB11, DNAJA1, DNAJC1), stress-induced phosphoprotein 1 (STIP1), and Fk506-binding protein 4 and 5 (FKBP4 and FKBP5). Other proteins observed in the pathway include antioxidant protein Ferritin (FTL and FTH1) and signaling protein MAPK14.
3.4.5 Ubiquitin-proteasome pathway
The up-regulation in both protein abundance and gene expression for several 26S proteasome regulatory subunits and several members of ubiquitin-ligase complex was observed (Fig. 6D), suggesting an increase in the activity of the ubiquitin-proteasome system (UPS). Such an increase in the UPS is common in response to cellular stresses such as trauma injury and infection. Our observation of the up-regulation of several HSPs such as DNAJB11, DNAJA1, and DNAJC1 (Fig. 6C) also supports the activation of the UPS since HSPs were also implicated in the increase of UPS activities 60. The importance of the proteasome activity in the nuclear factor kappa beta (NFκβ) activation, which inhibits neutrophil apoptosis in severe trauma, has also been reported 61, 62.
3.4.6 Apoptosis
Neutrophil numbers are known to be significantly increased in trauma patients with MODS compared to healthy controls and an inhibition of apoptosis or delayed apoptosis in severe trauma is anticipated 17, 62. A number of proteins in our dataset have been previously reported as potentially involved in apoptosis and their protein abundance and gene expression patterns are shown in Fig. 6E. The observed protein abundance increase of NFκβ suggests an increase in its activity, which is consistent with previously reported NFκβ-dependent inhibition of neutrophil apoptosis 62. Increased protein abundances in response to trauma were observed for most of these apoptosis-associated proteins and many of these proteins have been previously reported as antiapoptotic. These potential antiapoptotic proteins include caspase-1 (CASP1) 63, cyclin-dependent kinase (CDK2) 64, HSPs (HSPD1, HYOU1) 65, protein kinase B (PTK2B) 66, fatty acid synthase (FASN) 67, peptidylprolyl isomerase D (PPID) 68, HECT, UBA, and WWE domain-containing protein 1 (HUWE1) 69, nucleoside diphosphate kinase A (NME1) 70, and serine/threonine-protein kinases SRPK1 71, and TAOK3 72. The antiapoptotic implications of most of these proteins have not been reported in PMNs, thus representing potential novel regulators for PMN apoptosis. Only a few proteins were explicitly reported for their antiapoptotic roles in neutrophils. For example, caspase-1, one of the most up-regulated proteins in our data, was also reported as up-regulated for providing an inhibition of apoptosis of inflammatory neutrophils through activation of IL-1β 63. The inhibition of cyclin-dependent kinase (CDK) was shown to induce neutrophil apoptosis 64. It was also reported that protein kinase B (PTK2B) was involved in the inhibition of neutrophil apoptosis via the signaling through PI-3-kinase and downstream pathways 66.
In addition to the up-regulation of potential antiapoptotic proteins, we have also observed a significant down-regulation of several potential proapoptotic proteins, including arachidonate 15-lipoxygenase (ALOX15) 73, thymosin beta-10 (TMSB10) 74, and 40S ribosomal protein s3a (RPS3A) 75. Arachidonate lipoxygenases have been reported as essential regulators of cell survival and apoptosis 76 and inhibition of ALOX15 expression has been shown to prevent cancer cell apoptosis 73. A dramatic down-regulation (approximately tenfold) in both protein abundance and gene expression for ALOX15 was observed in PMNs in response to trauma, implying that ALOX15 may be a novel important regulator for neutrophil apoptosis. Taken together, the observation of up-regulation of many antiapoptotic proteins and down-regulation of proapoptotic proteins provides solid evidence of inhibited or delayed apoptosis in PMNs under trauma conditions.
3.4.7 Other novel proteins
Clinical Relevance
Polymorphonuclear neutrophils (PMNs) play an important role in mediating the innate immune response after severe traumatic injury. In order to gain understanding of the underlying molecular mechanisms of PMNs in response to trauma, it is necessary to study the cellular proteome response to the traumatic condition. Our study performed the first comparative proteome profiling of human PMNs from severe trauma patients and healthy controls by applying 2D-LC-MS/MS-based shotgun proteomics. Our observed proteome changes revealed increased neutrophil activation and inhibited neutrophil apoptosis in response to trauma. The study not only reveals an overall picture of functional neutrophil response to trauma at the proteome level, but also provides a rich proteomics data resource of trauma-associated changes in the neutrophils that may be valuable for further studies of the functions of individual proteins in PMNs and some of these proteins may even be potential candidate markers for predicting disease outcomes.
4 Discussion
The complexity of human diseases presents a significant opportunity for applying high-throughput technologies such as genome-wide gene expression profiling and proteomics to investigate the underlying mechanisms of human diseases. In this work, we demonstrate a comparative proteomics profiling of PMNs isolated from human trauma patients for revealing functional changes of immune cells induced by traumatic injury. In response to injury, we expect that there is somewhat of an overlap of changes induced by tissue injury and infection, which may be difficult to distinguish. To minimize the potential impact of infection, we have selected all the patients for this proteomics study without clinical evidence of infection. Although an occult infection could not be ruled out, none of these patients developed any evidence of infection during their entire clinical course.
Despite being the most abundant leukocyte and the first line of defense against intruding microorganisms, the importance of PMNs in the innate immune response has not been fully understood until recently 1, 10. Our work represents the first global comparative proteomics study of human patient PMNs with the aim to identify functional changes of PMNs in response to trauma. The study revealed significant neutrophil proteome response to traumatic injury with the abundances of 197 proteins significantly changed.
The overall good agreement between an independently acquired gene expression dataset and proteomics data provides a degree of support on the quality of this comparative proteomics data. Overall, the directions of abundance changes (up or down) for ∼67% proteins are consistent with those observed changes in mRNA abundance levels. More interestingly, the mRNA and protein abundance changes are nearly in perfect agreement for some specific functional categories such as protein biosynthesis and oxidative stress response as shown in Figs. 5 and 6, supporting the high confidence of the observed proteomic changes. Validation of selected proteins using targeted quantification in individual samples (Fig. 3) further supports the data quality.
This study also provides clear evidence that increased protein secretion to extracellular milieu is one of the major mechanisms for discrepancy observed between mRNA and protein abundance changes. Given the fact that increased protein secretion cannot be measured by whole cell proteomics alone without the measurement of blood protein concentrations, the concurrent observation of significantly increased gene expression and decreased intracellular protein abundances for secreted proteins represents an interesting and important example of the value for integrating proteomics and transcriptomics measurements.
The observation of imperfect correlation between protein and mRNA results is also in good agreement with previous investigations that also reported a rather poor correlation between mRNA and protein abundance changes 78, 79. These results suggest that the control mechanisms for regulating the mRNA and protein abundances are different. Protein abundances are known to be controlled by many posttranscriptional mechanisms. Therefore, it is not surprising that we did not observe significant protein abundance changes for many genes with significant changes in mRNA and vice versa.
Based on the functional implications of the observed significant proteins, the neutrophil response to trauma can be well consolidated into two main aspects: (i) increased neutrophil activation and (ii) improved neutrophil survival. The neutrophil activation is represented by the observed increase in known immune response associated pathways (Fig. 5), protein synthesis pathways including the EIF2 signaling and aminoacyl-tRNA biosynthesis pathways (Fig. 6A), and an increase in vesicular protein transport (Fig. 6B). Moreover, there are several well-known neutrophil activation markers such as high affinity Ig gamma Fc receptor I (CD64) and complement receptor type 1 (CD35) 80, 81. While CD64 and CD35 were not detected in our proteomics data presumably due to their low abundances, the up-regulation in gene expression 26 was observed for both CD64 and CD35 with 3.4- and 2.0-fold increases, respectively, further supporting neutrophil activation posttraumatic injury.
The increase in protein synthesis and vesicular protein transport supports the notion that secretory granule proteins are produced and secreted at a faster rate following neutrophil activation in response to trauma. The increased protein secretion through degranulation in response to inflammation is well known 45, and this is further supported by the observation of a decrease in intracellular protein abundances for these proteins (Fig. 4B). The observed significant increase in gene expression for known secretory proteins along with general activation of protein synthesis represents the cells’ compensatory mechanism to keep up the need for protein secretion. These observations fall well in line with the prior knowledge about the central role of granules and their associated proteins in antimicrobial functions of PMNs in providing a first line of defense against microorganisms 10.
The improved neutrophil survival is supported by the observation of the activation of NRF2-mediated oxidative stress response (Fig. 6C), and the observation of up-regulation in many antiapoptotic proteins and down-regulation of proapoptotic proteins (Fig. 6E). The integration of this pathway information strongly suggest that neutrophil apoptosis is inhibited or delayed under the trauma conditions being investigated, which is also in good agreement with recent studies on neutrophil apoptosis 61, 62.
While the functional implications of many of these proteins were reported in other cell types and disease conditions, the functions of most of proteins have not yet been reported in human PMNs. We anticipate that many of these proteins represent novel regulatory factors for neutrophil activation and survival. For example, as described in Section 3, the observation of significant down-regulation of ALOX15 suggests that it can be a novel regulator for neutrophil apoptosis 76. The significant up-regulation of fatty-acid synthase (FASN) suggests a role of fatty acids and lipid metabolism in neutrophil activation.
In summary, this comparative proteomics profiling of human PMNs revealed the overall functional changes of PMNs in response to trauma injury. While the observed proteome alterations generally correlate with the increased activation and survival of neutrophils, the functions of many of the altered proteins have not yet been reported for PMNs or inflammatory diseases. This dataset of trauma-associated neutrophil protein alterations provides a rich resource for further studies of the functions of individual proteins in PMNs and some of these proteins may even be potential candidate markers for predicting disease outcomes.
Acknowledgments
Portions of this research were supported by NIH grants U54 GM-62119–02 (to R.G.T.) and T32 GM-008256 (to R.G.T.), P41 GM103493 (to R.D.S.), DP2OD006668 (to W.J.Q.), and EMSL (Environmental Molecular Science Laboratory). EMSL is a national scientific user facility sponsored by the US Department of Energy (DOE) Office of Biological and Environmental Research on the Pacific Northwest National Laboratory (PNNL) campus in Richland, Washington. PNNL is operated by Battelle for the DOE under contract DE-AC05–76RLO-1830.
The authors have declared no conflict of interest.
5 References
6 Addendum
Henry V. Baker, Ulysses Balis, Timothy R. Billiar, Bernard H. Brownstein, Steven E. Calvano, Irshad H. Chaudry, J. Perren Cobb, Joseph Cuschieri, Bradley Freeman, Richard L. Gamelli, Nicole S. Gibran, Brian G. Harbrecht, Douglas L. Hayden, Laura Hennessy, Jureta W. Horton, Jeffrey Johnson, Matthew B. Klein, Stephen F. Lowry, John A. Mannick, Philip H. Mason, Grace P. McDonald-Smith, Michael N. Mindrinos, Joseph P. Minei, Ernest E. Moore, Avery B. Nathens, Grant E. O'Keefe, Laurence G. Rahme, Daniel G. Remick, Jr., David A. Schoenfeld, Michael B. Shapiro, Geoffrey M. Silver, John Storey, Robert Tibshirani, Mehmet Toner, H. Shaw Warren, Michael A. West.