Proteome of human endometrium: Identification of differentially expressed proteins in proliferative and secretory phase endometrium
Abstract
Purpose: To exploit the potential of proteomics to identify and study additional yet-unidentified important proteins present in human endometrium.
Experimental design: The proteome of human endometrium would be established using 2-DE and MALDI and the data analyzed to identify differential protein expression in the proliferative and secretory phase of the menstrual cycle using PDQuest software and MALDI.
Results: In the present work, 2-DE of human endometrium protein led to the resolution of over 200 spots. Subsequent MALDI analysis of 215 spots allowed the identification of 194 proteins. A total of 57 out of the 215 spots were found to be differentially expressed, out of which 49 could be identified using MALDI. These differentially expressed proteins included structural proteins, molecular chaperones, signaling proteins, metabolic proteins, proteins related to immunity, RNA biogenesis, protein biosynthesis and others. The differential expressions of seven representative proteins in secretory and proliferative phase endometrium tissue were confirmed by immunoblot analysis.
Conclusion and clinical relevance: This study establishes the 2-D proteome of human endometrium represented by 194 identified protein spots. The present data provides an important clue towards determining the function of these proteins with respect to endometrium related diseases.
1 Introduction
Endometrium, the mucosal lining of the uterus, is functionally involved in providing a suitable site for implantation and development of a fertilized oocyte. This highly dynamic tissue undergoes cyclic variation with every menstrual cycle during the reproductive years. The menstrual cycle is divided into three distinct phases – menstrual, proliferative (follicular) and secretory (luteal). During menstruation, the entire functional layer (functionalis) of the endometrium is shed with subsequent regeneration of the tissue from the remaining basal layer (basalis) 1. The proliferative phase begins after menses and terminates at ovulation. During this phase, under the influence of estrogen there is rapid cellular proliferation of all cell types and new extracellular matrix is laid down. Shortly after ovulation, in the secretory phase the endometrium undergoes progesterone dependent functional differentiation which provides a suitable environment for embryo implantation. Characteristically, the glands become increasingly tortuous with considerable secretory activity and the stromal cells begin a differentiation process, termed as decidualization, a prerequisite for successful implantation. The endometrium is receptive to embryonic implantation in the mid-secretory phase, approximately for a period of 5 days and this process coincides with a peak in the levels of progesterone. In the absence of pregnancy, a decline in progesterone levels in the late secretory phase leads to endometrial regression and menstruation 2, 3. Any abrogation in endometrial remodeling or endometrial physiology would lead to diseases like endometriosis 4-10, endometrial polyps, hyperplasia and endometrial cancer 11-14.
Earlier studies using the conventional approach of purifying and characterizing the proteins from the endometrium in different physiological states has led to the identification of a number of endometrium-specific proteins and the functions of some of these proteins like sialic acid binding protein (54 kDa glycoprotein) 15, glycodelin 16, IGF-binding proteins, PP14 17, 18 and CYP3A7, a cytochrome P450 isoform, which metabolizes estrogens 19 in human proliferative as well as secretory phase endometrium has been established. These earlier studies were limited with respect to the identification of only a few proteins. However, the advent of genomics and proteomics has led to the identification of several genes and proteins that are characteristic of the different phases of the menstrual cycle and some of the identified candidate proteins have been implicated in uterine receptivity and oocyte implantation 20-23. As on date only two studies have so far been carried out on the differential protein expression of endometrium both in proliferative and secretory phases 22, 23. One group has analyzed both the proliferative and secretory phases of the human endometrium by using ProICAT approach and identified 119 proteins, among which only five of the proteins showed consistent differential expression 22. The other group used DIGE and was able to identify 76 out of 196 differentially expressed proteins 23. Thus the two available studies do not provide a consistent profile of the proteome of the human endometrium. In an attempt to a get a more consistent profile of the human endometrium 2-D PAGE, followed by densitometry for relative quantification, and subsequent identification of proteins by MS and/or MS/MS analysis was carried out using human endometrium in the proliferative and secretory phase. A total of 194 proteins were identified which includes structural proteins, molecular chaperones and proteins related to metabolism, immunity, protein biosynthesis and RNA biogenesis. Further 57 proteins were differentially expressed in proliferative and secretory phase and 49 of these differential proteins were successfully identified by MALDI MS and/or MS/MS. This combined study has resulted in the identification of proteins which could give us new insights into endometrial development and remodeling.
2 Materials and methods
2.1 Human endometrial tissue samples
Endometrial tissue samples were obtained from women admitted to Infertility Institute and Research Centre, Hyderabad, India, for diagnostic purposes, including infertility, tubal re-enastomosis or pelvic pain. The tissue sample were obtained from proliferative (days 7–10; n=12) and secretory (days 20–24; n=12) phases of the menstrual cycle from women with normal menstrual cycle, normal hormonal profiles and free of uterine abnormalities. All tissue samples were collected during routine surgical procedures. Written consent was obtained for the sampling of eutopic endometrium for research purposes. Irregularly cycling, amenorrheic, postmenopausal women and those who had received steroid hormone therapy in the last three months were excluded from the study. The samples were frozen in liquid nitrogen and preserved at −80°C until protein was extracted. The accurate phasing of the endometrial samples was done by histopathology section using standard procedure 24. All experiments were performed in accordance with the guidelines of the Institutional Review Board of the Centre for Cellular and Molecular Biology, Hyderabad, India.
2.2 Proteins extraction and 2-D PAGE
The frozen endometrial tissue samples were thawed, washed with PBS and lysed with lysis buffer [containing 7 M urea, 2 M thiourea, 2% NP40, 50 mM DTT, 0.5% pharmalytes 3–10, and protease inhibitor cocktail (Roche, Mannheim, Germany)]. The suspension was then homogenized for approximately 5 min and kept at 4°C for 2 h. Subsequently, the lysate was centrifuged at 12 5000×g for 1 h at 4°C and the solubilized protein recovered carefully without disturbing the sediment. The supernatant was precipitated using trichloroacetic acid/acetone and resultant pellet was resuspended in lysis buffer and protein was quantified by amido black method 25. The protein (200 μg) was then loaded on to a commercially available IPG strip (7 cm, pH 4–7; Bio-Rad, Hercules, CA, USA) by passive rehydration for 12 h. Later IEF was performed at 50 μA/IPG strips at 8000 V for 20 000 Vh using a Protean IEF cell (Bio-Rad). After the IEF run was completed strips were equilibrated in buffer I (containing 6 M urea, 0.375 M Tris pH 8.8, 2% SDS, 20% glycerol and 2% DTT), followed by a second incubation in buffer II which contained all the ingredients of buffer I except that DTT was replaced with 2.5% iodoacetamide. Each equilibration step was carried out for 20 min under gentle agitation. Strips were then transferred onto a 10% SDS-PAGE gel (10×10.5×0.10 cm3) and embedded into the gel with 1% agarose containing a trace amount of bromophenol blue. SDS-PAGE was performed using vertical gel electrophoresis system (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) at 20 Amp/gel. The gels were stained with colloidal Coomassie stain 26.
2.3 Image capture and analysis
To compare the 2-D gels of proliferative (n=6; in duplicates) and secretory (n=6; in duplicates) phase endometrium, the stained gels were scanned using a Fluor-S™ Multi Imager (Bio-Rad) and transferred to PDQuest Advanced 2D Analysis Software Version 8.0.1 software (Bio-Rad). The semi automated routines available in this software were used to detect and quantify protein spots as well as to match the profiles across a gel series. Protein spots in the gels were identified after normalization based on the local regression model (LOESS). The protein spots from all the gels in the same group were matched for reproducibility analysis in our test system and the scatter plot tool was used to show the correlation coefficient among the gels in each group. For gel comparison, a statistical approach was applied when determining differentially expressed proteins using the PDQuest software. Mann–Whitney Unpaired 2-sample test was performed with 95% significance level to determine which proteins were differentially expressed between the proliferative and secretory phase endometrium gels. A minimum of 1.2-fold change was considered for the upregulated/downregulated proteins.
2.4 In-gel digestion and protein identification by MALDI
The protein spots were manually cut out from gels and processed for MALDI. The excised protein spots were destained for 1 h using 50% v/v ACN and 100 mM NH4HCO3 and then briefly washed with 100% ACN, vacuum-dried in a SpeedVac concentrator (Labconco Corporation, Kansas City, MO, USA) and then incubated in 15 μL of trypsin solution (10 μg/mL trypsin in 10 mM NH4HCO3) for 16 h at 37°C. The tryptic peptides were spotted on the MALDI plate and dried prior to the addition of 1 μL of 5 mg/mL of CHCA in 50% ACN. Protein spots were then identified by MALDI MS and/or MS/MS using MALDI TOF/TOF 4800 Proteomics Analyzer (Applied Biosystems, Foster city, CA, USA). Peptide mass calibration was performed with external mass standard (Calmix 5; Applied Biosystems). Spectra were analyzed using in-house GPS Explorer (TM) software, version 3.5 with fixed carbamidomethylcysteine and variable methionine oxidation. Database used was Homo sapiens, NCBI 2008. Proteins that identified by MALDI MS and/or MS/MS with significant score have been tabulated. The results were also manually validated based on protein pI and molecular weight.
2.5 Immunoblotting
Endometrium proteins from proliferative phase (n=6) and secretory phase (n=6) were resolved by 1-D PAGE and then electrotransferred onto an NC membrane at 100 V for 1 h using the wet transfer system (Hoefer Scientific Instruments, San Francisco, CA, USA). Subsequently, the membrane was stained with 0.1% ponceau S, to check for equal loading of the proteins. Membranes were then blocked with 5% w/v non-fat milk in TBST (Tween 0.1% v/v in TBS) for 1 h at room temperature, washed and incubated with the primary antibody prepared in TBST containing BSA 1% w/v for 1 h. Primary antibodies were diluted as follows: (i) HSP 27 (Stressgen, Ann Arbor, Michigan, USA) 1:1000; (ii) glucose-regulated protein 94 (GRP 94) (Abcam, Cambridge, UK),1:5000; (iii) Vinculin (Millipore, Billerica, MA, USA), 1:1000; (iv) Lamin B1 (Santa Cruz, CA, USA), 1:1000; (v) GRP 78 (Santa Cruz, CA, USA), 1:1000; (vi) ERp57 (Millipore), 1:1000; (viii) major vault protein 37 (MVP-37) (generous gift of George L. Scheffer, VU Medical Center, Amsterdam, the Netherlands), 1:1000 and (ix) glyceraldehyde phosphate dehydrogenase (Gapdh), loading control (Millipore), 1:5000. After the incubation period, the membranes were washed three times (10 min each wash with TBST) and incubated in the appropriate secondary antibody prepared in TBST containing BSA 1% w/v for 1 h at room temperature. The secondary antibody used was conjugated to HRP (Sigma) and was used at a concentration of 1:10 000. The blots were developed using the ECL kit (Amersham, Buckinghamshire, UK). Exposed films were scanned with Fluor-S™ Multi-Imager (Bio-Rad Laboratories), and band of interest were quantified using GeneTools version 3.06.04 from SynGene (Cambridge, UK).
3 Results
3.1 Proteome of human endometrium
To establish the proteome of the human endometrium, proteins were resolved on 2-D PAGE and protein spots were identified using MALDI MS and/or MS/MS. The total proteins of human endometrium tissue following 2-D PAGE was resolved into a number of proteins in the molecular weight range of 10–110 kDa and pI 4–7 (Fig. 1). A total of 215 consistently appearing protein spots were excised from the CBB stained 2-D gels of proliferative (n=3; in duplicates) and secretory (n=3; in duplicates) phase endometrium. Out of 215 spots, 194 protein spots were unambiguously identified by MS and/or MS/MS (Summarized in Table 1 and Supporting Information Tables 1–3) and categorized based on their functional properties as shown in Fig 2. The most abundant group corresponds to proteins that are structural in function (39%). The molecular chaperones constituted 20% of the proteins and the other important categories of proteins identified are those involved in signal transduction (10%), metabolism (9%), immunity (7%), protein biosynthesis, RNA biogenesis and nuclear proteins. In addition there are proteins which are of diverse function and are categorized as others.
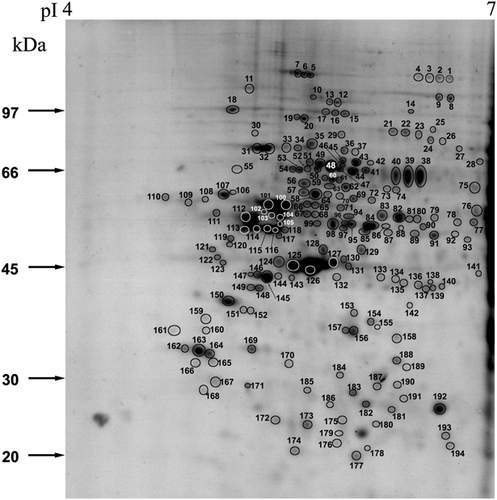
2-DE map of Human endometrial tissue proteins. The first dimension was performed by IEF on pH 4–7 IPG strips, the second dimension on 10% SDS-PAGE gels and the proteins were visualized by CBB G250. The indicated spots (1–194) were excised from the gel and identified by MALDI MS and/or MSMS as listed in Table 1.
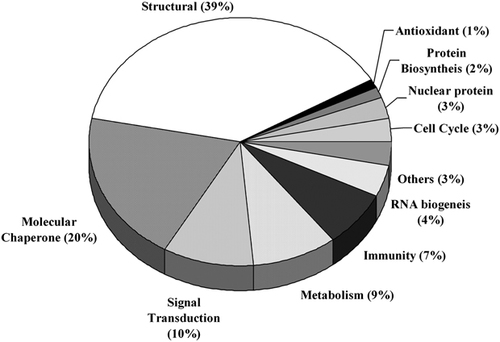
Functional distribution of the proteins isolated from the 2-DE gels and identified by MS and/or MS/MS in human endometrial tissue proteins.
| S. No. | Spot no.a) | Protein name | Accession no.b) | pI/mass | ID method | MOWSE scorec)/seq cov (%) | Peptide matched | Fold differenced) |
|---|---|---|---|---|---|---|---|---|
| Structural proteins | ||||||||
| 1 | 2 | α 2 type VI collagen isoform 2C2 precursor | gi∣115527062 | 5.85/109709 | MS | 127/19 | 22 | 1.8 |
| 2 | 5 | Collagen, type VI, α 1 precursor | gi∣87196339 | 5.26/109602 | MS/MS | 187/30 | 25 | −1.7 |
| 3 | 9 | Vinculin isoform meta-VCL | gi∣7669550 | 5.50/124292 | MS | 100/16 | 22 | 1.9 |
| 4 | 21 | Gelsolin isoform b | gi∣38044288 | 5.58/80876 | MS/MS | 209/32 | 25 | 1.5 |
| 5 | 84 | Keratin 8, isoform CRA_a | gi∣119617057 | 5.41/57829 | MS/MS | 173/43 | 22 | 2.0 |
| 6 | 110 | VIM | gi∣47115317 | 5.09/53604 | MS/MS | 172/51 | 20 | −1.7 |
| 7 | 118 | Dynactin subunit 2 | gi∣22096346 | 5.10/44318 | MS/MS | 183/50 | 19 | −2.0 |
| 8 | 121 | Vimentin | gi∣5030431 | 4.94/47062 | MS/MS | 137/45 | 17 | −2.2 |
| 9 | 122 | Vimentin | gi∣62414289 | 5.06/53676 | MS/MS | 295/68 | 31 | −2.0 |
| 10 | 131 | Actin, β | gi∣14250401 | 5.23/40116 | MS/MS | 161/48 | 17 | −2.1 |
| 11 | 162 | Tropomyosin 3 isoform 2 isoform 10 | gi∣73961081 | 4.77/27386 | MS | 84/48 | 12 | 2.5 |
| 12 | 184 | Gamma-actin | gi∣178045 | 5.20/28478 | MS/MS | 85/40 | 10 | 2.5 |
| 13 | 187 | F-actin capping protein β subunit | gi∣4826659 | 5.69/30952 | MS | 71/34 | 10 | 1.8 |
| Molecular chaperones | ||||||||
| 14 | 17 | Heat shock protein 90 kDa β, member 1 | gi∣4507677 | 4.76/92696 | MS/MS | 203/29 | 28 | −4.8 |
| 15 | 18 | Tumor rejection antigen (gp96) 1 | gi∣61656607 | 4.77/92567 | MS/MS | 241/33 | 32 | −1.7 |
| 16 | 34 | GRP78 precursor | gi∣386758 | 5.03/72185 | MS/MS | 258/41 | 28 | −2.0 |
| 17 | 42 | Heat shock 70 kDa protein 9 | gi∣12653415 | 5.97/73890 | MS/MS | 186/39 | 24 | −2.5 |
| 18 | 43 | Heat shock 70 kDa protein 9B precursor | gi∣62897075 | 5.97/73890 | MS/MS | 238/40 | 27 | −2.1 |
| 19 | 44 | HSP70-2 | gi∣4529892 | 5.48/70267 | MS/MS | 227/44 | 26 | 1.2 |
| 20 | 46 | Heat shock 70 kDa protein 8 isoform 1 | gi∣5729877 | 5.37/71082 | MS/MS | 236/44 | 29 | −1.8 |
| 21 | 58 | Mitochondrial heat shock 60 kDa protein 1 | gi∣189502784 | 5.83/60813 | MS/MS | 190/41 | 25 | −1.9 |
| 22 | 64 | Mitochondrial heat shock 60 kDa protein 1 | gi∣189502784 | 5.83/60813 | MS/MS | 202/49 | 25 | −4.3 |
| 23 | 69 | T complex protein1 subunit epsilon | gi∣194373691 | 5.37/51968 | MS/MS | 103/33 | 15 | −2.1 |
| 24 | 72 | Chaperonin containing TCP1, subunit 8 (theta) | gi∣62896539 | 5.42/60183 | MS/MS | 119/28 | 17 | −2.4 |
| 25 | 82 | Protein disulfide isomerase family A, member 3 | gi∣119597640 | 6.78/54468 | MS/MS | 244/48 | 25 | −1.5 |
| 26 | 116 | Protein disulfide isomerase-related protein 5 | gi∣1710248 | 4.95/46512 | MS/MS | 157/50 | 17 | 1.2 |
| 27 | 181 | Heat shock protein β-1 | gi∣4504517 | 5.98/22826 | MS/MS | 119/55 | 12 | 3.3 |
| 28 | 182 | Heat shock protein β-1 | gi∣4504517 | 5.98/22826 | MS/MS | 134/54 | 13 | 1.5 |
| 29 | 192 | Heat shock protein β-1 | gi∣4504517 | 5.98/22826 | MS/MS | 134/49 | 13 | 1.7 |
| Immunity-related proteins | ||||||||
| 30 | 15 | Fibrinogen gamma | gi∣223170 | 5.54/46823 | MS/MS | 225/53 | 21 | −2.5 |
| 31 | 16 | Fibrinogen gamma chain, isoform CRA_o | gi∣119625326 | 5.54/47971 | MS/MS | 205/60 | 20 | −2.5 |
| 32 | 35 | α-1-B-glycoprotein | gi∣69990 | 5.65/52479 | MS/MS | 141/36 | 17 | −3.2 |
| 33 | 161 | ANXA5 | gi∣49168528 | 4.94/35840 | MS/MS | 273/71 | 24 | 1.7 |
| 34 | 169 | ANXA5 | gi∣49168528 | 4.94/35840 | MS/MS | 203/52 | 20 | −1.7 |
| Metabolic proteins | ||||||||
| 35 | 36 | NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75 kDa | gi∣21411235 | 5.8/80415 | MS/MS | 201/36 | 25 | −2.3 |
| 36 | 90 | Mitochondrial Aldehyde Dehydrogenase | gi∣6137677 | 5.7/54394 | MS/MS | 157/37 | 19 | −1.8 |
| Signal transduction | ||||||||
| 37 | 12 | Major vault protein | gi∣19913410 | 5.34/99551 | MS | 230/31 | 28 | −1.4 |
| 38 | 166 | 14-3-3 Protein Epsilon | gi∣67464424 | 4.92/26912 | MS | 76/45 | 14 | −1.6 |
| 39 | 172 | Rho GDP dissociation inhibitor (GDI) β | gi∣56676393 | 5.1/23031 | MS/MS | 110/58 | 12 | −1.7 |
| 40 | 176 | RAB6A | gi∣49456921 | 5.42/23648 | MS/MS | 128/50 | 14 | 1.7 |
| 41 | 180 | Protein PP4-X | gi∣189617 | 5.65/36262 | MS/MS | 255/69 | 28 | 2.5 |
| 42 | 194 | RAB11B protein | gi∣148342496 | 5.87/24617 | MS/MS | 146/45 | 15 | −2.5 |
| RNA biogenesis, protein biosynthesis and nuclear proteins | ||||||||
| 43 | 52 | Lamin B1 | gi∣5031877 | 5.11/66653 | MS | 270/54 | 31 | −2.4 |
| 44 | 61 | Lamin B2 | gi∣27436951 | 5.29/67762 | MS | 185/39 | 27 | −2.0 |
| 45 | 91 | HNRPH1 | gi∣48145673 | 5.79/49384 | MS/MS | 83/39 | 12 | 1.5 |
| 46 | 148 | Heterogeneous nuclear ribonucleoprotein C isoform b | gi∣117190174 | 4.94/32375 | MS/MS | 169/47 | 22 | −2.3 |
| 47 | 149 | Heterogeneous nuclear ribonucleoprotein C (C1/C2), isoform CRA_c | gi∣119586801 | 10.22/19079 | MS/MS | 98/55 | 11 | −1.6 |
| 48 | 150 | B23 nucleophosmin (280 AA) | gi∣825671 | 4.71/31090 | MS/MS | 119/43 | 14 | −1.5 |
| 49 | 152 | FP1047 | gi∣33341656 | 6.76/69809 | MS | 99/15 | 15 | −1.8 |
- a) a) Spot code as in Fig. 1.
- b) b) Accession numbers are according to NCBI database.
- c) c) MOWSE protein score based on MS data. In all cases, a probability score <0.05 was obtained.
- d) d) A positive fold change indicates that protein expression was upregulated in the secretory phase. A negative fold change indicates that protein expression was upregulated in the proliferative phase.
3.2 Differential protein expression of endometrium tissue from secretory and proliferative phase
To identify proteins associated with proliferative and secretory phase eutopic endometrium, the 2-D proteome of endometrium tissue from proliferative (days 7–10; n=6) and secretory (days 20–24; n=6) phase in the molecular weight range of 10–110 kDa and pI range of 4–7 were compared and analyzed using PDQuest software. The reproducibility of 2-D gels was analyzed by the scatter plots generated in the same software. The correlation coefficient of >0.8 in secretory phase group gels and >0.8 in proliferative phase group gels indicated good reproducibility of the gels in each group. These gels were then used to synthesize a master gel. The synthesized master gel contained 215 discovered spots and was chosen for comparison with the proteomes of the proliferative and secretory phase endometrium. Those protein spots that showed significant changes of more than ±1.2-folds (p<0.05) across all individuals in the group were considered as differentially expressed. Fifty-seven protein spots were identified as differentially expressed on comparison of secretory and proliferative phase eutopic endometrium. Among these a total of 17 protein spots showed a significant increase, whereas 38 protein spots showed a significant decrease in expression in secretory phase and two protein spots were unique to proliferative phase. Out of a total of 57 differential protein spots, 49 protein spots were successfully identified by MALDI MS and/or MS/MS (Table 1 and Supporting Information Table 1). Table 1 gives the identity of the differentially expressed proteins and characteristics such as its pI, mass, peptides matched, sequence coverage and the fold difference compared to proliferative phase endometrium.
3.3 Validations of differential proteins by immunoblotting
To validate the differential protein expression in secretory and proliferative phase endometrium tissue identified by PDQuest analysis, the proteins were separated by 1-D SDS-PAGE and immunoblotting was performed with the respective antibody using a total of 12 samples representing six from each phase of the menstrual cycle. Figure 3 shows immunoblot and quantitation results of GRP 96, GRP 78, HSP27, ERp57, vinculin, major vault protein and lamin B1 of three individual samples from each phase. The results from the remaining six samples were also identical (data not shown). Gapdh was used as internal loading control. The band intensities were normalized with internal control and were compared as secretory versus proliferative. The level of significance for the differences in expression was determined by the Student's t-test (p<0.05). The results of HSP27 and vinculin revealed that there was about 1.5-fold and 1.4-fold upregulation respectively in the secretory phase endometrium compared to the proliferative phase, while GRP 94, GRP 78, ERp57 and lamin B1 were about 1.4- to 1.7-fold downregulated in the secretory phase. These results were consistent with the results of the seven differentially expressed proteins in 2-DE experiments.
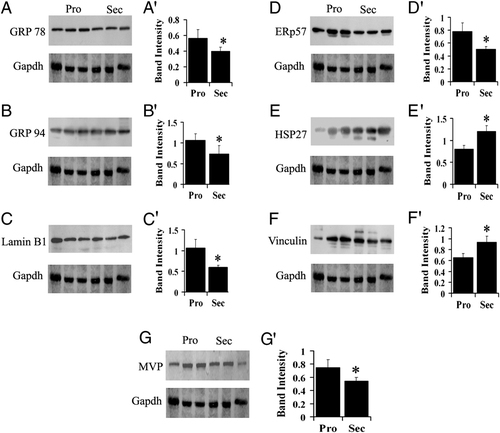
Immunobloting analysis of proliferative (Pro) and secretory (Sec) phase endometrium tissue protein lysates. Panels A-G shows representative immunoblots of GRP 78, GRP 94, lamin B1, ERp57, HSP27, vinculin and MVP, respectively. Panels A′ to G′ shows the graphical representation of the immunoblots A–F, which was obtained by densitometry analysis. The semiquantitative data of A to G immunoblots was normalized by relative intensity of Gapdh. *Asterisk indicates significant difference (p<0.05, as determined by Student's t-test) between the two phases. Experiments were performed with endometrial tissues from 12 different individuals representing six each in the proliferative and secretory phase, respectively.
4 Discussion
Human endometrium undergoes remarkable histological and structural changes throughout the menstrual cycle, in preparation for embryonic implantation and subsequent shedding and regeneration in non-conception cycles 1-3. Disturbance to this normal endometrial remodeling process could lead to a diseased condition such as endometriosis 4-10, endometrial polyps, hyperplasia and endometrial cancer 11-14. Therefore, there is a need to establish the proteome of human endometrium as well to study the differential protein expression between proliferative and secretory phase of the cycle.
In the present study, 194 proteins in the proteome of human endometrium were identified out of which 57 proteins were differentially expressed when the proteomes of the endometrium in the proliferative and secretory phase were compared (Fig. 1; Table 1 and Supporting Information Tables 1–3). Among these differentially expressed proteins, 17 proteins showed a significant increase while 38 proteins showed a decrease in expression in secretory phase of the cycle. The change in the expression levels of five representative downregulated proteins, GRP 78, GRP 94, ERp57, lamin B1 and MVP and two representative upregulated proteins namely, HSP27 and vinculin were further confirmed using immunoblot analysis (Fig. 3), which showed similar results as obtained by using PDQuest software. In the two earlier studies on the differentially expressed proteins in secretory versus proliferative phase endometrium, one group had identified 119 proteins by ICAT and online MS/MS. Out of 119 proteins, only five of the proteins showed consistent differential expression 22. The other group had identified 196 differentially expressed proteins but only 76 could be identified using DIGE and MS. They found 39 proteins were downregulated and 157 were upregulated in secretory phase 23. Among the 49 differential protein spots identified in this study, 20 proteins were consistent with the earlier two reports, thus implying the identification of additional differentially expressed proteins in the proliferative and secretory phase of the human endometrium. The reasons for the observed discrepancy in the results between the earlier and the present study could be possibly because of the different techniques used such as ICAT and DIGE used by the earlier workers and may also be due to the experimental conditions such as the gel size, the use of gradient gel and the tissue used in terms of days of the menstrual cycle. In addition, in our study it was interesting to observe that more proteins are downregulated than upregulated in the secretory phase. Similar observation have been noted previously by others using DNA microarray 21, 27. Downregulation of proteins in the secretory phase could be because these may be estradiol dependent proteins and require estradiol action which in the secretory phase is low 3. Therefore proteins that were upregulated by estradiol (E2) in the proliferative phase are now downregulated due to the loss of E2 action 28, 29. In addition, direct downregulation by progesterone and multiple progestomedins during the implantation window may result in more downregulated proteins compared with upregulated proteins. The proteins identified could be categorized into eleven groups based on their functions and are briefly discussed below.
4.1 Structural proteins
This is the most abundant group in human endometrium proteome and this is not surprising since the endometrium is a heterogeneous tissue which comprises not only epithelial cells, but also supportive stroma cells, blood and vessels which all contribute a heterogeneous array of structural proteins. Dynactin subunit 2 is one of the structural proteins that is upregulated in the proliferative phase of the menstrual cycle, which is characterized by rapid cellular proliferation. It is known that dynactin is involved in cell proliferation 30, 31. Two proteins are upregulated during secretory phase of the menstrual cycle and it includes vinculin and F-actin capping protein β–subunit, whereas two other proteins vimentin and actin are downregulated. It is of interest to note that the upregulated proteins, vinculin and F-actin capping protein β-subunit are known to be involved in cell adhesion, in maintaining cell morphology, cell proliferation and regulate cell migration 32-36 implying that these processes have a role in proliferative-to-secretory phase transition in endometrium. It is also not surprising that vimentin and actin are downregulated in secretory phase of endometrium when the endometrium undergoes structural and functional differentiation and reorganization to provide a suitable environment for embryo implantation.
4.2 Molecular chaperones
The next most abundant group of proteins in the human endometrium are the molecular chaperones which includes several members of the HSP90, HSP70, HSP60 and HSP27 including the HSP homologous GRPs. These findings show that human endometrium has a full complement of HSPs. These proteins are estrogen-regulated and are known to be involved in the correct folding and processing of proteins 37 and in the signal transduction of various steroid receptors, including the estradiol receptor and growth factor receptors 38 and other tyrosine kinases 39. Estrogen has been shown to regulate uterine mRNA levels of HSP90 28, 40, 41, GRP 94 41, HSP70 41, 42 and therefore it is not surprising that twelve proteins of this category were upregulated in proliferative phase (which includes GRP 96, GRP 78, mitochondrial heat shock 60 kD protein 1, isoforms of HSP70, T-complex protein 1 subunit epsilon, and protein disulfide isomerase family A, member 3). The role of HSPs as molecular chaperones and their interaction with steroid receptors and contribution to proliferation and anti-apoptosis 43 supports our findings. Isoforms of heat shock protein β-1 (HSP27) that are known to be involved in stress resistance and actin organization 44 and protein disulfide isomerase related protein 5 that catalyzes the rearrangement of -S-S- bonds during protein folding 45 were upregulated in secretory phase. Protein disulfide isomerase mRNAs are expressed in the endometrium and may play a role in activating immunoglobulin binding factor 46.
4.3 Immunity related proteins
Several proteins which are related to immunity have been identified. This group includes isoforms of MHC class I antigen, HLA-A α1 and α2 domains, fibrinogen γ and isoforms of annexin V. One of the isoform of annexin V (spot no. 161) was found to be upregulated in secretory phase. This protein has been implicated in ion-channel regulation 47, 48 and as an inhibitor of protein kinase C 49, 50, phospholipase A 51 and blood coagulation 52. Interestingly, we observed that the other isoform of annexin V (spot no. 169) was downregulated in secretory phase. This result suggests that from proliferative to secretory phase transition, annexin V might be undergoing post translational modifications 53. However, at this stage we could not explain the precise basis of these isoformic variations in expression. The proteins which are downregulated in secretory phase include isoforms of fibrinogen γ. These proteins play an important role in blood coagulation. In addition, various cleavage products of fibrinogen and fibrin regulate cell adhesion and spreading, and display vasoconstrictor and chemotactic activities 54, 55. The upregulation of annexin V and downregulation of fibrinogen γ during secretory phase underscores the importance of maintaining an environment for anticoagulation during implantation.
4.4 Metabolic Proteins
Majority of the proteins identified in this group are involved in energy production. Metabolic proteins like NADH dehydrogenase (ubiquinone) Fe-S protein 1 and mitochondrial aldehyde dehydrogenase were found to be downregulated in secretory phase. Apart from their likely role in glucose metabolism (via oxidative phosphorylation and glycolysis), they are also involved in oxygen sensing, apoptosis, cell cycle regulation, and immune recognition and response 56. However, the exact function with respect to endometrium is not clear.
4.5 Signal transduction
Proteins which are involved in cell cycle control, cell proliferation and anti apoptosis are downregulated in secretory phase endometrium and this group includes 14-3-3 protein subunit epsilon, MVP and RAB11B 57-63. In addition Rab family proteins, which play an essential role in membrane transport, mitosis and cytokinesis 64, 65 are found to be differentially expressed in secretory and proliferative phase endometrium. For instance Rab11, which is downregulated in secretory phase, has been implicated in cytokinesis 64 where as Rab6A, which is upregulated in secretory phase is required for the metaphase/anaphase transition 65. Annexin IV, which promotes membrane fusion, involved in exocytosis, anti-apoptosis and signal transduction 66 is found to be upregulated in secretory phase.
4.6 RNA biogenesis, protein biosynthesis and nuclear proteins
Proteins involved in RNA biogenesis such as heterogeneous nuclear ribonucleoprotein C, and B23 nucleophosmin are found to be downregulated in secretory phase of endometrium. These heterogeneous nuclear ribonucleoprotein family members are regulated by estrogen and may bind to other nuclear receptors 67-69 and have been linked to a variety of cellular processes, including mRNA translation, transcription, RNA processing, RNA shuttling and stabilization, chromatin remodeling, cell survival, cell proliferation, differentiation, apoptosis, and cell cycle regulation 70, 71. The B23 nucleophosmin is another protein which is found to be downregulated in secretory phase endometrium. B23 nucleophosmin has several potentially important roles in regulating cell function and signaling 72, 73. The expression level of B23 nucleophosmin is shown to be induced by estrogen in vascular smooth muscle cells 74 and MCF-7 breast cancer cells 75. FP1047, which is a translation elongation factor and which belongs to the EF-1-β/EF-1-γ family, is found to be downregulated in secretory phase. Lamin B1 and B2, which play a role in nuclear architecture, DNA replication and gene expression are essential for cell proliferation 76-78 are found to be downregulated in secretory phase.
4.7 Conclusions
In the present study, we have reported the proteomic identification of 194 proteins present in human endometrial tissue samples. We have applied proteomic techniques to study protein expression profiling between secretory and proliferative phase endometrium. A comparative analysis of the human endometrium tissue proteome by 2-D in combination with MS and/or MS/MS enabled us to identify 49 proteins that showed significant changes in expression levels. Furthermore, we have confirmed the downregulation of GRP 78, GRP 94, ERp57, lamin B1 and MVP and upregulation of HSP27 and Vinculin in secretory phase human endometrium by immunoblot. In this study we have identified additional 137 protein spots in 2-D of human endometrium that had previously escaped identification. This study establishes the 2-D proteome of human endometrium represented by 194 identified protein spots. However, more proteins could be present in this kind of tissue, which could not be identified due to problems related to acquiring sufficient amount of tissue. Further, since the tissue is limited in quantity it is a further hindrance to running bigger length gels. One could pool matched tissues and run bigger gels but one knows that this may not be the best approach since matched tissue do exhibit variations between individual patients. Therefore, it was decided to use small gels. The reported proteomic identification of proteins in the present work now provides the basis for subsequent studies on the physiological and pathological aspects of endometrium in aberrant condition such as endometriosis. Following this study, we are currently investigating both eutopic endometrial proteins in women with and without endometriosis, during both the proliferative and secretory phases of the menstrual cycle.
Clinical Relevance
Endometrium, a heterogeneous tissue lining the uterus, is highly dynamic and exhibits marked cyclical changes during the menstrual cycle. Any abrogation in endometrial physiology would lead to diseases like endometriosis, endometrial polyps, hyperplasia and endometrial cancer. Therefore, there is a need to understand the molecular basis of function of the endometrium per se. In the present study, we have established the proteome of human endometrium by 2-DE and MALDI, which comprises of 194 identified protein spots. A comparative analysis of proliferative and secretory phase human endometrium was also carried out, which enabled us to identify 49 out of 57 differentially expressed protein spots. This study has led to the identification of proteins which are characteristic of the two phases of the menstrual cycle and instigates further investigations into global changes in protein expression in disorders of the endometrium. Finally, this study may also set the stage to develop a screen for candidate proteins in patients with infertility and for targeted drug discovery for enhancing (or inhibiting) implantation for infertility treatment (or contraception).
Acknowledgements
Priyanka Rai and Venkatesh Kota are the recipients of CSIR fellowship from Government of India. We thank Dr. Archana B. Siva, Dr. Satish S. Bhande and Y. Kameshwari for their valuable suggestions and technical help. We sincerely thank Dr. George L. Scheffer for the anti-MVP-37 antibody.
The authors have declared no conflict of interest.




