SHIFTing goals in cystic fibrosis—managing extrapulmonary disease in the era of CFTR modulator therapy; Proceedings of the International Shaping Initiatives and Future Trends (SHIFT) Symposium
Jonathan E. M. O'Donnell and Lucy Hastings are joint first authors.
Chee Y. Ooi is the senior author.
Abstract
Background
Cystic fibrosis (CF) is a life-shortening multisystem genetic disease. Although progressive pulmonary disease is the predominant cause of morbidity and mortality, improvements in treatment for CF-related lung disease, with associated increase in longevity, have increased the prevalence of extrapulmonary manifestations1.
Methods
To discuss these issues, a multidisciplinary meeting of international leaders and experts in the field was convened in November 2021 at the Shaping Initiatives and Future Trends Symposium with the goal of highlighting shifting management paradigms in CF. The main topics covered were: (1) nutrition and obesity, (2) exocrine pancreas, (3) CF-related diabetes, (4) CF liver disease, (5) CF-related bone disease, and (6) post-lung transplant care. This document summarizes the proceedings, highlighting the key priorities and important research questions that were discussed.
Results
Improved life expectancy, the advent of cystic fibrosis transmembrane conductance regulator modulators, and the increasing appreciation of the heterogeneity or spectrum of disease are leading to a shift in management for patients with cystic fibrosis. Care should be individualized to ensure that increased longevity is accompanied by improved extra-pulmonary care and reduced morbidity.
Abbreviations
-
- A1AT
-
- Alpha-1 antitrypsin
-
- ALT
-
- Alanine transaminase
-
- APRI
-
- AST to platelet ratio index
-
- AST
-
- Aspartate aminotransferase
-
- BMD
-
- Bone mineral density
-
- BMI
-
- Body mass index
-
- BOS
-
- Bronchiolitis obliterans syndrome
-
- CF
-
- Cystic fibrosis
-
- CFBD
-
- Cystic fibrosis bone disease
-
- CFLD
-
- Cystic fibrosis-related liver disease
-
- CFRD
-
- Cystic fibrosis related diabetes
-
- CFTR
-
- Cystic fibrosis transmembrane conductance regulator
-
- CGM
-
- Continuous glucose monitoring
-
- CLAD
-
- Chronic lung allograft dysfunction
-
- DXA
-
- Dual energy X-ray absorptiometry
-
- FEV1
-
- Forced expiratory volume
-
- FFM
-
- Fat-free mass
-
- FM
-
- Fat mass
-
- GPR
-
- GGT to platelet ratio
-
- IBW
-
- Ideal body weight
-
- OGTT
-
- Oral glucose tolerance test
-
- PERT
-
- Pancreatic enzyme replacement therapy
-
- pwCF
-
- Person with cystic fibrosis
-
- RAS
-
- Restrictive allograft syndrome
-
- SHIFT
-
- Shaping Initiatives and Future Trends
1 INTRODUCTION
Cystic fibrosis (CF) is a life-shortening multisystem disease and one of the most common autosomal recessive genetic disorders worldwide. Progressive pulmonary disease remains the dominant cause of morbidity and mortality. Advances in CF care, including but not limited to the advent of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies, have resulted in increased life expectancy and improved quality of life.1, 2 However, alongside these welcome advantages are emerging challenges associated with the rising prevalence and appreciation of extra-pulmonary CF complications and age-associated co-morbidities. The proportion of patients with cystic fibrosis (pWCF) with extrapulmonary complications is rising, and care will shift from preventing mortality to preventing morbidity.
Extra-pulmonary disease is increasingly realized as an important disease modifier. An understanding of those who are at risk of extrapulmonary disease and complications through an appreciation of the heterogeneity or spectrum of disease will be key in future management and allow for individualized patient care.
1.1 Current state of nutritional management
Malnutrition and underweight have historically had a strong association with mortality and morbidity. PwCF with normal range weight-for-age and height-for-age, have better pulmonary function (percentage predicted forced expiratory volume in 1 s – FEV1) and long-term survival.3 However, nutrition in CF is complex and dependent on multiple factors, including physical, psychological, and socioeconomic factors as well as personal preferences. Decreased lung function, tachypnoea, and chronic pulmonary infections increase energy needs, while impaired gastrointestinal absorption of nutrients, decreased appetite, presence of gastrointestinal symptoms, dysmotility, and intestinal inflammation with subsequent reduced appetite contribute to negative energy balance.4
In 1988 Corey et al.5 championed a high-fat, high-energy diet with aggressive pancreatic enzyme replacement therapy (PERT) noting increased height and weight with improved long-term survival. For the last 30 years (along with routine salt and fat-soluble vitamin supplementation), this “legacy” diet prevailed as the gold-standard in CF care. For those who cannot access modulators or suffer from ongoing malnutrition, under-weight or high energy requirements, this CF legacy diet may still provide benefits, albeit with a focus on a healthier, nutrient-rich diet.
With the knowledge that nutrition and weight are important disease modifiers, along with the incorporation of gastroenterologists and dietitians into CF clinics, the percentage of underweight children and adults with CF has decreased over time.6, 7 In tandem, however, the percentage of individuals with overweight or obesity has increased—even before the introduction of modulator therapy.8, 9 The reason for this increase in overweight/obesity is multifactorial. Along with an improved understanding of the need to avoid malnutrition by increasing energy intake, there is clear heterogeneity in CF, with those who have milder disease on the basis of lung function, pancreatic function, or genotype having higher rates of overweight/obesity.7 Such patients with milder disease also have increased survival, with a survival effect in adults possibly influencing overweight/obesity rates.
With the introduction of CFTR modulators, body mass index (BMI) has been increasing further.10 This is likely due to improved lung disease with an associated reduction in pulmonary complications, causing a decrease in energy expenditure. Intestinal absorption is also increasing, possibly secondary to increased bicarbonate secretion in the pancreas and gut, resulting in increased motility, leading to increased lipolysis within the intestinal lumen, decreased gastrointestinal dysbiosis, and decreased inflammation.11 This weight gain has been associated with higher rates of hypertension, triglyceride, cholesterol, and low-density lipoprotein levels.12
There is clear evidence that many pwCF consume diets with increased added sugar, higher glycaemic load and saturated fats, with reduced dietary fiber compared to healthy controls.13, 14 Additionally, there are increasing rates of hypertension in those on modulators,12 postulated secondary to reduced hypersecretion of salt or salt loss, if the traditionally high salt intake has not been modified. These are all likely a consequence of the traditional “high fat, high energy, high salt diet” in pediatric and adult CF care. With the advent of modulators and increased survival of individuals with cystic fibrosis, it is time to re-examine the diet recommended to pwCF. The legacy “high fat high calorie high salt” diet for all may now have negative long-term consequences for pwCF in the era of improved survival—such as an increased risk of colorectal cancer,15 metabolic disease, dyslipidaemia, type 2 diabetes, and cardiovascular disease.16
1.1.1 How should we assess nutrition?
Classically, BMI has been used as a marker of nutritional status3, 17 and is more sensitive than percentage ideal body weight (%IBW)17 at predicting FEV1 in children. For pWCF aged 2–20 years, CF nutrition guidelines recommend the use of the BMI percentile method for children and adolescents and that optimal nutritional status is associated with a BMI ≥ 50th percentile. For those less than 2 years, weight-for-length ≥50th percentile is recommended.17 For adults, optimal nutritional status is, for women, a BMI of at least 22 kg/m2 and for men at least 23 kg/m2. BMI is easy to obtain and can identify at-risk patients in the low and high extremes—and historically, BMI > 50th percentile has been a key performance indicator of CF care.
Despite its routine use in clinical practice, there are limitations with BMI. While BMI is associated with improved disease outcome, research has shown that FEV1 improved with increasing BMI up to 23 kg/m2, but FEV1 improvements beyond that are marginal.6 Notably, BMI measurements do not account for the body's distribution into fat mass (FM) and fat-free mass (FFM). More specifically, BMI may fail to detect excess FM in an individual with a BMI in the healthy range—also termed “normal weight obesity (NWO),” which is conversely associated with FFM depletion. BMI may also risk misclassifying an individual as overweight or obese when they have high FFM (e.g., an athlete).10 The discrimination between FM and FFM in their relative contribution to body weight is clinically important. Higher body fat percentage with normal BMI is associated with decreased FEV1. Depletion of FFM is similarly associated with reduced lung function, increased pulmonary exacerbations, lower bone mass, and increased inflammation.18-22
Dual-energy X-ray absorptiometry (DXA) is an accepted reference method for assessing body composition by measuring adiposity. It is fast, uses low-dose radiation, and allows differentiation between tissues based on attenuation.23 Many centers already use DXA for bone mineral density (BMD) assessment in CF; however, accessibility and cost are potential issues with its implementation, along with the need for repeat scans for serial monitoring. Other indicators of lean muscle mass emerging as predictors of mortality that may be applicable to cystic fibrosis include hand-grip strength,24 mid-arm circumference,25 bioelectrical impedance analysis,26 quadriceps muscle thickness,27 and CT/MRI scans.28 Longitudinal studies on newer markers of nutrition are lacking— however the STRONG-CF study currently underway (https://www.clinicaltrials.gov/study/NCT05639556), will assess these markers cross-sectionally and compare them to DXA to evaluate emerging nutritional and body composition parameters and link them to clinical outcomes.
1.1.2 Time for the era of personalized nutritional care in CF
There are many challenges moving forward in individualizing nutrition care in cystic fibrosis for both clinicians and patients. The first challenge will be to identify those at risk of malnutrition who require a high energy, high salt diet, and those who are at risk of overweight/obesity and the associated cardiovascular and metabolic complications that may arise in adulthood as a consequence of this diet. The difficulties related to changing dietary habits in adulthood that were established as a child, make dietary advice provided to children with CF even more important. With improving survival, it is expected that healthy balanced diets that emphasize foods with positive health outcomes in the general population will become standard practice in CF care: consumption of “healthy” fats (unsaturated fats) and nutrient-dense foods, and limiting energy-dense-nutrient poor foods (high-sugar, high salt foods, and beverages with low nutrient density) will become standard practice for most pwCF. The second challenge will be enacting these dietary changes—this will entail a significant increase in resources for dietitians working with pwCF to provide individualized dietary advice. Third, there is a need to focus more on body composition as a marker of nutrition in CF than on BMI alone. BMI, as the traditional measure of nutrition, will miss those with excess fat mass/NWO and its attendant risk of cardiovascular and metabolic complications.29 Finally, there will be a need to set up screening programs for those with CF who, in adulthood, will be at increased risk of cardiovascular, metabolic, and oncological complications of the disease, previously unrecognized with reduced life expectancy masking long-term complications.30
1.2 Exocrine pancreatic disease
Pancreatic changes evident in CF may start in utero, with reports of fibrosis in foetal pancreatic tissue as early as 26 weeks.31, 32 In post-mortem infants, there are changes in acinar dilatation and cysts, with flattened epithelium and eosinophilic material noted in pancreatic tissue. Immunoreactive trypsinogen (IRT), a marker of pancreatic inflammation used in newborn screening, is elevated in those with CF, which then declines over time.33 This is thought to represent progressive pancreatic disease and fibro-fatty replacement of pancreatic tissue, a harbinger of the onset of endocrine pancreatic insufficiency.34 It is important to note that pWCF born with meconium ileus may have normal range IRT due to more advanced pancreatic damage.
Historically, it was thought that once significant pancreatic damage had occurred, this was irreversible. Yet given the progressive pancreatic disease that occurs in those who subsequently progress to exocrine pancreatic insufficiency, there is a potential therapeutic window to restore pancreatic exocrine function and speculated, but yet unproven plasticity in progenitor cells within the pancreas. This is supported by the fact that despite the anatomical and histological maturity of the pancreas at term, exocrine pancreatic function is still developing in the postnatal period with relative deficiencies in enzymatic secretion.35, 36
1.2.1 Window of reversibility of pancreatic injury in the era of modulators
Studies have suggested that CFTR modulators may result in the recovery of markers of pancreatic function.37, 38 The early institution of modulators may be paramount. The ARRIVAL study38 showed improved faecal elastase and IRT in those with gating mutations aged between 12 and 24 months. The KIWI study showed some reversibility up to age 6 years37—but the PROMISE study suggested little potential for recovery from age 12 years.39 The case report of an infant with homozygous F508del CF exposed to CFTR modulators in utero and subsequently born pancreatic-sufficient,40 is supported by similar evidence in animal studies in which in utero treatment of G551D CF ferrets with CFTR modulator was protective from meconium ileus and pancreatic insufficiency at birth.41 This improvement in function has been noted at the coal-face in some pwCF—which has led to some being able to reduce or cease PERT.42, 43
It is important to note that exocrine pancreatic insufficiency remains a clinical diagnosis made by a combination of patient symptoms, weight gain, fat-soluble vitamin levels, and stool tests (e.g., faecal elastase). All of these should be taken into account when initiating PERT. Furthermore, even individuals with improvement in markers of pancreatic exocrine function may still have acinar and ductal function that does not match those of healthy controls44 and may gain clinical benefit from PERT.
1.2.2 Pancreatitis in the era of modulators
With the potential of CFTR modulators to improve pancreatic function, there may be increased potential for children previously classified as “pancreatic insufficient” to develop acute, acute-recurrent, or chronic pancreatitis. It is known that those with milder CFTR mutations, determined by genotype, are more likely to have enough acinar function for continued enzyme synthesis but impaired ductal flow, hence predisposing to pancreatitis.45 If CFTR modulators have the potential to delay or reverse pancreatic decline, there is the theoretical potential for these children, with previously insufficient residual acinar tissue, to develop pancreatitis. This is supported by several case reports of those on modulators developing pancreatitis.46 Conversely, in those who are pancreatic-sufficient, CFTR modulation may reduce the risk of pancreatitis.47 It is important to note that normal lipase values may be lower in CF, which is relevant as the traditional definition of pancreatitis is two out of three of 2× upper limit of normal (ULN) lipase rise, radiographic findings, or exam findings. Therefore, the ULN may require individualization to a pwCFs pretreatment lipase or baseline lipase as opposed to a normal population reference range.
For those who develop pancreatitis on CFTR modulators, the recommended approach is an individual-based decision taking into account the effect of modulator therapy on lung function as well as other organ function. Finally, the additional effect of other non-CF genetic variants on the development of pancreatitis should always be considered.
1.2.3 Biomarkers for monitoring exocrine pancreatic function
Due to the potential of reversible pancreatic damage, pancreatic exocrine function should be monitored every 6–12 months, in addition to anthropometry. Currently, faecal elastase is a readily available binary biomarker of pancreatic function, with a cut-off of 200 mcg/g used by most clinical laboratories, but the 100 mcg/g cut-off gives the greatest specificity for exocrine pancreatic insufficiency.48 Limitations include intestinal failure with steatorrhea and diarrhea with false positive results, especially within the 100–200 mcg/g range. Other biomarkers include serum trypsin,49 which may have the potential as a longitudinal biomarker for pancreatic function in children with cystic fibrosis. However, it is not readily available.
1.2.4 Personalization of pancreatic-directed therapy
Challenges moving forward will be ensuring early initiation of CFTR modulators for maximum pancreatic reversibility (particularly in those with delayed diagnosis due to negative newborn screening), ensuring monitoring for the potential return of pancreatic sufficiency, as well as how and when to safely cease PERT therapy.
Further research is needed into the optimum timing of CFTR modulation to salvage pancreatic function. It is highly likely that there will be a move to early CFTR modulation in pwCF. Since the introduction of modulators, the age of commencement has slowly been reduced by regulatory bodies, making these medications available earlier and theoretically increasing pancreatic reversibility. There may also be patient groups currently not eligible for modulator therapy, such as those with CFTR-related recurrent pancreatitis, that may benefit from improved pancreatic ductal flow (as CFTR is predominantly expressed in pancreatic ductal vs acinar cells).
For those on modulators within a window of pancreatic function reversibility, it is recommended that pancreatic function be monitored with 6–12 monthly faecal elastase with a level of >200 μg/g indicative of pancreatic sufficiency.48 For those who become pancreatic sufficient as indicated by good BMI/body composition, a faecal elastase in the pancreatic sufficient range, normal fat-soluble vitamins, and an improvement in gastrointestinal symptoms, it may be biologically plausible to consider reduction or cessation of PERT in a pwCF. In pwCF who still require PERT, transition to a healthy balanced diet from a high energy, high fat diet may itself reduce gastrointestinal symptoms and PERT requirements accordingly.
However, there is speculation that continuation of PERT to reduce theoretical pancreatic stress could be considered among those who become pancreatic sufficient, particularly during periods of life with changing nutritional physiology—such as when transitioning to larger meals and eating solid food. Further research is required regarding PERT therapy in the era of modulators; however, best practice will involve individualizing therapy to pwCF.
1.3 Current state of cystic fibrosis-related diabetes (CFRD)
Studies have convincingly demonstrated that there is increased morbidity and mortality in pwCF who have CFRD as defined by oral glucose tolerance test (OGTT) criteria.50-54 More recently, however, it has become apparent that there is also a relationship between early hyperglycemia even before the development of CFRD and poor pulmonary outcomes, infection, inflammation and weight decline.55-60
1.3.1 Glycaemic targets in CFRD
According to current consensus guidelines, glycaemic targets for the treatment of CFRD are the same as those in Type 1 and 2 diabetes mellitus,61, 62 whereby large multicentre trials have established the benefits of target HbA1c < 7% in reducing microvascular and macrovascular complications in these specific populations. The 2019 consensus guidelines of the American Diabetes Association, however, highlighted certain high-risk populations, such as pregnant women, that require tighter target ranges.63 While pwCF and CFRD are not currently recognized as a high-risk cohort, hyperglycemia has been described as a risk factor for pulmonary damage through various mechanisms; airway surface liquid glucose fueling bacterial growth, promotion of advanced glycation end-products leading to inflammation, glycaemic variability promoting oxidative stress, and chronic hyperglycemia potentially leading to autonomic neuropathy affecting the phrenic nerve and diaphragmatic function.64-69 In light of the evidence for direct hyperglycemia causing pulmonary damage, tighter glycaemic target ranges in CFRD may be required to optimize nutrition and pulmonary outcomes in CFRD70; however, studies confirming the benefit of tight glycemic control are greatly needed.
1.3.2 Definition and management of early dysglycaemia
Early glycaemic abnormalities (dysglycaemia) that do not meet the current definition of CFRD have been associated with poorer pulmonary outcomes and weight gain. The current definition of CFRD is based on criteria developed for type 1 and 2 diabetes. Clearer definitions of what is clinically significant dysglycaemia and how best to define optimal blood glucose targets relevant to CF-specific outcomes such as weight gain and lung function, need to be determined. Continuous glucose monitoring (CGM) technology is rapidly evolving with improved accuracy so robust characterization of glycaemic patterns is possible. In combination with large prospective trials, this will no doubt aid in establishing consensus guidelines around CF-specific glycaemic targets and the identification of individuals likely to benefit most from earlier introduction of insulin and tighter control despite the additional burden for pwCF, who are already managing complex care requirements. The results of a randomized controlled trial of insulin treatment for subjects with early dysglycaemia (CF-IDEA) are expected in the near future (https://clinicaltrials.gov/study/NCT01100892).
1.3.3 Glycaemic abnormalities in very young children with CF
It has been recognized since the 1960s that very young children with CF, even those under 1 year of life, can have abnormal insulin secretion leading to diabetes.71, 72 In recent years, further characterization of the glycaemic profiles of very young children with CF has shown that those with “indeterminate glycaemia” (abnormal values in OGTT before 2 h), are at 11 times higher risk of developing CFRD compared to those with a normal OGTT,73 they have poorer lung function measured via FEV1, and have a significant inverse correlation between peak blood glucose and weight z-score.74 These early glucose abnormalities in young children have also been shown to correlate clinically with findings on bronchoalveolar lavage, which showed a statistically significant relationship between CGM variability and the proportion of neutrophils in the lavage as a marker of pulmonary inflammation.55 Further clouding the definition of clinically relevant glycaemic targets in children are data showing that these children can fluctuate between the diabetic and nondiabetic ranges.74 Overall, the uncertainty around optimal CGM criteria for diagnosing children with CFRD persists, and further characterization of the data points most likely to be associated with clinically relevant outcomes is required.
1.3.4 CGM as a replacement for OGTT
OGTT is still the gold standard for both screening and diagnosing CFRD (while making allowance for the diagnosis in specific settings outside of an OGTT, such as fasting glucose ≥7 mmol/L or random glucose levels ≥11.1 mmol/L with diabetic symptoms).61, 62 CGM is widely used for the management of insulin-treated pwCF. There is, however, currently no endorsement of CGM as a diagnostic tool for CFRD in the ISPAD consensus guidelines. Despite this, CGM is increasingly being used in some centers for early detection of dysglycaemia due to its many advantages, such as the avoidance of fasting or timed conditions, the capture of free-living glycaemic data, and the absence of fingerpricks or venous sampling. A recent study found that CGM measures of hyperglycemia reliably distinguished those with and without diabetes in adults with CF, including when these cut-offs were applied in separate validation sample.75 However, another study found that CGM performed poorly at discriminating between those with and without diabetes in a younger population of predominately adolescents.76 These conflicting studies highlight the need for larger, longer prospective studies to determine the clinical significance of specific CGM thresholds before CGM can be endorsed as a replacement for OGTT in the diagnosis of CFRD.
1.3.5 CFRD in the CF modulator era
The development of diabetes in CF is likely multifactorial, including progressive insulin deficiency due to pancreatic exocrine disease, as well as increased insulin resistance due to significant illness, inflammatory cytokines, hypercortisolaemia, and corticosteroid use. There is possibly also a higher future risk associated with overnutrition in pwCF on modulator therapy. The potential mechanisms by which CFTR modulators may affect glycaemia in pwCF are still being elucidated, but there is some evidence that there are both direct and indirect effects of CFTR modulators on beta cells that improve their function.77-80 Most of the published clinical studies are limited by small numbers, but evidence suggests ivacaftor may improve insulin secretion,81, 82 that the prevalence of CFRD is lower in pwCF treated with ivacaftor, and that elexacaftor/tezacaftor/ivacaftor can improve average glucose, glycaemic variability and time in target range measured by CGM.83 Meanwhile, studies focusing on the effect of lumacaftor-ivacaftor on glycaemia have been mixed.84, 85 Larger longitudinal studies are needed to better understand the impact of CFTR modulators on dysglycemia and the progression of CFRD.
1.3.6 Integrating CFRD care in the CF clinic
There are many different models of care in CF clinics; however, a common challenge is integrating the expertize required for managing diabetes, especially from a diabetes technology perspective. The burden of the disease of CF may be heavy and complex for pwCF and their families. The additional burden of CFRD treatment is significant, and for most pwCF to be managed optimally, this requires the input of diabetes-specific accredited practising dietitians, credentialed diabetes educators, endocrinologists and CF physicians with expertize in the management of CFRD. For example, in those with CFRD, an individualized diet is required to meet the patient's dietary needs of both diabetes and CF depending on overall health status. Some centers have found that by integrating clinicians from the diabetes team directly into the CF clinic, the service can achieve more tailored care and more timely diabetes management, based on two-way education and communication between the CF and diabetes teams. This model also achieves more integrated and streamlined care for pwCF and their families who are already trying to balance significant healthcare needs and contact with health professionals along with jobs, family life and other personal commitments.
1.4 Current state of CF liver disease (CFLD)
Liver involvement in CF refers to a spectrum of hepatobiliary problems with varied pathogenesis and severity resulting in clinical, biochemical, or radiological liver abnormalities—or a combination of the three. These may include benign intercurrent illness or drug effect, or more serious drug-induced liver injury, given the range and number of drugs taken by pwCF.
Advanced CFLD is a leading non-pulmonary cause of mortality in CF86-88 and refers to disease secondary to underlying CFTR defects. CFLD remains a diagnosis of exclusion, with other infectious, metabolic and structural causes ruled out. Risk factors include severe genotype, male gender, F508del homozygosity or other “severe” mutations89 and meconium ileus,90 with the alpha-1-antitypsin Z allele associated with increased risk of severe disease.91 Traditionally, CFLD was thought to primarily affect pwCF in the first decade of life, with 41% of children with CF diagnosed with CFLD before age 12 years.92 However, the incidence of CFLD has been reported to continue to increase in later adolescence and early adulthood.89 Focal biliary cirrhosis was considered the pathognomonic lesion in CF, with onset in childhood that could progress to portal hypertension and multilobular biliary cirrhosis with its related complications.
Many theories for the advanced CFLD have been proposed, such as common bile duct stenosis and, in the adult population, chronic hepatic congestion of cardio-pulmonary origin. The prevailing theory remains that CFTR dysfunction in cholangiocytes causes pH dysregulation, inspissated biliary secretions, bile duct plugging—resulting in the production of proinflammatory cytokines causing peribiliary inflammation and progressive periportal fibrosis. An area of recent investigation relates to the role that a disordered gut-liver axis may play in the development of cirrhosis in pwCF. The leak of pathologic endotoxins from the gut to portal circulation and liver together with the altered innate immune response of cholangiocytes might explain the development and progression of the biliary form of the liver disease. Supportive of this is that the CF population is vulnerable to unique bacterial colonization and infections, early and chronic antibiotic use, and a GI tract that is more acidic, more permeable mucosa with known dysbiosis.93
More recently, porto-sinusoidal vascular disease, secondary to obliterative portal venopathy has been described as an important contributor of “non-cirrhotic” portal hypertension in CFLD, mainly in adults—but also children.89 The pathophysiology of this “non-cirrhotic” CFLD is yet to be elucidated, but may be secondary to micro-thrombotic phenomena, platelet dysfunction, or endothelial dysfunction. Recognition of the spectrums of disease in CFLD—cirrhotic and non-cirrhotic—are essential to diagnosis and management since the latter may vary between the two entities.
Hepatic steatosis, whilst not classically considered CFLD due to lack of association with hepatic cirrhosis, is present in up to 70% of pwCF.94 Pathogenesis is unclear and theorized to relate to malnutrition, fat malabsorption (leading to fat soluble nutrient deficiencies, such as choline, linoleic acid), and increasing BMI. Whilst CFTR modulators may reverse steatosis,95 with increasing prevalence of obesity rates in the CF population, metabolic-associated fatty liver disease incidence may rise. Given the known risk this presents for progression to fibrosis and cirrhosis, this further highlights the impetus for rigorous nutrition optimization in CF.
1.4.1 Detection of CFLD
Early detection of CFLD, particularly in those at risk of developing portal hypertension secondary to cirrhosis or porto-sinusoidal disease is paramount—as therapeutic interventions may have the potential to be more effective in pwCF with early disease. However, as CFLD is normally subclinical until advanced changes are present and phenotype is heterogenous with cirrhosis reported in 5%–10%, and mild steatosis in 10%–70%, yearly screening is recommended.
Diagnostic tools for detecting cirrhotic CFLD include liver biochemistry, APRI (AST to platelet ratio index), GPR (GGT to platelet ratio), FIB-4 scoring systems (surrogates for significant fibrosis96), and ultrasound97 (may detect changes such as steatosis and heterogeneous echotexture before serological anomalies as well as cirrhosis and portal hypertension). CT and MRI are not currently recommended as screening tools as they give little additional information other than a clarification of steatosis. Biomarkers such as microRNA have also been proposed as screening tools,98, 99 but confirmatory studies are required. Porto-sinusoidal or “non-cirrhotic” CFLD is suggested by splenomegaly, signs of portal hypertension without abnormalities in hepatic parenchyma or nodular regenerative hyperplasia.94
Elastography is emerging as a potential noninvasive measure of liver stiffness and has good accuracy in detecting fibrosis and discriminating liver disease severity.100 It is increasingly widespread in monitoring the progression of liver disease and evaluation of treatment. This modality may play a key role in differentiating the differing spectrums of CFLD, with classical “cirrhotic CFLD” noting increased liver stiffness with signs of portal hypertension and “non-cirrhotic CFLD”, or porto-sinusoidal vascular disease noting lower liver stiffness with signs of portal hypertension.94 In these cases, liver biopsy can be considered to confirm the diagnosis of porto-sinusoidal vascular disease.
Recommendations are that annual screening for CFLD should occur in all pwCF, including total bilirubin, AST, ALT alkaline phosphatase, GGT, platelets count, along with physical examination for hepatosplenomegaly. Ultrasound with doppler should be performed 1–2 yearly. For those who have elevated biochemistry, abnormal clinical or radiographic findings, evaluation of other causes of concomitant CF-unrelated disease such as autoimmune hepatitis, A1AT deficiency, Wilson's disease, chronic hepatitis, and coeliac disease should be performed. Depending on availability, elastography can be considered.
1.4.2 Treatment of CFLD
In those with portal hypertension, there are no specific recommendations with respect to primary prophylaxis of portal hypertension-related gastrointestinal bleeding compared to the general population. Lung disease may contraindicate beta-blockers and repeated general anesthesia for screening endoscopy. TIPS is an effective treatment for the complications of portal hypertension as a bridge to liver transplant; however, the increased risk of hepatic encephalopathy, particularly in those children on high-calorie/high-protein diets should be considered. Finally, there has been some success with distal splenorenal shunts in avoiding or delaying transplant.101
Pre-emptive transplant in cirrhotic CFLD before occurrence of severe portal hypertension and nutritional deterioration remains controversial. There is no evidence for the use of ursodeoxycholic acid, although it is widely used and appears to reduce elevated transaminase levels.102
1.4.3 CFLD in the era of modulators
Initially, elevation in liver transaminases was described in 5%–15% of pwCF as a side effect of CFTR modulators, yet only elexacaftor-texacaftor-ivacaftor has shown elevation in ALT levels compared to placebo.10, 37, 103-105 It is thought that modulators may have advantages in reducing rates of CFLD through reversibility of disease pathophysiology. Ivacaftor may have the potential to improve enterohepatic regulation of bile acids in pwCF, lumacaftor may restore defective phosphatidylcholine secretion of hepatocytes in some Class III gating mutations106 and lumacaftor/ivacaftor may restore biliary fluid secretion to normal levels.107 Indeed, improvement in hepatic steatosis108 improved liver transaminases and bilirubin,109, 110 including in those with multinodular liver and portal hypertension have been described. However, the reversibility and delay of advanced CFLD are currently unknown.
For those with advanced liver disease, there is the potential that modulators may be able to be used without dose adjustments in patients with Child-Pugh class A (mild hepatic impairment) or patient-specific consideration of dose reduction in patients with Child-Pugh class B (moderate hepatic impairment) but with great vigilance given the potential for altered pharmacokinetics and absorption. Modified dosing in those with abnormal liver function has been trialed,111 without necessarily impacting drug levels and clinical benefit in other CF-affected organs.
Overall, there is a need for a more stringent definition of CFLD, increased recognition of cirrhotic and non-cirrhotic CFLD, increased early screening tools, biomarkers to identify who is at risk of bile duct injury, portal hypertension or cirrhosis, and improved preventative therapies.
1.5 Cystic fibrosis bone disease
As median survival in CF has improved, CF-related bone disease (CFBD) has become increasingly prevalent, most often in adults, but children and adolescents with CF may also demonstrate low BMD.112 Both vertebral and non-vertebral fractures can occur in those with CFBD, but vertebral fractures are particularly problematic as they lead to pain, ineffective chest physiotherapy, reduced airway clearance and decline in lung function. Whilst severe lung disease and malnutrition are recognized as risk factors for CFBD, the pathophysiology is poorly understood. Diagnostic tools for CFBD are limited, and there are few treatment studies, so risk factors need to be recognized and addressed early and comprehensively to prevent progressive problems with fractures, pain and associated decline in respiratory function and well-being. Contributing factors are many and may include CFTR dysfunction, vitamin D and K deficiency, calcium malabsorption, delayed puberty/hypogonadism, smoking, alcohol and caffeine intake, and possibly most importantly—malnutrition, poor growth and low lean body mass (even with normal BMI).113-115 To achieve weight and lean body mass adequate for optimizing bone health, there needs to be sufficient protein intake, pancreatic enzyme replacement and adequate physical activity, all of which need to be actively managed. Furthermore, the increase in proinflammatory cytokines in respiratory infections and systemic inflammation can lead to bone remodeling and accelerated bone resorption.116 Treatment with corticosteroids is often required in CF management, which may reduce bone formation, reduce calcium absorption and lower sex steroids. In essence, the etiology of CFBD is multifactorial and needs a comprehensive approach in its prevention and management by addressing these modifiable risk factors early and proactively.
1.5.1 Screening for and monitoring of bone disease in CF
The recommended test for detecting and monitoring CFBD is a DXA scan; however, guidelines across the world differ in their recommendations about when to commence screening for CFBD and how often to perform it. In general, a DXA scan of the Total Body, AP Spine, and proximal femur should be performed in all adults with CF and in children with CF from 8 to 10 years of age who have risk factors for compromised bone health, with a repeat scan considered every 2 years; acknowledging that some groups opt to stretch screening to 5 years in low-risk patients.115, 117 Meanwhile, those with existing low BMD should have DXA assessments annually, ideally with a vertebral fracture assessment to exclude the need for a separate spine X-ray. The laboratory work-up for CFBD includes baseline calcium and phosphate, liver function tests including alkaline phosphatase as a measure of bone turnover, 25OH-vitamin D, parathyroid hormone, sex steroids, and thyroid stimulating hormone. Selected pwCF may benefit from further laboratory evaluation depending on risk factors and comorbidities.115
1.5.2 Pitfalls of pediatric DXA scans
While DXA is the gold standard for monitoring BMD in children, interpretation must be done carefully, accounting for the impact of stature, pubertal development, and skeletal maturation on BMD and bone area. As such, pediatric DXA scans are interpreted using BMD Z-scores generated from gender, age, size, pubertal stage (and optimally ethnicity) matched reference data.115, 118-120 Pitfalls other than using inadequately matched comparative data include inadequate calibrations of equipment and disregarding the impact of differences between DXA machines. A comprehensive pediatric DXA report should include standard deviation scores (Z-scores) adjusted for age, sex, and size, in addition to raw BMD, BMC, and bone area.
There are no adequate alternatives to DXA scans in screening for CF bone disease. Multiple factors in CF, such as protein malnutrition and corticosteroid treatment, can make the interpretation of bone turnover (e.g., P1NP and CTX) difficult by falsely lowering their values. Hence, while bone turnover markers may have some use in monitoring response and adherence to certain therapies, such as bisphosphonates, they are not useful screening tools in CFBD.
1.5.3 Managing CFBD
The management of CFBD is first and foremost nonpharmacological, including optimizing nutrition, calcium intake, vitamin D and K, sex hormones, and exercise. Exercise is critical for bone health, just as it is for general and respiratory health in CF. The type of exercise required to best optimize bone health is still under discussion, but weight-bearing activities and resistance are important as the signaling pathway for remodeling initiation and synthesis of new bone is induced by osteocytes that behave as mechanoreceptors. Ultimately, any exercise is beneficial for overall health, but it is apparent that tailoring exercise to the individual is necessary to improve bone and body composition as a whole.
Calcium malabsorption is not fully corrected by PERT. Compounding this are multiple factors specific to CF, including high salt diet-induced natriuresis causing high urinary calcium, vitamin K deficiency impairing the activation of osteocalcin, and vitamin D deficiency leading to reduced intestinal calcium absorption and increased bone resorption. There is broad variation in the recommendations regarding calcium and vitamin D supplementation, but targeting total calcium intake (from diet and if required, supplement) of 1300–1500 mg and aiming for vitamin D level of at least 50–75 nmol/L is likely sufficient.
Pharmacologic treatment, including antiresorptive therapy, is reserved for those with osteoporosis, the definition of which is different for adults versus children. While fracture efficacy data in children and young adults are lacking, there is good evidence that bisphosphonates increase bone density in children and adults with CF.117, 121, 122 There are no studies on treatment with denosumab or selective estrogen receptor modulators in CF populations, but some early data on the use of teriparatide (PTH analogue) in adults with CF are promising.123 This must be considered with caution, however, considering the risk of hypercalciuria, and the “Black Box” warning against use in children with open growth plates, or in pwCF of any age with prior radiotherapy or bone metastases.
In essence, CFBD is common and of multifactorial etiology. DXA is the gold standard for bone health assessment, but there are many common pitfalls that affect the production and reliable interpretation of DXA data. The treatment of CFBD is based on age, bone density, and fracture history, with nonpharmacological management being extremely important. There are currently limited studies on the efficacy and safety of antiresorptive and anabolic agents in this population, and further studies are needed to guide optimal monitoring and duration of treatment of these therapies.
1.5.4 CFTR modulators in CF bone disease
The positive impact of CFTR modulator therapy in CF bone disease is becoming increasingly apparent. This can largely be attributed to the impact of CFTR modulators on many of the modifiable risk factors/contributors to bone disease such as improved nutritional status and weight gain, improvement in lung disease with less infection and inflammation, less steroids and generally more capacity to partake in exercise and weight-bearing activities due to better general health. In addition to these indirect effects, CFTR is expressed in human osteoblasts and osteoclasts,124 and studies suggest that CFTR dysfunction itself may play a role in bone disease by influencing how bone cells (osteoblasts, osteoclasts, and osteocytes) communicate via the RANK ligand osteoprotegerin pathway and that this may be impacting the balance of bone formation and bone resorption in the skeleton. Following on from this are some early murine and human studies showing some retrospective improvement in bone health whilst on CFTR modulators.125-127 In essence, CFTR modulators may help improve CF bone health both directly and indirectly, but further studies are required to characterize this.
In addition to prospective studies on the impact of CFTR modulators on CFBD, future areas of interest include the effects of insulin (this may be the most physiological method by which BMD can be improved), growth hormone and sex steroids on BMD accrual, as well as the use of anabolic agents teriparatide (PTH analogue), abaloparatide (PTH related peptide analogue), and romosozumab (sclerostin inhibitor) and their safety and impact in pwCF.
1.6 Extra-pulmonary complications pre and post-lung transplant
Historically, 25% of all lung transplants are for CF,128 however, there are suggestions this rate is decreasing and may decrease further in the era of CFTR modulators. Extra-pulmonary complications influence patient selection for transplant but also transplant success.
PwCF undergoing lung transplant have a more complex course in the setting of pre-transplant extra-pulmonary complications; many of which are contra-indications or risk factors for transplant failure. These include relative contraindications such as renal dysfunction, liver cirrhosis with portal hypertension or synthetic dysfunction and recurrent infection.129 Noncompliance is also a relative contraindication, and possibly secondary to this, adolescents with CF have worse outcomes than those without.130 Other risk factors for poor long-term outcomes which may complicate care in those with CF undergoing transplant include gastro-esophageal reflux, osteoporosis, hypoalbuminaemia, poorly controlled diabetes,131 as well as BMI greater than 35 kg/m2 or less than 16 kg/m2. It is important to note that end-stage CF lung disease is itself a risk-factor for poor nutrition and BMI, which may, in fact, represent a non-modifiable risk factor that may be an indication of the need for transplant rather than the need for supplementing enteral nutrition.
Outcomes following lung transplantation lag behind that for other solid organ transplants, with 5-year survival quoted at around 70% and 10-year survival between 40% and 50%.128 Major short-term complications include infection and graft failure, with longer-term complications centered around chronic lung allograft dysfunction (CLAD) and a heightened risk of malignancy. CLAD remains the major hurdle limiting long-term survival—and prevention revolves around cessation of the lung injury cycle and abnormal repair causing airway (bronchiolitis obliterans syndrome) and/or interstitial fibrosis (restrictive allograft syndrome). This involves preventative titration of immunosuppression to minimize acute cellular rejection while managing the known side effects of immunosuppression, including an increased risk of infection, renal disease, aspiration, gastro-esophageal reflux, hypertension, and diabetes. To achieve this balance, immunosuppression is individualized to immunological risk (rejection history, age, HLA antibodies, and multi-visceral transplant) and recipient morbidity profile.
For the CF population, management of extra-pulmonary complications has a major impact on lung transplant survival and quality of life. Comorbidities such as renal disease with prior exposure to nephrotoxins (such as aminoglycosides) or diabetic nephropathy, may increase the risk of chronic kidney disease already quoted at 25% at 10 years post-transplant,108 which complicates immunosuppression and is associated with decreased quality of life. Nutrition can be complicated by exacerbation of underlying GI dysmotility with opioids and anxiolytics post-transplant, as well as the risk of gastroparesis with vagal nerve damage intraoperatively. This can result in worsening constipation, nausea and vomiting. Antibiotic use can also worsen GI dysbiosis and should be prescribed appropriately. GORD can lead to Barrett's esophagus and esophageal cancer in CF. Micro-aspiration is a risk factor for the development of CLAD—so treatment should be optimized pre- and post-transplant. Gastrointestinal (colorectal, small bowel, biliary, and pancreatic) malignancy occurs at increased frequency independently in CF and post-lung transplant populations with amplified risk in the CF post-transplant group. The current recommendation is for colonoscopy screening to commence from 30 years of age.132
It is important to note that poor glycaemic control post-transplant is a major risk factor for rejection, infection and allograft failure rates,133, 134 particularly relevant to CF with the risk of CFRD. Optimization of these modifiable risk factors is essential pre-transplant.
Post-transplant, there is a paucity of evidence for long-term effects of modulator therapy on disease outcomes. For those on modulators, there may a requirement to adjust tacrolimus dosing, given the potential for CYP3A inhibition, guided by tacrolimus levels. Dosing may be required to be withheld if bilirubin is greater than two times the upper limit normal and if liver transaminases are five times the ULN or greater.
1.6.1 Recommendations for post-lung transplant care
Early multidisciplinary optimization of extra-pulmonary complications has major implications on patient selection and long-term outcome in lung transplant for pwCF. Cooperative care through a multi-disciplinary CF clinic and multidisciplinary transplant center input is the ideal model of care to ensure optimization of risk factors for complications and ongoing surveillance.
2 SUMMARY/CONCLUSION
2.1 Individualise nutritional management of children with CF.
A paradigm shift is needed with a focus on individualized diet and lifestyle interventions based on disease phenotype, rather than the traditional legacy high-fat, high-calorie diet for all. PwCF should have periodic nutritional assessments after starting modulator therapy, with BMI and body composition being used to monitor for under and over-nutrition and to individualize dietary and nutritional advice (see Figure 1).
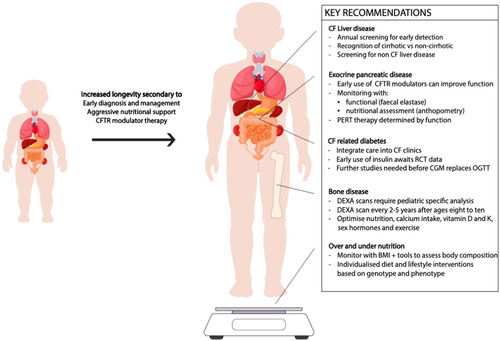
2.2 Window of pancreatic disease reversibility
Further research is needed into the optimum timing of CFTR modulation to salvage pancreatic function. Early postnatal treatment at birth, or potentially in utero, is likely to offer the greatest potential for any degree of clinically significant exocrine pancreatic recovery. The exocrine pancreatic function should be monitored with pancreatic function assessment (6–12 monthly faecal elastase) as well as with secondary markers on nutrient absorption (anthropometry—BMI/body composition and fat-soluble vitamins).
PERT will need to be individualized, with reduction or cessation guided by markers of exocrine pancreatic function.
2.3 CGM is a promising tool in the diagnosis and management of CFRD, but further studies are needed
Further studies are required to identify the clinical significance of specific CGM thresholds before CGM can be used to replace the OGTT as a diagnostic tool for CFRD.
2.4 Potential benefits of insulin therapy for treating early dysglycaemia in PwCF are yet to be determined
Early dysglycaemia (before the onset of CFRD as it is currently defined) has been associated with poor outcomes in CF patients. The results of a randomized controlled trial of early insulin treatment are expected in the near future.
2.5 Integrating CFRD care into the CF clinic
CFRD-specific expertize should be integrated into the existing CF clinic, including providers familiar with newer diabetes technology such as CGM.
2.6 Current status of liver disease
Screening for CF-related liver disease should begin in early childhood to anticipate those who will develop complications. Regular clinical examination and blood tests are recommended, although sensitivity and specificity for predicting advanced disease is limited. Ultrasound is a widely available and relied upon modality for the detection of advanced liver disease, but nonspecific changes are common. Elastography is more sensitive for detection, monitoring and progression of liver disease. Screening for concomitant CF-unrelated liver disease is essential when transaminases rise, but a second chronic liver disease is uncommon in the CF population.
2.7 Optimize risk factors for CFBD
Diagnostic tools for CF bone disease are limited, and there are few treatment studies, but DXA is the most widely used method to assess BMD in adults and children. Risk factors for compromised skeletal health need to be recognized and addressed early to prevent progressive problems with fractures, which can lead to significant pain and potential decline in respiratory function and well-being. These risk factors include glucocorticoid treatment, lung inflammation, vitamin D and K deficiency, calcium malabsorption, delayed puberty or hypogonadism, physical inactivity, and malnutrition.
2.8 Pediatric-specific DXA
Standardized, pediatric-specific acquisition and analysis of DXA data is essential for reproducible and clinically significant interpretation of BMD and body composition data in children and adolescents.
2.9 Extrapulmonary complications of transplant
Optimal and timely management of extra-pulmonary complications in CF is associated with improved health outcomes and survival post-transplant. Coordinated care through a multi-disciplinary CF center with extra-pulmonary CF specialists and transplant center input is the ideal model of transplant care.
THE SHIFT SYMPOSIUM 2021
John F. Engelhardt: Department of Anatomy and Cell Biology, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City, USA.
Tamarah Katz: Department of Nutrition and Dietetics, Sydney Children s Hospital, Sydney Australia.
Susannah King: Nutrition Department, Alfred Health, Melbourne, Victoria, Australia.
Peter J Lewindon: Department of Gastroenterology, Hepatology and Liver Transplant, Queensland Children s Hospital, Brisbane Australia; Faculty of Medicine, The University of Queensland, Australia.
Angela G. Matson: CF Endocrine Service Adult Cystic Fibrosis Centre (ACFC) The Prince Charles Hospital, Australia.
Catherine M. McDonald: Primary Children s Hospital, Salt Lake City, USA.
Amir Moheet: Department of Medicine, University of Minnesota, USA.
Michael R. Narkewicz: Digestive Health Institute, Children s Hospital Colorado and Section of Pediatric Gastroenterology, Hepatology and Nutrition, University of Colorado School of Medicine, Aurora, Colorado, USA.
Mark Oliver: Department of Gastroenterology, Royal Children's Hospital, Australia; University of Melbourne, Australia.
Grant A. Ramm: Faculty of Medicine, The University of Queensland, Australia.
Hepatic Fibrosis Group, QIMR Berghofer Medical Research Institute, Australia.
Zachary M. Sellers: Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Stanford University, Palo Alto, California, USA.
Michael Wilschanski: Pediatric Gastroenterology, Hadassah University Hospitals, Jerusalem, Israel.
AUTHOR CONTRIBUTIONS
Jonathan E. M. O'Donnell: Conceptualization; investigation; writing—original draft; writing—review and editing. Lucy A. Hastings: Conceptualization; investigation; writing—original draft; writing—review and editing. Julie N. Briody: Conceptualization; writing—review and editing. Christine L. Chan: Conceptualization; writing—review and editing. Carla Colombo: Conceptualization; writing—review and editing. Tonia A. Douglas: Conceptualization; writing—review and editing. Steven D. Freedman: Conceptualization; writing—review and editing. Tanja Gonska: Conceptualization; writing—review and editing. Jerry R. Greenfield: Conceptualization; writing—review and editing. Daniel H. Leung: Conceptualization; writing—review & editing. Adeline Y. L. Lim: Conceptualization; writing—review and editing. Antoinette Moran: Conceptualization; writing—review and editing. Bernadette J. Prentice: Conceptualization; writing—review and editing. Melissa S. Putman: Conceptualization; writing—review and editing. Michael Trotter: Conceptualization; writing—review and editing. Elizabeth Tullis: Conceptualization; writing—review and editing. Glen P. Westall: Conceptualization; writing—review and editing. Charles F. Verge: Conceptualization; writing—original draft; writing—review and editing. Claire E. Wainwright: Conceptualization; investigation; writing—original draft; writing—review and editing. Chee Y. Ooi: Conceptualization; investigation; writing—original draft; writing—review and editing; supervision; resources. SHIFT Symposium: writing—review and editing.
ACKNOWLEDGMENTS
CYO is funded by the National Health and Medical Research Council (NHMRC, Australia) Investigator Grant (2020/GNT1194358). Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The SHIFT symposium was supported by Vertex Pharmaceuticals, with no involvement in manuscript. This was inclusive of honoraria to JB, CLC, TD, AYLL, BP, MP, MT, ET, CEW, CYO, JFE, TK, SK, PL, AMo, MW, MO, CV. CLC has received grant funding from the Cystic Fibrosis Foundation and honorarium from Vertex Pharmaceuticals as a consultant, donations from Dexcom, Insulet for research supplies. CC has received honoraria payment for advisory boards on Cystic Fibrosis. TD has received honoraria from Vertex Pharmaceuticals for educational activities, lectures and presentations. DHL has received grants from the Cystic Fibrosis Foundation, NIDDK and honoria from Vertex Pharmaceuticals. SD Freedman has received grants from NIH, Massachusetts Life Sciences, Cystic Fibrosis Foundation. AM has received grants from Abbott Diabetes, consulting fees from Abata, has participated in data safety monitoring for Novo Nordisk, received research equipment from Medtronic. BP was involved with TSANZ Cystic Fibrosis SIG as co-convenor. MP has received grants from Vertex Pharmaceuticals, NIH, Cystic Fibrosis Foundation, consulting fees from Anagram Therapeutics, involved with Cystic Fibrosis Foundation data safety board member, Associate Editor for Endocrine Practice: the Journal of the American Association of Endocrinologists. ET has received grants from Vertex Pharmaceuticals, Spyryx, consulting fees and speaking fees from Vertex Pharmaceuticals. JFE has received grants from NIDDK, NHLBI, honoria from Vertex Pharmaceuticals. TK has received consulting fees and honoraria from Vertex Pharmaceuticals. SK was a member on the Steering Committee, Australian Cystic Fibrosis Data Registry 2017-2021. AGM has received grants from TPCH, ACFC; travel subsidies from Vertex Pharmaceuticals. AMo has received grant funding from the Cystic Fibrosis Foundation. MN has received grants from the Cystic Fibrosis Foundation, Abbvie, Gilead, consulting fees from Vertex Pharmaceuticals ZMS has received grants from NIH, Cystic Fibrosis Foundation, Cystic Fibrosis Research Institute, NASPGHAN Foundation, consulting fees from Renexxion, Vertex Pharmaceuticals, Abbvie. Was on the NASPGHAN Pancreas Committee Vice Chair/Chair, CF Foundation CFLD Guidelines Committee Co-Chair. MW has received research funding from Vertex Pharmaceuticals, consulting fees from Anagram Therapeutics TG has received grants from Vertex Pharmaceuticals and Canadian Institute of Health Research. CV has received research grants from NHMRC, CF Australia, Sydney Children's Hospital Foundation, Pfizer, research support from Novo Nordisk, Abbott, Medtronic, honoraria from Vertex Pharmaceuticals. CEW is Associate Editor Respirology, Associate Editor Thorax, on the International Advisory Board for Vertex Pharmaceuticals. CYO has received research funding from the National Health and Medical Research Council (NHMRC Australia) Investigator Grant (2020/GNT1194358), research funding from Vertex Pharmaceuticals, consulting fees from Vertex Pharmaceuticals, is on an advisory board for Vertex Pharmaceuticals. w Article.
Open Research
DATA AVAILABILITY STATEMENT
Data is available on request from the authors.




