A screening tool to identify risk for bronchiectasis progression in children with cystic fibrosis
This study has been previously presented in a conference as an abstract, Am J Resp Crit Care Med 193;2016: A5580.
Abstract
Background
The marked heterogeneity in cystic fibrosis (CF) disease complicates the selection of those most likely to benefit from existing or emergent treatments.
Objective
We aimed to predict the progression of bronchiectasis in preschool children with CF.
Methods
Using data collected up to 3 years of age, in the Australian Respiratory Early Surveillance Team for CF cohort study, clinical information, chest computed tomography (CT) scores, and biomarkers from bronchoalveolar lavage were assessed in a multivariable linear regression model as predictors for CT bronchiectasis at age 5–6.
Results
Follow-up at 5–6 years was available in 171 children. Bronchiectasis prevalence at 5–6 was 134/171 (78%) and median bronchiectasis score was 3 (range 0–12). The internally validated multivariate model retained eight independent predictors accounting for 37% (adjusted R2) of the variance in bronchiectasis score. The strongest predictors of future bronchiectasis were: pancreatic insufficiency, repeated intravenous treatment courses, recurrent lower respiratory infections in the first 3 years of life, and lower airway inflammation. Dichotomizing the resulting prediction score at a bronchiectasis score of above the median resulted in a diagnostic odds ratio of 13 (95% confidence interval [CI], 6.3–27) with positive and negative predictive values of 80% (95% CI, 72%–86%) and 77% (95% CI, 69%–83%), respectively.
Conclusion
Early assessment of bronchiectasis risk in children with CF is feasible with reasonable precision at a group level, which can assist in high-risk patient selection for interventional trials. The unexplained variability in disease progression at individual patient levels remains high, limiting the use of this model as a clinical prediction tool.
1 INTRODUCTION
Cystic fibrosis (CF) is a severe genetic disease characterized by progressively destructive lung disease resulting in bronchiectasis and respiratory failure.1 There is marked heterogeneity of disease expression and progression, even among patients with the same CFTR genotype.2 Therefore, evaluating variables that predict accelerated disease progression, has been a focus of a considerable number of studies in patients with CF.3
In young preschool children, predicting accelerated disease progression is further complicated by the availability of adequate outcome measures to inform disease progression.3, 4 Spirometry, which is considered a major outcome in adults, is difficult to perform, maybe unreliable5 and is usually normal despite significant structural lung damage as seen on computed tomography (CT).5, 6 Other measures, such as the lung clearance index (LCI), are increasingly being studied in young children, and are used as a surrogate endpoint in clinical trials.7, 8 Still, the overall mild lung disease in most preschool children with CF limits the practicality of even using LCI to routinely assess a treatment effect on lung disease. Indeed, recent trials evaluating the efficacy of novel CFTR modulators in preschool children present effects on nutritional status and sweat chloride measurements but not respiratory outcomes.9-12
Structural lung disease, and especially bronchiectasis, are a definite endpoint in lung disease progression in CF and have been shown to be associated with quality of life, mortality, and predict future lung function decline.13-15 Thus, correctly identifying young children at increased risk of structural lung disease progression, in addition to its clinical importance, may improve patient selection for clinical trials. This will allow better assessment of treatment effects on lung health in this population.
Using data from the well-phenotyped Australian Respiratory Early Surveillance Team (AREST) CF birth cohort with follow-up until 6 years of age, we aimed to optimally predict the increase in CT bronchiectasis score to enable identification of children with the highest risk for rapid progression of structural lung disease.
2 METHODS
2.1 Study population
Infants diagnosed with CF after newborn screening at the CF clinics of the Princess Margaret Hospital and at the Royal Children's Hospital have been recruited in the AREST CF study. Comprehensive examinations were performed at the “annual visits” at age 3 months, 1 year, and yearly thereafter, including a bronchoscopy under general anesthesia with bronchoalveolar lavage (BAL). Since 2005 a chest CT under general anesthesia was also included in the annual follow-up in children up to the age of 5 years in Melbourne and up to the age of 6 years in Perth. The study was approved by the Ethics Committee of the Child and Adolescent Health Service (approval no. 1762EPP) and Royal Children's Hospital (approval no. 25054) and parents provided informed consent. During the study, none of the children were on CFTR modulator therapy.
2.2 Data collection
At each clinical follow-up, a respiratory symptom and medication questionnaire were completed, and physical examination was recorded. BAL fluid was cultured for the detection of pathogens and analyzed for markers of inflammation (neutrophil elastase, interleukin 8, % neutrophils). Chest CT scans consisted of two volume-controlled limited slice scans obtained at end inspiration (transrespiratory pressure = 25 cmH2O) and end expiration (transrespiratory pressure = 0 cmH2O). Data on the number of admissions and intravenous (IV) antibiotic courses were collected from the Australian CF Data Registry.
2.3 Outcome variable
CT images were scored using the CF–CT scoring method.17 The outcome variable at end of follow-up was defined as the CF–CT bronchiectasis score at age 6 years if available, alternatively at age 5, and analyzed as a continuous score using linear regression. After the multivariable prediction model was built and validated using this continuous score, the median score at age 5–6 was used to dichotomize the predicted outcome in high versus low-risk groups.
2.4 Predictor variables
Baseline predictor variables included homozygosity for CFTR p.Phe508del mutation, residual CFTR function, sweat chloride concentration, meconium ileus, pancreatic sufficiency, and socioeconomic status (socioeconomic index for areas 2011 census data according to postal codes at birth).18
Age-specific variables used as predictors included data collected at 3 months of age and at each annual follow-up thereafter until 3 years of age. These predictors included CF–CT bronchiectasis and air trapping scores, BAL results of infection and inflammation, the number of IV antibiotic courses in the year before the annual visit, and anthropometric measurements. Bacterial pathogens known to elicit a clear proinflammatory response in the lower respiratory tract (Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, and Aspergillus species) were also defined as a combined variable of proinflammatory pathogens.19
2.5 Statistical analysis
Univariate associations between candidate predictor variables up to 3 years of age, and the outcome of bronchiectasis score at 5–6 years were investigated. To avoid selection bias, missing data were imputed using the “multivariate normal regression procedure” in STATA. Predictor variables were taken into account in constructing 100 multiple imputed datasets. A data-driven approach was used to select the best combination of predictors, by means of backward stepwise linear regression with likelihood ratio statistics as a criterion for selection.20 Akaike's information criterion was used to select predictors equivalent to p < 0.157.21 The model's predictive ability was assessed using adjusted R2, calibration (graphically), and Bland–Altman plot. Internal model validation was performed using a bootstrap resampling technique.22 This enabled us to estimate the bias (i.e., optimism) caused by overfitting and to correct performance estimates as well as the regression coefficients (shrinkage) accordingly.23 All analyses were performed using STATA 13.0.
3 RESULTS
3.1 Study population
A total of 171 children completed 5 or 6 years of the AREST CF program and were included in the current study. The overall proportion of missing data on the selected candidate predictor variables at baseline (n = 8) was 12%, and for the age-specific candidate predictor variables (n = 10) at Age 1 33%, at Age 2 28%, and at Age 3 25%. Additional patient characteristics and associations with bronchiectasis at 5 or 6 years of age are presented in Table 1.
| n/N (%) or median (range), N | The difference in score bronchiectasis 5–6 years | p | |
|---|---|---|---|
| Predictor variables at baseline | n/N (%) | ||
| Gender (male) | 86/171 (50%) | 0.81 (−0.37 to 1.99) | 0.178 |
| Pancreatic insufficiency | 135/161 (84%) | 2.90 (1.30 to 4.50) | <0.001 |
| Research centre (Perth) | 67/171 (39%) | 0.67 (−0.55 to 1.88) | 0.280 |
| Genetic abnormality | |||
| Homozygous p.Phe508del | 85/169 (50%) | 0.32 (−0.88 to 1.51) | 0.602 |
| Heterozygous p.Phe508del | 75/169 (44%) | Combined as referencea | |
| No p.Phe508del | 9/169 (5%) | ||
| Meconium ileus (present) | 32/160 (20%) | 1.58 (0.05–3.10) | 0.042 |
| Sweat chloride (<60)b | 14/83 (17%) | 0.40 (0.06–0.73) | 0.020 |
| SEIFA quintile scorec | N = 170 | −0.23 (−0.66 to 0.20) | 0.297 |
| Age-specific predictor variables | Median (IQRd), N | ||
| BMI age 1 | 16.9 (15.9–18.2), 111 | −0.36 (−0.77 to 0.04) | 0.078 |
| BMI age 2 | 16.3 (15.3–17.6), 107 | 0.04 (−0.39 to 0.48) | 0.838 |
| BMI age 3 | 16.3 (15.3–17.0), 107 | −0.68 (−1.19 to −0.17) | 0.010 |
| CF–CT scores | |||
| Bronchiectasis score age 1 | 0 (0–0), 93 | 1.05 (0.50–1.59) | <0.001 |
| Bronchiectasis score age 2 | 0 (0–1), 101 | 0.58 (0.25–0.91) | 0.001 |
| Bronchiectasis score age 3 | 1 (0–3), 120 | 0.66 (0.45–0.88) | <0.001 |
| Air trapping score age 1 | 2 (1–5), 92 | 0.37 (0.06–0.68) | 0.019 |
| Air trapping score age 2 | 3 (1–6), 100 | 0.59 (0.37–0.80) | <0.001 |
| Air trapping score age 3 | 2 (0–6), 120 | 0.48 (0.28–0.68) | <0.001 |
| BAL fluid inflammation (continuous) | |||
| % Neutrophils age 1e | 16.5 (7.7–34.0), 124 | 0.38 (0.03–0.73) | 0.032 |
| % Neutrophils age 2e | 19.5 (7.0–41.3), 134 | 0.38 (0.12–0.65) | 0.005 |
| % Neutrophils age 3e | 20.0 (9.3–48.0), 140 | 0.60 (0.38–0.81) | <0.001 |
| BAL fluid inflammation (yes/no) | n/N (%) | ||
| Neutrophil elastase present age 1 | 23/124 (19%) | 1.79 (0.87–3.49) | 0.040 |
| Neutrophil elastase present age 2 | 29/137 (21%) | 1.85 (0.33–3.38) | 0.017 |
| Neutrophil elastase present age 3 | 42/141 (30%) | 2.50 (1.19–3.80) | <0.001 |
| Interleukin 8 present age 1 | 113/124 (91%) | 2.56 (0.24–4.89) | 0.031 |
| Interleukin 8 present age 2 | 122/137 (89%) | 2.53 (0.55–4.52) | 0.013 |
| Interleukin 8 present age 3 | 129/140 (92%) | 1.71 (−0.59 to 4.00) | 0.144 |
| BAL fluid infection | |||
| Proinflammatory pathogen age 1 | 24/124 (19%) | 1.68 (−0.00 to 3.35) | 0.050 |
| Proinflammatory pathogen age 2 | 48/137 (35%) | 1.12 (−0.19 to 2.44) | 0.094 |
| Proinflammatory pathogen age 3 | 63/144 (44%) | 2.46 (1.27–3.64) | <0.001 |
| ≥ 2 Proinflammatory pathogens age 0–2 | 21/146 (14%) | 1.28 (0.45–2.12) | 0.003 |
| ≥ 2 Proinflammatory pathogens age 0–3 | 45/152 (30%) | 1.68 (0.98–2.39) | <0.001 |
| Any IV AB's last year age 1 | 29/131 (22%) | 0.94 (0.00–1.87) | 0.049 |
| Any IV AB's last year age 2 | 29/142 (20%) | 1.10 (0.17–2.02) | 0.021 |
| Any IV AB's last year age 3 | 32/147 (22%) | 1.29 (0.35–2.24) | 0.008 |
- Note: Complete case analysis using all available data per variable. The bold values indicate p < 0.05.
- Proinflammatory pathogens: Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, or Aspergillus species.
- Abbreviations: AB, antibiotic; BAL, bronchoalveolar lavage; BMI, body mass index; CF, cystic fibrosis; CT, computed tomography; IQR, interquartile range; IV, intravenous; SEIFA, socioeconomic index for areas.
- a Association analyzed as dichotomous variable homozygous p.Phe508del versus not homozygous p.Phe508del.
- b Association analyzed as continuous variable, per 10 mmol chloride/ml increase.
- c SEIFA defined in quintiles and association analyzed per one-step increase in quintile.
- d IQR, 25th to 75th percentile.
- e Association analyzed per 10% increase in percentage neutrophils.
3.2 Outcome bronchiectasis score at 5 or 6 years
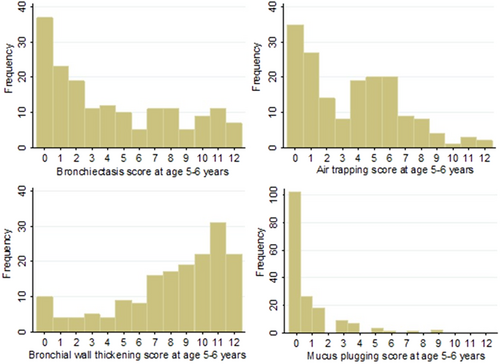
The distribution of all CF–CT subscores at Age 5–6 are given in Figure 1. One hundred and thirty-three children out of 171 (78%) had evidence of bronchiectasis (median score = 3, interquartile range = 1–8). There was a moderate to strong statistically significant correlation between bronchiectasis and all other CF–CT sub scores; air trapping score rs = 0.54 (p < 0.001), bronchial wall thickening score rs = 0.63 (p < 0.001), and mucus plugging score rs = 0.42 (p < 0.001).
3.3 Univariate analysis
Pancreatic insufficiency and meconium ileus were significant univariate predictors for later development of bronchiectasis. Homozygosity for p.Phe508del mutation was not a significant univariate predictor, using the CFTR2 database we also categorized genetic abnormalities according to their expected disease severity.24 However, as this is mainly defined by expected pancreatic insufficiency the resulting variable did not differ substantially from our existing variable on actual pancreatic insufficiency. Sweat chloride can also be considered a functional measure of residual CFTR function and was a significant univariate predictor. As expected, CF–CT subscores were all predictive of bronchiectasis scores at 5–6. To reduce the number of variables and the risk of collinearity, only bronchiectasis and air trapping (which showed the strongest association) were included in the imputed multivariate model. Other variables found to be significant predictors of bronchiectasis scores at 5–6 are presented in Table 1.
3.4 Multivariable analysis and model construction
Eight predictors were retained in the model: reduced body mass index (BMI), pancreatic insufficiency, homozygosity for p.Phe508del, two or more IV courses in the first 3 years of life, two or more lower respiratory proinflammatory infections in the first 3 years of life, CF–CT bronchiectasis, and air trapping scores at 3 years of age and percentage of neutrophils in BAL fluid at 3 years of age (Table 2). Neutrophil elastase did not remain an independent predictor in the multivariable model despite its strong univariate association, reflecting positive correlations with other predictors (i.e., the percentage neutrophils in BAL [p < 0.001]).
| Predictor variable | The difference in bronchiectasis score (CI95%) before internal validationa | The difference in bronchiectasis score (CI95%) after internal validationa | p | ||
|---|---|---|---|---|---|
| 1 | CF–CT subscores age 3 | ||||
| 1a Bronchiectasis (Bx) score | 0.35 (0.12–0.58) | 0.25 (0.09–0.41) | 0.003 | ||
| 1b Air trapping (AT) score | 0.18 (−0.04 to 0.39) | 0.13 (−0.03 to 0.28) | 0.107 | ||
| 2 | BAL fluid % neutrophilsb age 3 | 0.33 (0.10–0.56) | 0.24 (0.08–0.41) | 0.005 | |
| 3 | ≥2 Proinflammatory pathogens age 0–3 | 1.19 (−0.03 to 2.42) | 0.86 (−0.02 to 1.74) | 0.056 | |
| 4 | ≥2 IV AB courses age 0–3 | 1.27 (−0.06 to 2.59) | 0.91 (−0.04 to 1.86) | 0.060 | |
| 5 | Pancreatic insufficiency | 1.55 (−0.04 to 3.13) | 1.11 (−0.03 to 2.26) | 0.056 | |
| 6 | BMI age 3 | −0.28 (−0.62 to 0.06) | −0.20 (−0.45 to 0.04) | 0.105 | |
| 7 | p.Phe508del homozygosity | −0.86 (−1.98 to 0.27) | −0.62 (−1.43 to 0.19) | 0.133 | |
| Adjusted R2 | 40% | 37% | |||
| Validated prediction model for Bx score at age 5 or 6 years: | |||||
| 4.60 + 0.25 × Bx-score3 years + 0.13 × AT-score3 years + 0.24 × BAL %-neutrophils3 years + 0.86 × N proinflammatory pathogens0–3 years + 0.91 × N IV AB courses0–3 years + 1.11 × pancreatic insufficiency − 0.20 × BMI3 years − 0.62 × Delta508 homozygosity | |||||
- Note: Proinflammatory pathogens: Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, or Aspergillus species.
- Abbreviations: AB, antibiotic; AT, airtrapping; BAL, bronchoalveolar lavage; BMI, body mass index; BX, bronchiectasis; CI, confidence interval; CF, cystic fibrosis; CT, computed tomography; IV, intravenous.
- a Internal validation was performed using the bootstrapping method.
- b Association analyzed per 10% increase in percentage neutrophils.
3.5 Model validation and assessment
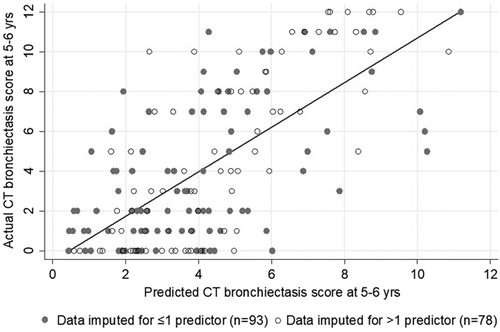
The discriminative power of the imputed multivariate model represented by adjusted R2 was 40%. Figure 2 graphically shows the predicted and actual CF–CT bronchiectasis score at 5–6 for children with complete and incomplete data. Bootstrap validation techniques to correct for overoptimism caused by overfitting estimated a validated adjusted R2 to be 37%. Coefficients for individual predictors are also reported after shrinkage by bootstrap internal validation to prevent overoptimistic predictions in new populations (Table 2).
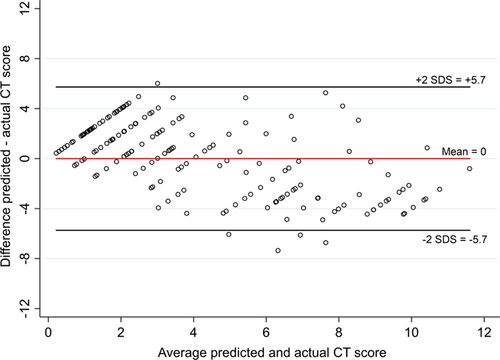
To further assess the model's predictive ability in individual patients, a Bland–Altman plot was constructed (Figure 3), showing good agreement on average between the predicted and the observed bronchiectasis scores. However, the 95% limits of agreement ranging from minus to plus 5 are wide. Furthermore, the plot seems skewed with a relative overestimation of bronchiectasis scores in children with lower actual scores and underestimation of scores in children with higher actual scores. This would limit the clinical utility of the rule in individual patients and is an explanation for the relatively wide limits of agreement.
3.6 The impact of different variables from the prediction tool
To appreciate the clinical impact of the adjusted and validated predictors, their effect size on the outcome of bronchiectasis at 5 or 6 years of age was estimated comparing the 25th versus the 75th percentile for each variable for continuous variables and presence or absence for dichotomic variables (Table 3). This shows that pancreatic insufficiency, two or more IV treatment courses and two or more lower respiratory infections in the first 3 years of life and percentage of neutrophils in BAL fluid at age 3 years, are the strongest predictors of bronchiectasis at 5 or 6 years of age. Each with an impact that is larger than that of pre-existing bronchiectasis at age 3.
| Predictors (continuous) | Mean | 25th percentile | Median | 75th percentile | The difference in predicted BE score at 5–6 for 25th versus 75th percentile |
|---|---|---|---|---|---|
| Bronchiectasis score at age 3 | 2.2 | 0 | 1.1 | 3.1 | +0.79 |
| Air trapping score at age 3 | 3.2 | 1 | 2.7 | 4.9 | +0.50 |
| BAL fluid % neutrophils age 3 | 31.5 | 10.3 | 26.0 | 49.0 | +0.93 |
| BMI age 3 | 16.1 | 15.2 | 16.2 | 17.0 | −0.37 |
| Predictors (dichotomous) | % Yes | % No | The difference in predicted Bx score at 5–6 for yes versus no | ||
| p.Phe508del homozygosity | 50.0 | 50.0 | +0.50 | ||
| Pancreatic insufficiency | 84.9 | 15.1 | +1.11 | ||
| ≥2 Proinflammatory pathogens age 0–3 | 38.5 | 61.5 | +0.86 | ||
| ≥2 IV AB courses age 0–3 | 26.5 | 73.5 | +0.91 | ||
| Outcome | Mean | 25th Percentile | Median | 75th Percentile | |
| Bx score at 5–6 | 4.3 | 1.0 | 3.0 | 8.0 | |
- Note: Proinflammatory pathogens: Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, or Aspergillus species.
- Abbreviations: AB, antibiotic; BAL, bronchoalveolar lavage; BX, bronchiectasis; BMI, body mass index; IV, intravenous.
3.7 Use of the internally validated model
Dichotomizing the CT scores assist in identifying groups of patients with a higher risk of developing more severe bronchiectasis. This could be of particular interest when selecting patients for an interventional trial in which prevention of bronchiectasis is the primary objective. Thus, we assessed the ability of the model to identify subjects with a CF–CT bronchiectasis score above the median (CF–CT bronchiectasis score > 3) at 5–6 years of age.
Of the 85 children (50% of the total group) with a predicted bronchiectasis score of above the median, 68 children had an actual bronchiectasis score of >3 at 5–6 years. This resulted in positive and negative predictive values of 80% (95% confidence interval [CI], 72%-86%) and 77% (95% CI, 69%-83%), respectively, with a diagnostic odds ratio of 13 (95% CI, 6.3–27).
4 DISCUSSION
Using data from the prospective longitudinal AREST CF cohort 8 clinical variables present at 3 years of age were selected to estimate the risk of progression of bronchiectasis at 5–6 years. The presence of pancreatic insufficiency, lower respiratory inflammation, and recurring need for treatment with IV antibiotics (recurrent infections and significant exacerbations) were the strongest predictors of bronchiectasis development in the subsequent 3 years. These variables could be used for the early identification of a group of children at high risk for lung disease progression. Disease prediction in individual patients to guide day-to-day clinical decisions remains difficult, despite the use of a comprehensive model.
Factors associated with worse CF lung disease have been previously reported. Most of these studies predicted CF sequelae in adolescence or adulthood13, 25-29 and only a minority have included young children.4, 30 From these studies, several risk factors for disease progression have been uniformly recognized. Forced expiratory volume in the 1 s at baseline is the most consistently reported predictor of future lung disease4, 13, 25, 26, 28-30 but is unavailable for use in young children. Other classic predictors of future lung disease, more relevant for young children include Pseudomonas aeruginosa colonization,4, 25, 27, 30 low BMI,4, 25, 27-30 pancreatic insufficiency,25, 27 pulmonary exacerbations,4, 28, 30 and structural lung disease.14, 16, 31
In our analysis, due to the low prevalence of pseudomonas in the first 3 years of life32 pseudomonas infections were considered in the total number of proinflammatory pathogens from BAL and not included as a separate variable. Our data show that in this very young age group, the presence of proinflammatory pathogens (not uniquely pseudomonas), contribute to the development of lung damage, as has also been recently reported.33 This suggests that early proinflammatory infections should be a target for early intervention.
Our data also support the relevance of recurrent pulmonary exacerbations as a predictor of worse lung disease and suggest that even in infancy the need for IV treatment is associated with worse structural lung damage at least up to 5–6 years of age.
As expected, we found CT bronchiectasis score at 3 years to be highly predictive of bronchiectasis at the age of 5–6 years. We also show that air trapping CT score at 3 years is predictive of later bronchiectasis, independent of concomitant CT bronchiectasis score, supporting the hypothesis that air trapping is an early consequence of inflammation and obstruction of the smaller airways, eventually leading to irreversible structural lung damage.34 The association between structural lung damage at 3 years and structural disease at 5 or 6 years is obvious. However, our data show that markers of both inflammation and infection outweigh the effect size of early structural lung disease on predicted bronchiectasis at 5-6 years. On the basis of our model, a child with pancreatic insufficiency, high BAL neutrophils, and recurrent infections (positive BAL cultures and recurrent IV courses) is expected to have twice as much progression until the age of 5–6, regardless of pre-existing structural abnormalities on CT scan. This implies that an effective intervention in a selected group of high-risk patients could be used to improve lung disease outcomes regardless of the presence or absence of early structural abnormalities at baseline.
In the current study, using the aforementioned clinical parameters, disease biomarkers, and imaging scores, we have successfully developed a model to identify those at risk for a more rapid progression of their lung disease. One previous study by VanDevanter et al.,4 in preschool children also developed a tool to predict school-age outcome (lung function at 6 years of age) using clinical variables. Despite this different outcome, the selected predictor variables were rather similar and differences were likely explained by the different predictor variables that were available in the two studies. For example, a physical exam (clubbing) was included only in the study by VanDevanter et al.,4 but CT scores and BAL outcomes were not. Importantly, the score in that study showed only a weak correlation in the validation group.4 Similarly, after validation, our detailed model including recurrent invasive assessments in all children was still expected to explain only 37% of the variance in bronchiectasis progression. Both the current and VanDevanter et al.4 studies show us that the developed scores may allow identification of high-risk groups of individuals but, at the same time, that despite the use of comprehensive and prospectively collected data, a large proportion of variability in disease progression in individual patients cannot be accurately predicted at baseline. Other factors not evaluated in the current study, such as treatment adherence and differences in management can possibly explain at least part of this variability. Thus, clinicians and researchers should take note of the most important and consistent predictors for future disease progression and can use them for group-based follow-up schedules, or for the selection of high-risk subgroups in intervention trials. It should at the same time be realized that significant lung disease may still develop in some children with favorable prognostic indicators possibly leading to significant bronchiectasis (CF–CT scores of 8 and above, as seen in a quarter of children from our cohort).
As previously mentioned, given the overall mild lung disease in preschool children with CF recent clinical trials evaluating CFTR modulators did not assess respiratory indices as primary outcomes.9-12 As bronchiectasis in CF patients is in general nonreversible and in school-aged children has been shown to consistently predict relevant clinical outcomes.14, 16, 31, 35 The use of a model, such as the one presented in this study may aid in preschool patient selection for clinical trials and allow assessment of a relevant respiratory outcome.
The strengths of our study lie in the prospective longitudinal design of the AREST CF cohort, the use of CT as a sensitive indicator of early lung damage, and the 99% participation in the AREST CF annual surveillance program. However, several limitations should be mentioned: first and foremost, differences in CF patient populations or management between CF centers in Australia and other parts of the world might limit generalizability, although registry data suggest that the AREST CF cohort are representative of newborn screened populations elsewhere. Second, the use of BAL biomarkers of neutrophilic inflammation and chest CT scores, are not available in many centers, limiting the full use of our prediction score. However, we also showed that simple clinical risk factors, not requiring chest CTs or BALs (pancreatic insufficiency, BMI, pulmonary exacerbations, and infections) may identify patients at high risk for more severe disease and help target treatment. Third, it should be noted that even though we performed internal validation of the model, ideally the model should be externally validated before use in clinical care.
In conclusion, our data suggest that in young children with CF, identifying individuals at high risk for disease progression is possible from 3 years of age. The use of risk assessment can be an important tool to help select high-risk children for interventional trials. However, our model had only modest ability to predict structural lung disease progression in individual patients, underlying the need to find novel and more accurate biomarkers to better monitor CF lung disease in clinical care.
ACKNOWLEDGMENTS
Australian Respiratory Early Surveillance Team Cystic Fibrosis program was supported by the NHMRC Grants APP1000896 and 1020555, as well as Cystic Fibrosis Foundation USA and Australia. Daan Caudri received grant support from the Rothwell Foundation, the Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences, and a 2014 Research Fellowship from the Sophia Children's Hospital Fund. None of the funding bodies were in any way involved in the data collection, interpretation of the data, or writing of the manuscript. The full authorship includes the members of AREST CF. The full list of AREST CF members can be found at www.arestcf.org.
CONFLICT OF INTERESTS
Stephen M. Stick reports grants from the NHMRC and the USCF Foundation during the conduct of this study. Other authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Please set as per style here.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




