CFTR, bicarbonate, and the pathophysiology of cystic fibrosis
Summary
The gene that encodes for the cystic fibrosis transmembrane regulator protein (CFTR) was identified in 1989, yet major pathophysiologic questions remain unanswered. There is emerging evidence that CFTR is a bicarbonate channel, a driver of chloride-bicarbonate exchange and through its action on local pH, a regulator of other ion channels and of proteins that function optimally in a neutral environment. In both the respiratory and gastrointestinal (GI) tracts, bicarbonate drives ionic content and fluid on epithelial surfaces, allows mucins to unfold and become slippery, and contributes to innate immunity. In the GI tract bicarbonate neutralizes gastric acid to support digestion and absorption. When CFTR is dysfunctional, lack of bicarbonate secretion disrupts these normal processes and thus leads directly to the clinical symptoms and signs of CF. This article synthesizes evidence from cell, animal, and human investigations that support these concepts. Bicarbonate secretion does not seem to be the same in all tissues and varies with physiologic demand. Thus, tissue type and whether conditions are baseline or stimulated needs to be taken into account when evaluating the evidence concerning the role of bicarbonate in the pathophysiology of CF as a regulator of local pH. Basic and applied research that focuses on the role of CFTR-mediated bicarbonate secretion helps explain many of the diverse clinical manifestations that are CF. Pediatr Pulmonol. 2015; 50:2S4–S30. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
The gene that encodes for the cystic fibrosis transmembrane regulator protein (CFTR) was identified in 1989, yet major pathophysiologic questions remain unanswered. Why do patients with cystic fibrosis (CF) develop thick, sticky secretions in the airways, and GI tract? Why do patients become colonized with pseudomonas aeruginosa and develop microbial imbalance (dysbiosis) in both the airways and gastrointestinal (GI) tract? Why do many patients have severe pancreatic insufficiency while others do not? Why does pancreatic insufficiency develop in utero while lung disease does not? There is emerging evidence that CFTR's role as a bicarbonate channel, a driver of chloride-bicarbonate exchange, and thus a modifier of local pH may be at the heart of these disruptions in normal physiology and lead directly to the clinical symptoms and signs of CF. Although bicarbonate may be an important contributor to disease in other tissues, this review will focus on its role in the airways and GI tract.
Our bodies cannot function unless the acid–base balance in blood remains within a narrow range. However, in various lumens there are oscillations in the concentration or activity of hydrogen ions (pH) that require local adaptations. For example, the airways are exposed the alkalinizing effects of no acid, or very low acid, during inhalation and more dramatically, there is a massive influx of highly acidic gastric contents into the duodenum after meals that must be neutralized. In this article I will synthesize how investigations into CFTR in these epithelial-lined conduits via cell and animal model experiments have led to a new appreciation of the role CFTR plays as a regulator of local pH. As referenced below, the ability to improve CFTR function in humans with the CFTR potentiator ivacaftor has led to additional clinical validation of this concept.
Measurement of chloride in sweat is used to demonstrate CFTR dysfunction and remains the key diagnostic test for CF; however, skin faces the outside world and does not require mucus for protection as do invaginated epithelial structures. It is relatively easy to analyze sweat for chloride concentration but more difficult to do so for bicarbonate.1 For these two reasons and perhaps others, the role of bicarbonate in the pathophysiology of CF may have been overlooked in past decades. Bicarbonate secretion does not appear to be the same in all tissues and may vary with physiologic demand. Thus, tissue type and whether conditions are baseline or stimulated needs to be taken into account when evaluating the evidence concerning the role of CFTR as a regulator of local pH. Nonetheless, many processes that are abnormal in patients with CF are similar in different organ systems and seem to be explained by abnormalities in intraluminal bicarbonate, emphasizing its central role as a driver of disease. These are summarized in the Table 1.
| Bicarbonate drives ionic content and fluid on epithelial surfaces |
| Bicarbonate allows mucins to unfold and become slippery |
| Bicarbonate contributes to innate immunity |
| Slippery mucins are needed to trap microorganisms and transport them away from epithelial surfaces |
| Neutral pH is needed for optimal bacterial killing by antimicrobial proteins |
| In the GI tract bicarbonate neutralizes gastric acid |
| Neutral environment is needed for pH optima of pancreatic enzymes |
| Neutral environment is needed for micelle formation |
IONIC CONTENT AND FLUID ON EPITHELIAL SURFACES
Through its role in secreting bicarbonate and chloride and through chloride-bicarbonate exchangers, CFTR appears to influence local pH and thus ionic content and fluid on epithelial surfaces. In addition to direct conductance of ions through CFTR, local pH influences proteins that act on other ion channels, most notably in the airways via the epithelial sodium channel ENaC (see below). Normal airway surface liquid (ASL) has a pH of around 7.0 (estimated bicarbonate concentration of approximately 10–20 mMol). A recent review describes the evidence that ASL pH is controlled by bicarbonate secretion via CFTR and other secreted proteins.2 CFTR in airway cells derived from a lung cancer cell line (Calu-3) will secrete either bicarbonate or chloride, depending on the activity of basolateral membrane cotransporters.3 In whole animal systems (small airways of healthy pigs), β-adrenergic-(cAMP) and purinergic (Ca(2+))-mediated agonists each will stimulate bicarbonate secretion, likely via CFTR and calcium-activated chloride channel-dependent processes.4 In CF pigs and in newborns with CF, airway pH is lower than in controls providing supportive evidence that dysfunctional CFTR leads to abnormal airway acid–base balance.5, 6 However, paracellular flow of bicarbonate down its concentration gradient from the blood to the airways seems to limit the pH difference between CF and normal airways to <0.5 U.7 It is possible that airways clearance is also affected by changes in pH based on the observation that transient acidification of normal ASL increases rate of ASL absorption by the epithelia while alkalinization of ASL leads to a slowing of ASL absorption and transiently restores ASL height into the normal range.7 ENaC plays a major role in ionic content and fluid on the airway epithelial surface.8 Prostasin, a membrane-anchored serine protease with trypsin-like substrate specificity, likely is required to activate ENaC. However, prostasin may be inactive below pH 7.0. Another regulatory protein, secreted protein short palate lung and nasal epithelial clone 1 (SPLUNC1, also known as BPIFA1) has been shown to be a pH-sensitive regulator of ENaC that is unable to inhibit ENaC in the acidic CF airway environment.9 Thus, CFTR likely controls ENaC by regulating local acid–base balance.
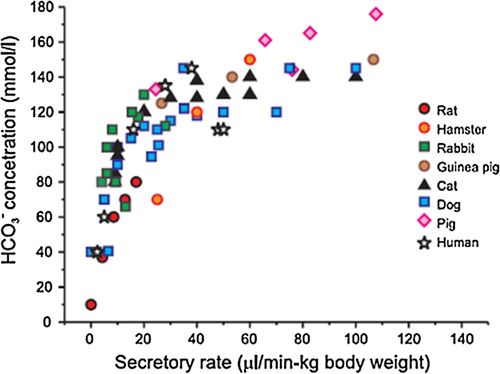
In the GI tract, bicarbonate has a larger role in fluid secretion than in the airways, partly because the volume of fluid and the amount of luminal neutralization needed to counteract gastric acid is so great. In a wide range of animals and humans, bicarbonate is a major driver of pancreatic duct fluid secretion10 (Fig. 1). The intraluminal bicarbonate concentration in pancreatic ductular fluid is much higher than in the airways, up to 140 mMol. CFTR itself has a limited permeability to bicarbonate, but it has been postulated that bicarbonate secretion, and thus fluid secretion, may be achieved by exchange of one chloride molecule for two bicarbonate molecules via an apical chloride-bicarbonate exchanger in the SLC26 family.11,1112 There does not appear to be paracellular backflow of bicarbonate in the pancreatic duct, contributing to the high electrochemical gradient of bicarbonate. Human pancreatic duct cells have high levels of kinases that respond to a low chloride environment (with-no-lysine kinase [WNK1], oxidative stress-responsive kinase 1 [OSR1], and sterile 20/SPS1-related proline/alanine-rich kinase [SPAK]). Activation of the WNK1-OSR1/SPAK pathway appears to be the molecular switch to generate bicarbonate-rich fluid in the human pancreatic duct.13 Interestingly, CFTR variants associated with pancreatitis but not typical CF (CFTR R74Q, R75Q, R170H, L967S, L997F, S1235R, and D1270N) and some associated with a variable clinical course (R117H, D1152H) had normal chloride but not bicarbonate permeability and conductance with WNK1-SPAK activation,14 suggesting that the failure of bicarbonate but not chloride secretion leads to hyperconcentration in the pancreatic duct.
Pancreatic insufficiency occurs before birth in the majority of infants with “severe” CFTR mutations. Meconium ileus (MI) also occurs prenatally but does not correlate directly with those mutations. A search for modifier genes suggests that MI is not related to mutations in mucin genes. However, mutations in various solute carrier (SLC) genes increase the risk of developing MI and pancreatic insufficiency, among them SLC26A9, a chloride-bicarbonate exchanger regulated by WNK kinases.15 Although little is known about prenatal pancreatic fluid and electrolyte secretion, it is reasonable to speculate that neutralization of pancreatic duct contents must start in the second trimester, since trypsin and chymotrypsin are produced in increasing levels starting at approximately 20 weeks of gestation16 and mechanisms to prevent their intraductal activation would be needed. If SLC activity is not present when needed in prenatal life, it may contribute to pancreatic ductal blockage, enzyme activation, autodigestion of the pancreas before birth and subsequent pancreatic insufficiency.
In summary, both the airways and the GI tract secrete bicarbonate, which regulates ionic content and fluid secretion, although the amount and mechanisms likely are different.
MUCIN SECRETION AND ACTION ON THE EPITHELIAL SURFACE
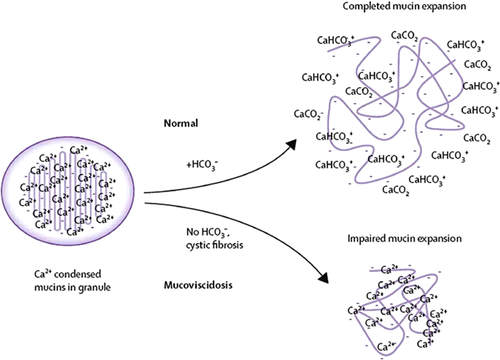
Hippocrates recognized that an excess of mucus was a sign of bad health and could lead to death.17 Mucus, a viscous colloid containing antibacterial defensins, immunoglobulins, inorganic salts, mucins, and other proteins, is a key component of innate immunity. Mucins either are tethered or float freely as a gel. Tethered transmembrane mucins are on the apical side of epithelial cells, with gel-like mucins layered on top to trap micro-organisms and particulate matter so they can be transported out of the lumen. In the respiratory tract, tethered mucins are in the periciliary space and may act as a protective barrier. In the GI tract, they form the glycocalyx. Although we think of mucus as needing to be hydrated to be slippery, there are physical chemical events unrelated to hydration that need to take place for mucins to perform optimally. These physical events are pH-dependent and failure of CFTR to secrete bicarbonate has been shown to lead to abnormally compacted mucins.18 Prior to secretion, the very large mucin molecules are packaged as small granules with their electrical charges masked by calcium. When secreted in a neutral environment, it has been hypothesized that bicarbonate complexes with calcium allowing the negative charges to be unmasked. These charges repel each other, creating slipperiness and a lubricating effect19, 20 (Fig. 2). In the CF airway, diminished rates of mucociliary transport occur by a mechanism that is independent of ciliary hydration and function but seems to be related to increased mucus viscosity. By selectively inhibiting either bicarbonate or chloride transport, it was demonstrated that bicarbonate regulates this abnormal relationship.21, 22
In both the airways and the GI tract, mucins are secreted from goblet cells as well as submucosal glands. Goblet cells secrete mucins and are found in the trachea, bronchi, and bronchioles, as well as in the small and large intestine. In mouse small intestine goblet cells, normal mucus formation requires concurrent exocytosis of mucin granules and an independent cAMP-mediated, CFTR-dependent, bicarbonate secretion that appears mainly to enhance the extracellular phases of mucus excretion.23
As opposed to simple goblet cells, submucosal glands are complex organs containing at least four distinct regions and at least that many cell types. Submucosal glands in the respiratory tract are found in the sinuses, trachea, and bronchi. In the GI tract, submucosal glands are in the proximal duodenum (Brunner's glands), and the pancreas can be thought of structurally as the largest submucosal gland in the body. Individual airway submucosal glands have been studied in sheep and mice and show decreased basal rates of secretion in mice when bicarbonate is removed from the environment.24, 25
Mucins in the CF mouse small intestine ileal mucosa adhere strongly to the epithelium and are denser than that of wild-type mice, but these abnormalities can be normalized when secreted into a high concentration sodium bicarbonate buffer.17 Mucins appear to be released as long strands. As in the airways, local pH affects proteins that act in the lumen. Meprin β is a metalloproteinase that has a neutral pH optimum. Meprin β cleaves the portion of the mucin that remains anchored to the submucosal gland, thus when bicarbonate is not secreted, long sticky strands of mucins remain attached to the intestine.26 A similar finding of long, adherent strands of mucin have been observed in airways as well. After treatment with ivacaftor, individuals with G551D CFTR had a remarkable increase in mucociliary and cough clearance.27 However, at present we lack a direct biomarker to clarify whether this is due to an improvement in local airway pH or other effects of CFTR potentiation.
PROTECTION OF EPITHELIAL SURFACES AGAINST DYSBIOSIS
Invaginated epithelial surfaces remain in contact with the outside world and thus must defend themselves against microbes. Mucins help trap microorganisms but submucosal glands also secrete defensins, which are naturally occurring antimicrobial proteins. Airway colonization with pseudomonas aeruginosa, staphylococcus aureus and other pathogens has long been recognized as a hallmark of CF, although the reason for this observation never has been clear. Various defensins function optimally at a neutral or alkaline pH and a lower pH impairs both their activity and synergism between them (8.0 ↔ 6.8).5, 28 In a cell system, the efficiency of anti-pseudomonal activity of Calu-3 secretions was greater at a neutral pH and it was observed that that alkaline oscillations simulating tidal breathing were associated with lower bacterial viability than when cells were exposed to the same time-averaged pCO2,29 consistent with the oscillations observed in ASL height described above.7 One can speculate that these normal oscillations are lost in patients with dysfunctional CFTR. Furthermore, the pH-sensitive SPLUNC-1 protein has antimicrobial properties in addition to regulating ENaC.30 It has been shown to bind to bacterial lipopolysaccharide and inhibit the growth of P. aeruginosa.26 CF pigs are unable to kill bacteria introduced into their airways, even shortly after birth. The airway surface pH is lower in CF pigs than in wild-type pigs and sub-optimal functioning of defensins in this acidic environment is the leading hypothesis for the lack of bacterial killing.5 These observations from cells and animals suggest that modulation of CFTR-dependent bicarbonate defect in the airways may be a mechanism to explain airway colonization with pathogens in patients with CF. In the observational study of patients with G551D CFTR who were treated with ivacaftor, there was evidence of decreased colonization with pseudomonas, suggesting that improving CFTR function improves the ability of the airways to support normal microbiota.31
Dysbiosis is also found in the GI tract, both in the small intestine and in the colon. Investigations with CF knockout mice revealed small intestinal bacterial overgrowth (SIBO),32 a finding that is also seen in patients with CF.33 When pathogenic bacteria are present in the small intestine they can metabolize normal nutrients making them unavailable to the host and transforming them into toxic substances that can cause enterocyte damage and may contribute to fat malabsorption. In one study of pathogenic Escherichia coli strains, binding to the small bowel mucus layer was significantly greater at pH 5.7 than at pH 7.4 or 8.0,34 again pointing to the role of bicarbonate in the protection of epithelial surfaces against dysbiosis. In contrast to the small intestine, the normal physiologic state is to have a large and diverse microbiota in the colon. However, examination of the fecal microbiome in CF knockout mice35 and humans with CF36, 37 shows that whole gut microbiota is altered and is less diverse when compared to healthy control subjects. How CFTR dysfunction causes these alterations is unclear; however, in light of the mechanisms described above the role of bicarbonate should be explored.
NEUTRALIZATION OF GASTRIC ACID
There is one function of CFTR as a bicarbonate channel that is unique to the proximal GI tract and does not have a correlate in the airways. Gastric acid, which has a pH of 1–3, must be neutralized rapidly after contents leave the stomach and enter the proximal duodenum. The healthy adult human pancreas secretes approximately 1–3 L/day of bicarbonate-rich fluid in response to meals. This fluid acts as a vehicle for pancreatic pro-enzymes to flow from the pancreatic acini into the duodenum, protects the ducts against premature enzyme activation, creates an intra-intestinal pH optimum for pancreatic enzyme function38 and the formation of micelles,39 and is a source of fluid for movement of bulk contents along the GI tract. Biliary and intestinal glandular secretions also contribute to neutralization of gastric acid. In the pancreatic duct, CFTR likely functions directly as a bicarbonate channel that enables secretion of bicarbonate at high concentrations to decrease retention of intraductal pancreatic pro-enzymes.40, 41
Abnormal CFTR function leading to intestinal hyperacidity has clinical implications. Intestinal acidification interferes with digestion by inhibiting pancreatic enzyme activity and in the case of exogenous pancreatic enzyme replacement therapy, delaying dissolution of enteric coating.42 Post-lipolytic absorption of nutrients may be sub-optimal because intraluminal precipitation of bile acids impairs mixed micelle formation. In addition, lack of bicarbonate leads to abnormally compacted and dessicated mucus along the small intestinal absorptive surface. Furthermore, because of the direct relationship between bicarbonate secretion and high-volume fluid secretion from the pancreatic duct, CFTR dysfunction likely contributes to diminished intraluminal fluid in both the small and large intestine, leading to abdominal discomfort, constipation, and even intestinal obstruction.
CFTR mutations associated with pancreatic insufficiency in patients with CF do not support bicarbonate transport, and those associated with pancreatic sufficiency (CF-PS) show reduced bicarbonate transport.43 In vivo, using direct pancreatic stimulation testing, it has been shown that patients with CF-PS have decreased volume and concentration of bicarbonate compared to healthy controls; however, flow is sufficient to allow pancreatic enzymes to reach the duodenum in quantities sufficient to allow digestion,44, 45 suggesting a “dose effect” of CFTR activity on bicarbonate secretion.
Patients with CF have been shown to have a more acidic small intestine than controls,46 especially in the critical initial time period following gastric emptying.47 Notably, this delay in proximal intestinal acid neutralization was ameliorated in patients with G551D CFTR after taking ivacaftor, suggesting a direct effect of CFTR on bicarbonate secretion.48 The effect of CFTR on bicarbonate secretion as a pathophysiologic link may be easier to study in vivo in the GI tract than in the airways because the pH changes in whole units rather than tenths of units, making shifts in values easier to observe. However, because ivacaftor potentiation is systemic, the purported improvements in bicarbonate secretion in the GI tract after CFTR modulation likely are not organ-specific.
CONCLUSION
This exposition began by asking whether focusing on CFTR's role in bicarbonate secretion could answer some of the basic questions in the pathophysiology of CF and concludes with a resounding yes. Bicarbonate has a direct role in creating thick, sticky secretions in the airways and GI tract through its effects on the ionic content and fluid on epithelial surfaces and the physical properties of mucins. There is evidence that patients may become colonized with pseudomonas aeruginosa and develop dysbiosis in the airways and GI tract through bicarbonate-dependent abnormalities of two properties of the innate immune system, the barrier effect of normal mucus and the action of antimicrobial proteins. Patients with CF who develop pancreatic insufficiency have almost complete absence of bicarbonate transport, while those with CF-PS have bicarbonate secretion that is diminished but still present. Last, one can speculate that because fetal tidal breathing with amniotic fluid does not result in oscillations in airway pH, lung disease may not develop in utero but that prenatal pancreatic insufficiency may occur when bicarbonate secretion is needed but is dysfunctional. In The Sum of all Logic, William of Ockham wrote, “What can be explained on fewer principles is explained needlessly by more.” As we attend conferences and read journals, we should pay attention to basic and applied research that focuses on the role of CFTR-mediated bicarbonate secretion to help us understand the diverse clinical manifestations that are CF.
ACKNOWLEDGMENT
The thoughtful comments of Drs. Michael Duffey and Daniel Sheehan are greatly appreciated.




