People with dementia: what is known about their experience of cancer treatment and cancer treatment outcomes? A systematic review
Abstract
Objective
The objective of the study is to report a systematic review of what is currently known about the experience of cancer treatment and cancer treatment in adults with dementia.
Methods
The analytic plan and inclusion/exclusion criteria were specified in advance of the search process in a protocol.
Searches were conducted in MEDLINE, CINAHL, PsycINFO and the Cochrane Library for publications about people with cancer and a pre-existing dementia. Limits were English language; 2000 to 12/2015; adults; >18 years old.
The search identified 5214 titles and abstracts that were assessed against eligibility criteria and 101 were selected for full-text examination by two researchers who agreed inclusion of nine papers, extracted data independently then conducted a content analysis and narrative synthesis.
Results
Nine studies conducted in four resource rich countries were included in the review. These studies evidence that when compared with other cancer patients, those with dementia are diagnosed at a later stage, receive less treatment, are more likely to experience complications from treatment and have poorer survival.
The experience of supportive care and preferences of people with dementia receiving cancer services and cancer treatment have not been investigated. Research into how the cancer team manage the particular needs of people with dementia and their family members has been limited to one study that reported how a cancer team managed the particular needs of seven people with dementia.
Conclusion
Further work is needed to establish practice guidelines for the management of cancer in people with dementia.
Copyright © 2016 John Wiley & Sons, Ltd.
Introduction
Worldwide there are an estimated 44 million people with dementia, which affects one in 20 people over the age of 65 years 1. At the same time, because of an ageing population and improvements in diagnostic procedures and treatments, more people are living with cancer. It is predicted that by 2035 the number of new cases of cancer will have risen by 70% 2. An increasing number of people will therefore experience the challenge of living with both cancer and dementia. This study examines what is currently known about cancer treatment and care for people with dementia.
Background
Dementia is a term for conditions that impair mental function, which present as memory difficulties and other problematic behavioural changes 3. Psychosocial and behavioural interventions are recommended as first line treatment for all but the small number who put themselves or others at risk of harm, when pharmacology is then appropriate 3, 4. This recommendation is underpinned by a psychological perspective that assumes that problem behaviours arise because of unmet need 5. Psychological and behavioural interventions can address unmet needs aiding with the management of challenging behaviours, where such behaviours impact cancer treatment and care then there is potential for psychosocial and behavioural interventions to contribute to improving outcomes in people with dementia who develop cancer.
The proportion of people with cancer and a confirmed diagnosis of dementia vary by cancer site and age. Epidemiological studies report a pre-cancer diagnosis of dementia in 10% of colon cancer patients, 7.4% of breast cancer patients and 5.1% of prostate cancer patients aged 68 years or older 6 and 0.5% prevalence of dementia in women of all ages with breast cancer 7. Population-based studies have also found higher mortality rates in people with cancer and dementia 6-8 compared with those without dementia. What these studies do not tell us is whether people with dementia are receiving the same cancer treatments, care and services as other cancer patients. In the UK, the National Institute for Health and Care Excellence 3 provides guidelines for good practice in dementia care, which includes the recommendation that people with dementia should not be excluded from interventions or services. It is important to know if the poorer survival in people with dementia is the consequence of different access to cancer services, under treatment and inadequate supportive care.
Purpose
The purpose of this paper is to report what is known about the experience of cancer treatment and cancer treatment outcomes in adults with dementia.
Design
An analytic plan and inclusion/exclusion criteria were specified in advance of the search process in a protocol. This protocol (available from the first author) sets out a plan to conduct a mixed-methods systematic review. The search methodology and methods were informed by the NHS Centre for Reviews and Dissemination guidance for undertaking reviews in health care 9. The reporting follows the Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) guidelines 10.
Methods
Search methods for identifying data sources
Relevant studies were identified by conducting a search of electronic databases: CINAHL, MEDLINE, PsycINFO and the Cochrane Library. Limits were English language; 2000 to 12/2015; adults; >18 years. Conference abstracts were included. The search strategy was iterative, in that references of the included studies were searched for further relevant publications.
Study selection
The search strategy was developed for MEDLINE (Appendix A) and then translated into other databases. It combined selected MeSH terms and free text terms, using search terms relating to dementia (and other equivalent terms, for example, Alzheimer's), memory problems (and other equivalent terms, for example, cognitive impairment) and cancer (and other equivalent terms such as neoplasm).
Eligibility criteria
- Inclusion criteria
- Cancer and dementia
- Adult >18 years
- English language
- Primary data
- Cancer treatment
- ○ Radiotherapy/chemotherapy/surgery/hormone therapy
- ○ Non-pharmacological interventions (supportive or palliative)
- ○ Other
- Experiences and/or
- ○ Physical symptoms
- ○ Negative / positive emotions
- ○ Existential threat
- ○ Disruption in social life
- Outcomes
- ○ Behaviour change (e.g. adherence)
- ○ Change in symptoms (e.g. intensity)
- ○ Change in symptom management
- ○ Survival
- ○ Other physical function
- ○ Quality of life
- ○ Other
- Exclusion criteria
- Teenagers/children <18 years
- Pharmacological interventions for dementia
- Single clinical case studies
- Non-illness-related family problems
- End of life/terminal care
- Cancer or cancer treatment causing memory loss
- Pre 2000
Data extraction and management
All titles and abstracts identified during the search were assessed for relevance to the review by two reviewers (J. H. and R. M. or A. K. and R. M.), a process aided by a screening tool devised for the study from the inclusion/exclusion criteria. If either reviewer considered a citation to match the inclusion criteria, the full paper was obtained. Full papers were examined independently, and not blinded, by two reviewers (J. H. and R. M. or A. K. and R. M.) for conformity with the inclusion criteria, with disagreement being resolved through discussion. Data were then extracted from included papers by two reviewers (J. H. and R. M.). The design of the data extraction sheet was based on published guidelines 9, 11. Studies identified from reference list searches were assessed for relevance in the same way but with the start point being the study title. Quality assessment of included papers was conducted by two reviewers (J. H. and D. E.) using the Effective Public Health Practice Project (EPHPP) quality assessment tool designed for evaluating quantitative studies, including cohort/survey research 12.
Analysis
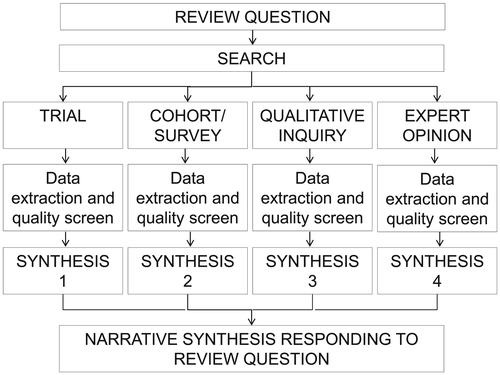
Heterogeneity in study design, methodology and methods was expected; therefore, meta-analysis was not planned. The analytic plan was to extract relevant information, to organise and synthesize it within four separate methodological streams, write a narrative description for each stream, identify cross-stream themes 13 and present the analysis as a narrative synthesis 14, 15 (Figure 1). The search found only cohort/survey research; hence, the analysis and synthesis reported in the succeeding text are within a single methodological stream.
Results
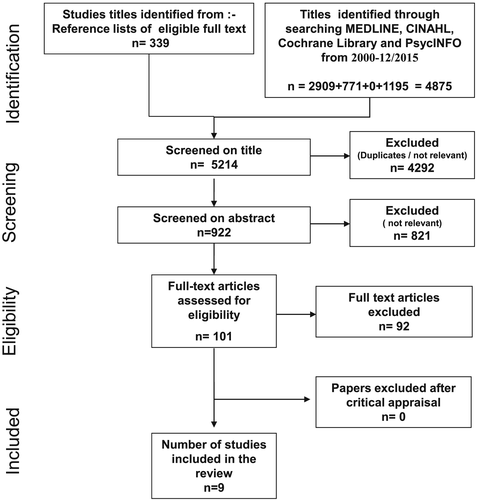
The systematic search found nine empirical studies about the experience and outcomes of cancer treatment in people with dementia 16-24. A flow diagram of the review process is provided in Figure 2. The search of electronic databases from 01/2000 to 12/2015 found 4875 titles. Also screened were a further 339 titles in the reference lists of included studies. Of the total 5214 titles screened, 4292 were not relevant or were duplicates. The abstracts of the remaining 922 were screened and a further 821 excluded as not relevant. The full text of 101 publications was assessed for eligibility of which a further 92 were excluded. The main reasons for these 92 exclusions were a study of people across the continuum of cognitive impairment where the sample included an unspecified number with dementia (n = 15) or people with dementia were excluded (n = 6), review (n = 15), treatment causing cognitive impairment (7) or delerium (n = 3), end of life care (n = 5) and population level study of association between cancer and dementia with treatments not specified (n = 11).
The search identified only cohort/survey studies. Of note is that, no qualitative research exploring the experience of people with dementia receiving cancer treatment was found. The characteristics of the nine selected studies are shown in Table 1. The quality of the studies ranged from strong to weak. The five strong studies 18, 19, 21, 23, 24 and two of moderate quality 17, 20 were retrospective cohort studies of large geographical areas that included samples taken from country-wide databases. The two weaker studies 16, 22 were case control studies in single hospital sites.
| First author | Study aim/objective | Design | Sample and setting | Intervention for dementia | Outcomes | Results | Author comment | Quality |
|---|---|---|---|---|---|---|---|---|
| Abe 16 Japan | To examine the clinical outcomes and prognostic factors of acute myeloid leukaemia in patients 75 years and older | Retrospective note review |
31 patients with acute myeloid leukaemia who received chemotherapy between 2002 and 2009 Age 75 to 94 (median 79) 7/31 had Alzheimer disease Mini-mental state examination (MMSE) was performed on patients with suspected depressed cognitive function and a dementia diagnosis made based on magnetic resonance tomography (MRI) and single photon emission computed tomography (SPECT) |
Donepezil hydrochloride for behavioural and psychological symptoms of dementia (BPSD) Family member present to provide support during chemotherapy |
Survival Treatment selection and tolerance |
4/7 had mild Alzheimer 3/7 moderate Alzheimer Induction therapy was performed in all seven cases. Complete remission was obtained in the three moderate cases, but consolidation therapy was discontinued because of exacerbation of BPSD including night delirium in all three. |
Treatment decisions in patients with moderate dementia need to take quality of life into account Induction therapy exacerbates BPSD. The mechanism for this needs to be found. |
Weak |
| Alibhai 20 Canada | To confirm that androgen deprivation therapy (ADT) in men with prostate cancer increases fragility fractures and to investigate if clinical variables before initiating the therapy are associated with increase fracture risk. | Matched cohort study |
24 518 men with prostate cancer diagnosed 1 Jan 1995 to 31 Dec 2005 (excluding those with metastasis) who received at least 6 months of ADT (medical or surgical). 19 079 appropriate matched non-users of ADT with prostate cancer. Age 66 and older Linked administrative data at the Institute for Clinical Evaluative Sciences in Ontario Number with dementia not reported. |
N/a |
Fracture, stratified into fragility and non-fragility types. Fracture associated with dementia. |
11 778 (9%) of ADT users compared with 1157 (5.9%) of nonusers had a fragility fracture (hazard ratio 1.65, 95% CI 1.53 to 1.78, p < 0.0001). 3387 (17.2%) of ADT users compared with 2495 (12.7%) of nonusers had any fracture (hazard ratio 1.46, 95% CI 1.39 to1.54, p < 0.0001) In addition to ADT predictors of time to fracture included prior dementia. (fragility fracture, hazard ratio 2.14, 95% CI 1.61 to 2.86, p < 0.05; any fracture, hazard ratio 1.56, 95% CI 1.22–2.00, p < 0.05; adjusted for collapsed ambulatory diagnostic group, income quintile and rurality) |
Clinicians should counsel patients on the increased risk of facture, as a consequence of ADT and consider preventative measures, particularly in those at greatest risk such as those with dementia. | Moderate |
| Baillargeon 17 USA | To evaluate the extent to which pre-existing mental disorders influence diagnosis, treatment and survival in older adults with colon cancer | Retrospectivecohort study |
80 670 people with colon cancer diagnosed from 1 Jan 1993 to 31 Dec 2005 with a diagnosis of a mental illness in the 2 years before the cancer diagnosis. Register of cancer incidence and treatment in US (SEER registries) and the Medicare claims data system linked to enable a population-based analysis of likelihood of treatment and survival. Age 67 and older 7267 patients had dementia Dementia was identified from ICD-9-CM codes. |
N/a |
Likelihood of diagnosis and treatment varying with mental disorder Survival |
Participants with dementia were more likely to be diagnosed with colon cancer at an unknown stage (24.3% compared with 6.2% in no mental disorder, p < 0.001) and more likely to be diagnosed at autopsy (8.1% compared with 1.1%, p < 0.001). Those who survived for 6 months or more after diagnosis (n = 61,942) were at greater risk of non-receipt of treatment (13.3% compared with 2.6%; risk ratio 2.47 95% CI (2.08 to 2.93) adjusted for age, ethnicity and co-morbidity), and to be less likely to have chemotherapy (78.9% compared with 38.7%; risk ratio 3.23 (95% CI 2.66 to 3.91) adjusted for age, ethnicity and co-morbidity) A diagnosis of dementia was associated with greater colon cancer-specific mortality in the patients with dementia not diagnosed at autopsy; hazard ratio 1.41 (95% CI 1.34 to 1.48) adjusted for age, ace and ethnicity, sex, marital status, region, income, year of diagnosis, stage at diagnosis and comorbid disease. |
Findings are consistent with those of other studies and may be because of the complex informed consent process, the inability of the patients to follow medical recommendations, such as adhering to appointments and treatments, and concerns for staff about additional time and resource required. | Moderate |
| Gorin 18 USA | To describe breast cancer treatment in patients with Alzheimer's disease | Retrospectivecohort study |
50 460 women with Stages I–III breast cancer diagnosed between 1 Jan 1992 and 31 Dec 1999. Age 65 and older. Register of cancer incidence and treatment in US (SEER registries) and the Medicare Claims Data System linked to enable population-based analysis of breast cancer diagnosis and surgery, chemotherapy and radiotherapy treatments. Alzheimer's and related dementias identified from ICD-9 codes. 1935(3.8%) had a diagnosis of Alzheimer's before or up to 6 months after cancer diagnosis. Subjects with Alzheimer's were older (12.9% > 90 vs 2.7% > 90, p < 0.001), more likely to be African American (9.8% vs 5.4%, p < 0.001), more frequently widowed (57.8%, vs 40.1%, p < 0.001), had more co-morbidities (7.1% vs 2.4%, p < 0.001). |
N/a | Type of therapy for breast ancer |
Women with Alzheimer disease were less likely to receive treatment compared with women without Alzheimer disease, with the exception of breast conserving surgery. 3.7% of women with Alzheimer's had no record of treatment compared with 0.9% of patients without Alzheimer's (OR = 0.26, CI = 0.21 to 0.33). 96.4% received surgery compared with 99.0% of patients without Alzheimer's (OR = 0.3, CI = 0.24 to 0.38). They were less likely to receive mastectomy (62.3% compared with 71.0%) and more likely to receive breast conserving surgery (37.7% compared with 29%) than patients without dementia. 11.7% received radiation therapy compared with 36.8% without Alzheimer's disease (OR = 0.24, CI =0.21 to 0.27). 3.3% received chemotherapy compared with 10.5% without Alzheimer's disease (OR = 0.3, CI = 0.23 to 0.38) Patients with Alzheimer's disease were more likely to be diagnosed at a later stage (10.8% vs 6.6% at Stage III, p < 0.001) and with lymph node involvement (29.1% vs 25.9%, p < 0.1) and had significantly lower odds of any treatment (OR = 0.55, CI = 0.42 to 0.74) adjusted for age, stage, co-morbidity and poverty level. Treatment differences persisted across all ages. The greatest differences in treatment of people with Alzheimer's disease compared with those without Alzheimer's disease occurred between the ages of 80 and 89. |
Hormone therapies are often administered as first-line therapies in older women with breast cancer and would not have been reliably captured using this study methodology. The findings of reduced treatment in women with Alzheimer disease is consistent what is known about other groups, such as ethnic minorities and other chronic conditions. It may be appropriate to offer less aggressive treatment to women with Alzheimer's disease, although the identified pattern suggests opportunities are missed to reduce non-Alzheimer's-related morbidity. |
Strong |
| Gupta 21 USA | To determine the prevalence of dementia in older people diagnosed with colon cancer and to examine the influence of co-morbid dementia on presentation, diagnosis and treatment. | Observational cohort study to look back 2 years for a diagnosis of dementia preceding a diagnosis of colon cancer. |
17 507 patients with pathologically confirmed Stages I–IV adenocarcinoma of the colon diagnosed between 19 Jan 1993 and 30 Dec 1996. 16 323 patients (6823 men) without dementia mean age 67 or older. 1184 patients (396 men) with dementia mean age 67 or older. Data collated from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End-Results (SEER) composed of 11 population-based cancer registries linked to Medicare data enabling evaluation of in-patient and outpatient care. Procedure codes indicating administration of chemotherapy were used to identify those considered to have been treated with 5FU. Prevalence of dementia 6.8% (1184/17 507 people with colon cancer) Patients with dementia tended to be older, female, reside in low income/urban neighbourhoods and have other co-morbidity. Dementia was identified from dementia-related ICD-9-CM codes. |
N/a |
Antecedent dementia on likelihood of being diagnosed with colon cancer after death. Surgical resection. Odds of receiving adjuvant 5FU. |
Dementia patients were twice as likely to have colon cancer reported only after death. Adjusted OR 2.31, 95% CI 1.79 to 3.00. Adjusted for age, race, marital status, neighbourhood poverty, urban residence and non-dementia co-morbidity. Patients with dementia and cancer before death (n = 1070) were twice as likely to be diagnosed using non-invasive methods rather than direct tissue biopsy with histology confirmed. Adjusted OR 2.02, 95% CI 1.63 to 2.51, p < 0.001. Adjusted for age, race, marital status, neighbourhood poverty, urban residence and non-dementia co-morbidity Patients with dementia were twice as likely to have their cancer reported as unstaged. Adjusted OR 2.12, 95% CI 1.77 to 2.55, p < 0.001. Adjusted for age, race, marital status, neighbourhood poverty, urban residence and non-dementia co-morbidity Stages I–III disease (n = 12 728) patients with dementia were 43% as likely to receive surgical resection. Adjusted OR 0.43, 95% CI 0.33 to 0.70. Adjusted for age, race, marital status, neighbourhood poverty, urban residence, non-dementia co-morbidity and geographical location. Resected Stage III (n = 3386) patients with dementia were 20% as likely to receive adjuvant 5FU. Adjusted OR 0.21, 95% CI 0.13 to 0.36. Adjusted for age, race, marital status, neighbourhood poverty, urban residence, non-dementia co-morbidity, geographical location and histological grade. |
The cohort of patients with dementia in this study was less likely to be treated with curative intent. Treatment of locally advanced colon cancer may prevent cancer-related consequences, but little data are available relating to benefits, risks and tolerance of anti-cancer treatments in patients with dementia at different stages of the disease. | Strong |
| Iritani 22 Japan | To compare behaviours of cancer patients with and without dementia with respect to (i) the process of cancer discovery (ii) the recorded pain complaints excluding those clearly unrelated to cancer (iii) the documented use of analgesics and narcotics |
Review of nursing and medical records of all patients admitted to a surgical ward for cancer evaluation and treatment Comparison of cancer patients without and with dementia. |
134 patients with cancer who were communicative about pain experience and admitted to a public psychiatric hospital with a facility to treat physical conditions in Japan from 1993 to 2004. 121 patients had gastrointestinal cancers. Most of the patients had abdominal surgery. 84 patients (46 men) without dementia mean age 72.1 (range 61.6 to 82.6) 50 patients (29 men) with dementia mean age 74.1 (range 66.0 to 82.2) Diagnosis of dementia based on patient history, clinical evaluation, CT scan and psychiatric consultation. Severity of dementia assessed according to DSM-IV-TR. Twenty patients were assessed to have severe dementia. |
N/a |
Mode of cancer detection Pain: assessed as absent or present from the nursing records. Recorded use of non-narcotic and narcotic analgesia. |
63% of non-demented patients with cancer sought a medical opinion pre-diagnosis because of discomfort compared with 8% of the patients with dementia. Pain was recorded in 76% of patients without cognitive impairment compared with 22% of patients with dementia. Analgesic use increased with stage progression in cancer patients without dementia. Non-narcotic analgesia was required for 64% patients Stages I and II and 84% patients Stages III and IV, narcotic analgesia was required by 41% Stages III and IV. For patients with dementia, non-narcotic analgesia was required for 11% Stages I and II and 13% patients Stages III and IV, and only one received narcotics. |
Dementia greatly reduces patient'’ voluntarily seeking help. Dementia patients with cancer complain less frequently of pain associated with cancer and accordingly require less analgesia. Possibly because they do not have the cognitive functions to interpret, recall and communicate pain then engage in problem solving behaviours. | Weak |
| Kimmick 23 USA | To examine the relationship between co-morbidity and guideline-concordant care for early-stage breast cancer. | Cross-sectional retrospective record review of randomly selected cases across strata of race, ethnicity and location-specific factors. |
6439 women with Stages I–III breast cancer diagnosed in 2004. Guideline concordant treatment included components of surgery, adjuvant radiotherapy, lymph node surgery, chemotherapy and endocrine therapy. The guideline was devised from international guidelines for breast cancer treatment based on tumour size, node status and hormone receptor status. Mean age 58.7 (range 20 to 99) Data collated from the National Programme of Cancer Registry composed of seven population-based cancer registries and details of treatment from physician office records physicians and outpatient facilities Number of the 6439 women with dementia is not reported. |
N/a | Treatment concordance in 26 co-morbid conditions, including dementia, compared with no co-morbidity. |
Treatment was guideline concordant for 69.5% without co-morbidity. Less concordance with treatment guideline associated with dementia (OR = 0.45, 95% CI = 0.24-0.83, p < 0.05) |
Less guideline concordant care may be justified given the known relationship between co-morbidity, poorer life expectancy, but also because of the financial and quality of life burdens of treatment. | Strong |
| Louwman 19 the Netherlands | To describe the prevalence of serious co-morbidity in patients with breast cancer and its impact on treatment and effect on prognosis, independent of the patient's age and stage of disease | Observational cohort study |
8966 patients with breast cancer diagnosed 1995–2001 (follow up to 2004) Data collated from the population-based Eindhoven Cancer Registry and Central Bureau for Genealogy (register of deceased Dutch citizens) Recorded treatments were surgical procedure, radiotherapy, chemotherapy and hormone therapy 70/8966 (1%) patients had a recorded dementia at diagnosis. Of these 70 patients, 0/2313 was age less than 50, 3/4185 were age 50 to 69 24/1668 were age 70 to 79, 43/800 were age 80 or older. |
N/a |
Prevalence of co-morbidity analysed by age group. Difference in treatment in those with and without co-morbidity analysed by age group. Crude survival stratified by age at diagnosis. Estimate of independent prognostic effect of co-mor bidity adjusting for age, stage of disease and treatment. |
Co-morbidity affected treatment in all age groups, but effect was smaller before the age of 70 years. For example, in those age 80 or older, 70% with no co-morbidity received radiotherapy compared with 54% of those with one co-morbidity (p < 0.01)(data are reported for any co-morbidity not specifically for a co-morbid dementia) Crude survival in patients 70 years of older at 1 year was 93% (SE 0.8) compared with 83% (SE 6.2) for those with co-morbid dementia and at 5 years 68% (SE 1.6) compared with 27% (SE 8.8) for those with co-morbid dementia. Survival of the 70 patients with dementia was shorter compared with those without co-morbidity (HR 2.34, 95% confidence interval 1.6 to 3.5, p 0.0001 adjusted for age, disease stage and treatment) |
The presence of co-morbid conditions alters the therapeutic regimen, independent of patient's age and disease stage. This may or may not be good clinical practice. Risk of death from breast cancer is higher in those with dementia at diagnosis, independent of age or disease stage and treatment. This maybe the result of dementia or under treatment. |
Strong |
| Patnaik 24 USA | To measure associations between specific co-morbidities and overall survival and all cause mortality amongst older women diagnosed with breast cancer. | Retrospective cohort study |
64 034 women with breast cancer diagnosed 1 Jan 1992 to 31 Dec 2000. Aged 66 and older. Median age at diagnosis 75 years. Register of cancer incidence and treatment in US (SEER registries) linked to Medicare Claims Data System enable a population-based analysis of all cause mortality and survival. 887 (1.4%) had dementia at diagnosis |
N/a |
All cause mortality Survival |
Women with breast cancer and dementia were at higher risk of all cause mortality compared with those without dementia (hazard ratio of death 1.96, 95% CI 2.02 to 2.41, p = 0.01 adjusted for age, race and ethnicity, tumour stage and grade, oestrogen receptor status, treatment and other co-morbidity). Patients with dementia diagnosed with Stages I and II breast cancer had survival experiences similar to those with Stages III and IV disease and no co-morbidity. |
Including co-morbid conditions in the assessment of prognosis may be important and attention to management of co-morbid conditions, as well as the patient's cancer may result in longer overall survival for older patients with breast cancer. | Strong |
Description of the studies
The nine selected publications were about studies conducted in the USA 17, 18, 21, 23, 24, Japan 16, 22, the Netherlands 19 and Canada 20. Although all were published from 2005 onwards, data were collected in 2004 for one study and over periods of time from 1992 to 2005 in the other eight. Study samples included subsamples of people with cancer and dementia that ranged from 7 to 7267 (median 887, with no cancer and dementia subsample size reported in two publications). The age of those studied ranged from 20 to 99 years (with 7 of 9 studies including only patients 65 years and older). Four of the studies were about breast cancer and dementia, two about colon cancer and dementia, one about prostate cancer and dementia, one about myeloid leukaemia and dementia and one about the pain experience of people with cancer and dementia.
There is consistency in the results of the five strongest studies 18, 19, 21, 23, 24 and a sixth study of moderate quality 17. They evidence that people with dementia who are treated for either breast cancer or colon cancer are diagnosed at a later stage 18, unknown stage 17, 21 or autopsy 17, receive less treatment compared people without dementia 17-19, 21, 23 and have poorer survival 17, 19, 24. The studies evidence a greater likelihood of diagnosis using non-invasive methods 21 a lesser likelihood of breast cancer treatment concordant with guidelines 23, with older women with dementia less likely to receive cancer treatment than younger 19, length of time since diagnosis of Alzheimer disease related to likelihood of treatment 18. Also, women with breast cancer and dementia were found more likely to be older, African-American or have other co-morbidities 18 and patients with colon cancer and dementia more likely to be older, female, or be on low income/live in urban areas, and have other co-morbidities 21. Survival outcomes for dementia patients with Stage I/II tumours have been found equivalent to those for patients with Stage III/IV tumours without dementia 24.
Two of the other included studies evidence increased likelihood of complications from cancer treatment in people with dementia. Men 66 years and older with prostate cancer and on androgen deprivation therapy for at least 6 months have an increased risk of fracture if they have dementia compared with those without dementia 20. In seven patients with Alzheimer's disease who were 75 years and older, all were able to receive remission induction therapy for myeloid leukaemia according to protocol. Interventions were in place to manage the behavioural and psychological symptoms of dementia, which were Donepezil and to have a family member present to provide support during the administration of chemotherapy. In five cases, the treatment had an adverse effect on the behavioural and psychological symptoms of dementia and of the three who went into remission; follow-on consolidation treatment (to reduce the risk of recurrence) was discontinued because of this adverse effect, which included night delirium 16.
The final study to meet the inclusion criteria for this review examined pain in people with dementia who had surgery for cancer. Patients with (n = 50) and without dementia (n = 84) admitted to a unit for cancer surgery were compared for pathway to diagnosis and pain management. Patients with dementia were found more likely to be diagnosed by chance rather than in consequence of seeking medical help and less likely to report pain or receive analgesia for pain 22.
Discussion
This review found that compared with cancer patients who do not have dementia, people treated for cancer who have a pre-existing dementia are diagnosed at a later or unknown stage, receive less treatment, are more likely to experience complications from treatment and have poorer survival. The reasons are currently unknown.
The review is interesting for what it did not find. It found no reported research investigating the supportive care needs and preferences of people with dementia in receipt of cancer treatment and only one study that reported how a cancer team managed the particular needs of seven people with dementia 16. The role of family carers seems to have been overlooked, in spite of the known importance of informal support in the lives of people with dementia 25. The review did find data on survival outcomes for those treated, but no robust study of other clinical or quality of life outcomes, such as physical function, or impact on symptoms of dementia. This information would give insight into and may help explain the observation that people with dementia are less likely to receive protocol compliant treatment, are more likely to experience treatment complications and have poorer survival. It would also enable comment on whether or not policy and clinical guidelines for dementia care are relevant to and/or adhered to in the delivery of specialist cancer treatment and care.
The authors of the nine studies included in this review discuss possible explanations for their results:
Possible explanation of poor survival
Under-treatment is a suggested possible explanation of poorer survival 18, 19. However, we found no studies based on treatment approaches tailored to people with dementia. It is not known if more treatment in those with dementia is practical or if it improves survival and/or other treatment outcomes such as quality of life. People with dementia have a shorter life expectancy than those without dementia 26, so poorer survival might be explained by factors independent of cancer. Furthermore, more aggressive treatment might exacerbate behavioural and psychological symptoms of dementia and other problems, thereby compromising both quality of life and survival.
Possible explanation of late diagnosis
People with dementia are recognised to have compromised problem solving ability, which hampers their recognition of potential health problems. They may not seek help as soon as people without dementia or be able to seek help at all 22. Late diagnosis may thus arise from of compromised ability to seek help.
Possible explanation of less cancer treatment
Abe et al.16 conclude that treatment in older people can be beneficial but for those with moderate to severe dementia treatment decisions should be informed by consideration of treatment impact on quality of life. Other factors may also impact treatment decisions. Cancer treatment in people with dementia can take additional staff time 17; the consent process can be complex and require agreement from clinical team members and someone who knows the patient well, such as a family member 17; staff may need to provide a higher level of through treatment monitoring and support 17; if impaired cognitive function is compromising ability to comply with self-care requirements, such as monitoring for side effects and attendance at follow-up appointments, complications of treatment may not be identified early 17, 22 limiting opportunity for intervention and further treatment options.
Seeking a solution to difficult decisions about cancer treatment in people with dementia
Treatment decisions in older people involve comparing costs and benefits in the absence of trial data on likely outcomes, as older people have often not been included in trials 27. Awareness of how to meet the needs of older people has been demonstrated to impact on cancer treatment and management. Chaibi 28 conducted an investigation of the impact of a geriatric assessment on treatment decisions in cancer. Comprehensive geriatric assessment (CGA) was conducted by a geriatric team for patients 75 years and older who were judged complex during cancer-site specific multidisciplinary team meetings. From Jan 2007 to Nov 2008, 161 patients were enrolled in the study. Forty-two (26%) were found to have cognitive impairment (the proportion with dementia is not reported) of whom 122 (76%) received one or more geriatric treatment interventions. A subsequent oncogeriatric multidisciplinary consultation resulted in 79 (49%) of patients being offered a treatment that differed from the treatment initially proposed. There was a trend towards lower dose intensity treatment in those with greater cognitive impairment. This suggests that different rather than more cancer treatment might be appropriate for people with dementia. It is thus possible that the observed lower treatment rates for patients with dementia are appropriate and that they are not under-treated, given their situation that can include problems complying with oral medication regimes and behavioural symptoms that make invasive treatments difficult.
Implications for practice
Cancer clinicians are faced with a difficult treatment decision when a person with dementia presents with cancer. This review supports an argument that treatment decisions should include consideration of life expectancy, potential impact of the treatment on the behavioural and psychological symptoms of dementia, severity of the dementia because of the implications for treatment compliance and management of side effects, and potential impact on quality of life. Patient and carer preferences may also be an important consideration 29 although no research was found examining these issues. The judgment of risk and benefit to the patient is complex, and there is a limited evidence base to inform decisions. Healthcare professional opinions on the best way to treat cancer in older people with dementia have been shown to differ 30. Oncologists have also been found to have had limited preparation for the task. In a survey of 64 oncology trainees in the UK, two-thirds (66%) had received no training in the needs of older people with cancer 31. Best practice in the assessment and supportive care of elderly people with dementia might usefully be adapted to the context of cancer care, facilitating treatment and aiding with the management of cancer symptoms and treatment side effects. This proposition should be tested empirically.
Limitations
This review only considered English language publications about adult cancer patients from 2000 to 2015. A small number of heterogeneous studies were found, with over representation of people with breast cancer and dementia, which limits the generalisability of the results. Dementia diagnostic rates are variable, for example, it is estimated that in the UK less than 50% of people with dementia have a diagnosis 32. It is likely the studies drawing on registries under-represent the population of people with dementia treated for cancer. Furthermore, the pathway to diagnosis was beyond the scope of our review, but examination may reveal additional explanations of the findings, such as cancer symptoms being mistaken as symptoms of dementia.
Conclusion
It may or may not be possible to improve survival and other treatment outcomes in people with dementia and cancer. It is important to find out if cancer treatments and psychosocial interventions are being put to full use in improving the lives of people with dementia and cancer and their family members. Further work is needed to establish guidelines for the management of cancer in people with dementia.
Funding
This work was supported by a Tenovus Innovation Grant (grant number TIG 2014-15).
Conflict of interest
The authors have no conflicts of interest to declare in relation to this work.
Appendix A: Search strategy: MEDLINE (OVID)
Searches
| □ | 1 | cancer.mp. or exp Neoplasms/ |
| □ | 2 | dementia.mp. or exp Delirium, Dementia, Amnestic, Cognitive Disorders/ or exp Dementia, Vascular/ or exp Dementia, Multi-infarct/ or exp Dementia/ or exp Frontotemporal Dementia/ |
| □ | 3 | exp Mild Cognitive Impairment/ or cognitive impairment.mp. |
| □ | 4 | exp Memory/ or exp Memory Disorders/ or exp Mental Recall/ or memory problem.mp. |
| □ | 5 | 2 or 3 or 4 |
| □ | 6 | 1 and 5 |
| □ | 7 | limit 6 to (english language and humans and yr = "2000 -Current" and "all adult (19 plus years)") |




