Staphylococcus aureus selective antimicrobial polymers based on an arginine-derived monomer
Abstract
The continuous spread of resistance genes among bacterial populations, in particular in hospital setting, threatens the advances of our medical systems and already costs more than 1 million lives per year. Antimicrobial polymers (APs) are a promising type of materials to counteract these developments, as they are non-susceptible toward resistance development, and even target resistant bacteria. We herein show the synthesis of an arginine-containing monomer and its polymerization via photo-induced reversible addition-fragmentation chain-transfer polymerization. The synthesis is straightforward, and the macromolecules possess two charged functions (i.e., guanidinium and ammonium) per repeating unit after deprotection. Assessment of the biological activity reveals that polymers inhibit the growth of methicillin-resistant Staphylococcus aureus (MRSA) while being relatively inactive against tested Gram-negative strains. Surprisingly, among the tested polymers, the homopolymer of the arginine-derived monomer is the best-performing material, even though it possesses no notable hydrophobic units. Toxicity tests against red blood cells and other mammalian cells show a good biocompatibility of polymers, leading to an overall excellent selectivity. Using membrane models, the mechanism of action was found to be membrane disruption. The good selectivity of the herein presented polymer for MRSA makes them promising materials for the development of therapeutic agents against such infections.
Graphical Abstract
1 INTRODUCTION
Antimicrobial resistance (AMR) is a growing threat to our society, and the World Health Organization defines AMR as one of the top 10 threats humanity must face.1 It is estimated that in 2019, over 1 million deaths occurred as a direct consequence of AMR, while many more were associated with resistant bacteria.2 Staphylococcus aureus (SA) is among the top six pathogens responsible for these numbers and methicillin-resistant Staphylococcus aureus (MRSA) alone is the cause of 100,000 deaths in 1 year. Its “capacity for genetic adaptation and the serial emergence of successful epidemic strains” are in part responsible for MRSA being a major threat to human health.3 The ease of MRSA to colonize surfaces,4 and its ability to colonize virtually any part of the human body, combined with the insusceptibility of MRSA to ß-lactam antibiotics, render it particularly dangerous in hospital settings.5 As such, developing new antimicrobials, targeting MRSA and other pathogens, without risking further resistance development is a worthwhile goal.
The susceptibility of antibiotics to AMR is in part based on their high target selectivity. As such, the development of novel antimicrobials, possessing a broader mechanism of action holds promise. Antimicrobial polymers (APs), which are mimics of host defense peptides are auspicious candidates, as their target is the bacterial cell envelope.6 A generally accepted mechanism of action comprises the attachment of cationic APs to the negatively charged cell membrane.7 This is followed by membrane insertion and hole formation caused by hydrophobic components of APs. A fitting amphiphilic balance is deemed to be of paramount importance for AP performance.8 But also other features such as the polymer length,9 or the polymer architecture,10 are essential for a beneficial bioactivity. The identity of the cationic functionality is another parameter that has tremendous influence on AP activity and selectivity, with primary amines generally being considered to be ideal in most cases.11
However, also guanidine-based charged units show excellent activity,12 can outperform their ammonium-based counterparts,13 and increase antifungal activity.14 While some of those APs show membrane permeabilization just as ammonium-based APs,12, 15 others are reported to leave the cell envelope intact while still being bactericidal. Fascinatingly, this behavior was linked to an intracellular mechanism, likely leading to condensation of DNA by the polymer.16, 17 In fact, it has been shown that guanidinium-based APs are able to penetrate the membranes of mammalian cells to eradicate intracellular SA infections.18
Arginine plays a special role as it is the key component in cell-penetrating peptides, able to cross cellular membranes with relative ease. This effect, also referred to as “arginine magic” is still not fully understood. However, the guanidine group is special among cationic functionalities in several aspects, as its charge is distributed over its three nitrogen atoms creating a Y-conjugated quasi-aromaticity, possesses amphiphilic properties, and can form like-charge ion pairs overcoming charge repulsion.19 The latter property was suggested to be important in the context of membrane pore formation.20 Translocation of arginine-rich compounds is described to proceed either via temporary pore formation,21 or by a combination of layering and membrane fusion.22 It has also been demonstrated that the combination of ammonium and guanidinium groups in mixtures of homopolymers or within one block copolymer shows synergistic effects.23 As such, we asked ourselves if this synergy can also be achieved when both functionalities are incorporated into the same repeating unit, and if we could use arginine, as a bio-based source for such polymers.
To answer these questions, an arginine-based acrylamide was synthesized. Indeed, the functionalization of acrylic monomers with amino acids is a well-known strategy to produce functional materials.24 Respective monomers were polymerized via reversible addition-fragmentation chain-transfer (RAFT) polymerization. The method is robust and enables macromolecular precision.25 Moreover, as molecular weight26 and dispersity9 of APs influence their activity, a precise control using a radical polymerization27 is desirable.
To decouple the polymerization from the reaction temperature and enable rapid synthesis of APs, photo-iniferter (PI)-RAFT polymerization can be used.28 Our group has recently developed a variation where xanthates are added as additional chain transfer agent (CTA), enabling rapid and living reactions under mild UV-light.29 It is worth noting that such strategies have also been used to control the dispersity of produced polymers.30 While this is not in focus here, the herein used method could be used to alter this parameter in a straightforward manner. In the context of photo polymerization, xanthates are excellent iniferters when activated via UV-light,31 and photo-mediated RAFT polymerization has been demonstrated to be a useful tool for AP production.32
Herein, we describe the synthesis of an arginine-derived monomer, its (co)polymerization via xanthate-mediated PI (XPI)-RAFT polymerization, as well as biological testing of resulting macromolecules. The presented materials show a strong preference toward MRSA, while leaving tested Gram-negative bacteria mostly unharmed.
2 RESULTS AND DISCUSSION
The first step toward arginine-derived APs was the design of a monomer, based on available arginine derivatives. We choose to use arginine where both the amino as well as the guanidine functions have been protected (with tert-butyloxycarbonyl (Boc) and 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl (Pbf), respectively). Both protecting groups are well established, for instance, in solid-phase peptide synthesis. A protected precursor enables selective monomer synthesis, as well as restricts issues during polymerization, where CTAs could undergo lysis in the presence of strongly nucleophilic functionalities.33 Both groups can be cleaved under acidic conditions after polymerization. The free acid group of arginine was subsequently converted in a Steglich esterification reaction (Figure 1). This is a commonly used reaction for the production of such monomers.24, 34 Hydroxyethyl acrylamide (HEAM) was used as alcohol and N,N′ Dicyclohexylcarbodiimid (DCC) as well as 4-(Dimethylamino)pyridine (DMAP) were used as coupling reagents. The reaction was performed in DCM where the respective dicyclohexylurea precipitates and a yield of 88% was achieved after 7 days at room temperature. The product was named ArgAM(P), where (P) refers to the presence of protecting groups.
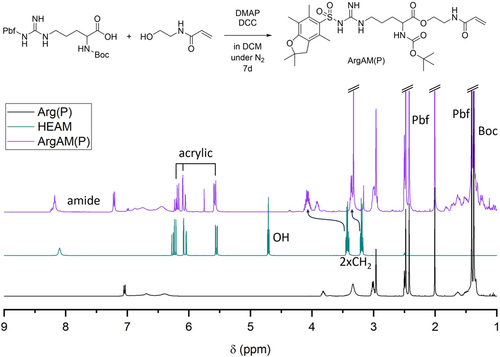
The 1H-NMR spectrum (Figure 1) shows the successful coupling of the educts as the product contains acrylic signals as well as peaks associated to the protecting groups. Moreover, the signals of the two CH2 groups associated to the ethyl spacer in HEAM shift significantly upon coupling, as they are directly adjacent to the OH group. A 13C spectrum of ArgAM can be found in the Supporting Information (Figure S1).
Initial attempts to polymerize ArgAM faced some issues regarding the solubility of the monomer. A list of solvents where ArgAM was not or insufficiently soluble at a concentration of least 0.1 mol L−1, or where the formed polymer precipitates can be found in the Supporting Information (Table S1). Ultimately, polymerizations were carried out in a mixture of toluene and isopropanol (1:4). In this mixture, the monomer and the polymer were soluble up to a degree of polymerization (DP) of 100 using an initial monomer concentration of 0.1 mol L−1. After optimization of the reaction medium, the homo and copolymerization of ArgAM were investigated using the XPI-RAFT methodology. An overview of the performed reactions can be found in Scheme 1.
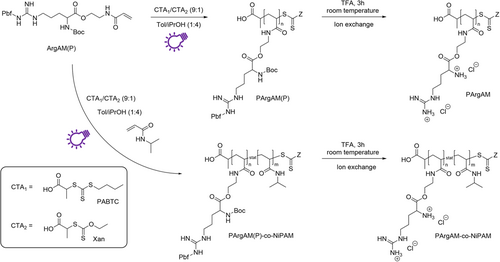
For copolymerizations, N-iso-propylacrylamide (NiPAM) was used as a comonomer in different ratios. NiPAM has previously been used as a mildly hydrophobic component in APs showing beneficial properties regarding antimicrobial activity, as well as reduced hemolytic activity.10, 35 To understand the amphiphilic balance in this comonomer system, only statistical copolymers were produced with more complex architectures like block copolymers representing an option for further investigations. Polymerizations were performed using a 1:9 mixture of O-Ethyl-S-(1-carboxy)methyl xanthate (Xan) and propanoic acid butyl trithiocarbonate (PABTC) as CTAs. While PABTC is excellently suited to control the polymerization of acrylamides,36 Xan represents a highly active iniferter, able to drive the reaction under UV-light (365 nm).31 Reactions were performed using an LED photo-reactor setup with a high power output (~100 W corresponding to approximately 75 mW cm−2 as detected in the reaction chamber). The reactor was cooled to maintain a temperature of 20°C.
To find suitable reaction times and assess whether the comonomers are both consumed in the case of the copolymerization, two kinetic reactions were performed. The DP was set to 50 and samples were taken at different time intervals to determine monomer conversions via 1H-NMR spectroscopy (Figure 2).
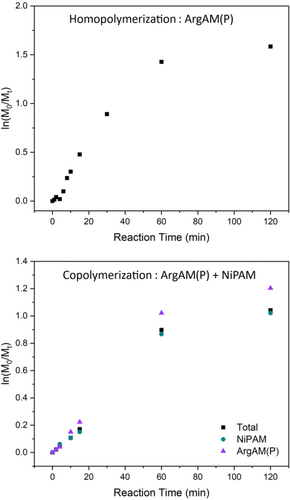
The homopolymerization proceeds rapidly in the beginning and slows down after 1 h, an effect that has been observed with other monomers using XPI-RAFT polymerization.29 The final conversion after 2 h of reaction time is above 80% which is satisfactory given the low overall monomer and CTA concentrations. The copolymerization only proceeds up to a conversion of 65% (ln(M0/Mt) = 1.04). However, both monomers were consumed at similar rates indicating a homogenous monomer distribution in the final product. For producing polymers on a larger scale for biological tests, a reaction time of 60 min was chosen as, within this time, the majority of the monomer is consumed.
Because of an application as AP, the polymers had to be deprotected, creating positively charged macromolecules. This was tested in neat trifluoro acetic acid (TFA) at room temperature over the course of 3 h. A successful deprotection was verified via the 1H-NMR spectra comparing the starting material and product of the reaction (Figure 3). The virtual absence of protecting group signals pointed toward a near-quantitative reaction. This was supported by the excellent water solubility of PArgAM. To rule out the influence of the counterion on later biological tests, the TFA anions were replaced with chloride via treatment with an ion exchange resin.
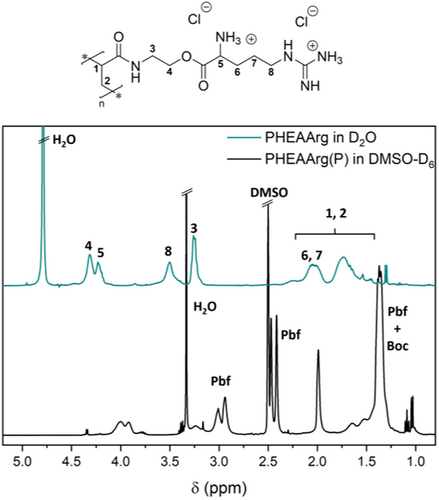
For antimicrobial activity, the overall charge of the polymer is of utmost importance to test whether the close proximity of charged units in the polymer leads to drastic alteration of the respective pKa values, PArgAM was titrated against NaOH (Figure S3). The homopolymer was chosen as coulombic repulsion is expected to be most intense here. The results show that both the guanidine (pKa = 10.3) and the amine (pKa = 6.6) are much lower than in the small molecule amino acid (pKa's = 12.1, 9.0). Still, the values are high enough to ensure that a majority of the groups are charged at physiological pH, with a share of the amino groups being unprotonated. While the presence of amino groups could lead to undesired aminolysis, the absence of a high molecular weight shoulder in the SEC traces after deprotection suggests that such reactions are absent as otherwise resulting thiols would couple in an oxidative fashion. While it has been described that CTA end groups are able to change biological activity,37 the herein used CTAs do likely not possess sufficient hydrophobicity to alter the membrane interaction.
Following the optimization of reaction conditions, four polymers were produced to be tested regarding their bioactivity. In addition to a homopolymer of ArgAM, three copolymers with NiPAM featuring different comonomer ratios were synthesized. Copolymers are referred to as PArgAM-co-NiPAM(X), where X refers to the amount of NiPAM within the copolymer in percent. The change in the ratio between ArgAM and NiPAM should lead to an altered amphiphilic balance of the respective polymers, which is a major parameter to be varied in AP design. Due to the high charge density of the monomer, it is expected that high amounts of NIPAM are beneficial for the planned applications. Thus, 50%, 80%, and 90% of NIPAM were targeted for copolymers. The SEC curves of polymers before and after deprotection can be found in Figure 4.
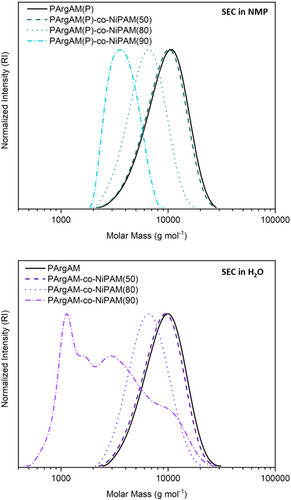
All polymers except PArgAM-co-NiPAM(90) showed unimodal molecular weight distributions and low dispersities (Table 1). The molecular weights were in the expected range and decreased with increasing NiPAM content, potentially as coiling is more pronounced when the charge density is reduced. The multimodal distribution of PArgAM-co-NiPAM(90) is likely associated with the low cationic comonomer content and the measurement conditions. The polymer consists mainly of PNiPAM which is known to possess an LCST-type behavior in water. As the column of the respective SEC setup was heated to 40°C, a coil-to-globule transition of the polymer was potentially responsible for the non-uniform SEC trace. It should be noted that for each polymer, the counterion was exchanged after deprotection using an ion exchange resin loaded with chloride. This was done to ensure the absence of TFA counterions that could alter the bioactivity of tested polymers.
| Sample | NiPAM contenta | Mnb | Ðb | Mnc | Ðc | Mn (theo.) | MIC50 (EC) | MIC50 (PA) | MIC50d (MRSA) | Hc10e | CC50f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (g mol−1) | (g mol−1) | (g mol−1) | (μg mL−1) | (μg mL−1) | (μg mL−1) | (μg mL−1) | (μg mL−1) | |||
| PArgAM | 0 | 9500 | 1.28 | 8300 | 1.19 | 17,200 | 512 | 128 | 16 | 512 | 870 |
| PArgAM-co-NiPAM(50) | 51 | 9200 | 1.23 | 7900 | 1.19 | 11,400 | 512 | 128 | 32 | 256 | 1007 |
| PArgAM-co-NiPAM(80) | 69 | 7200 | 1.18 | 6000 | 1.17 | 9100 | 512 | 256 | 64 | 256 | 612 |
| PArgAM-co-NiPAM(90) | 96 | 4300 | 1.15 | - | - | 6100 | 512 | 512 | 256 | 2048 | 953 |
- Note: The targeted DP for all polymers was 50. MIC50 values were determined using Escherichia coli (EC), Pseudomonas aeruginosa (PA), and methicillin-resistant Staphylococcus aureus (MRSA).
- a Determined via 1H-NMR spectroscopy comparing the signals of respective monomers.
- b SEC in NMP/LiBr (PS calibration) before deprotection.
- c SEC in water (0.3% formic acid, 0.1 mol L−1 NaCl, PEG calibration) after deprotection.
- d Concentration where optical density of bacterial cells was reduced to 50% or below growth inhibition assays.
- e Hemolytic concentration where 10% of red blood cells were lysed.
- f Concentration where cell viability was reduced to 50% or below in cytotoxicity tests.
The obtained polymers were then tested regarding their biological activity. As such, the first test that was performed was the determination of antimicrobial activity. This was accomplished by growth inhibition assays, where pathogenic bacteria were incubated with different concentrations of the respective polymers. The lowest concentration where less than 50% of growth (as determined by the optical density) was detected after 24 h is defined as the MIC50 value (Figure 5A). The concentration-dependent growth inhibition curves can be found in the Supporting Information (Figure S2). A panel of three pathogens was used to assess the antimicrobial potential of the polymers. Two Gram-negative strains (Escherichia coli [E. coli] and Pseudomonas aeruginosa [P. aeruginosa]) were utilized. P. aeruginosa possesses resistance against tetracycline, chloramphenicol, and kanamycin. As a Gram-positive pathogen, a methicillin- and oxacillin-resistant strain of Staphylococcus aureus (MRSA) was used.
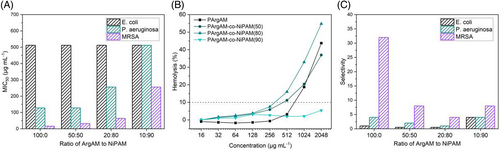
The polymers showed only limited activity against E. coli with MIC50 values of 512 μg mL−1 regardless of the composition. For the other two strains, the activity is clearly linked to the amphiphilic balance, with a decreasing activity for higher NiPAM contents. This is interesting as the polymers do not seem to require a distinct hydrophobic component to be antimicrobial active. Usually, the absence of hydrophobic components leads to a diminished antimicrobial performance, as found for different polymeric platforms.8, 38 The results are even more surprising as each ArgAM unit has two charged groups resulting in a highly charged homopolymer. However, there are also sufficient examples where the presence of a distinct hydrophobic functionality is not required.39, 40 Moreover, while the activity against P. aeruginosa is limited with MIC50 values of >100 μg mL−1, the activity against MRSA is relatively pronounced. Also here, a clear connection to the charge density is obvious with the homopolymer PArgAM being the best-performing material with an MIC50 of 16 μg mL−1. It could be argued that the direct accessibility of the plasma membrane in S. aureus enables these highly charged polymers to directly cause disruption, whereas in Gram-negative bacteria, the outer membrane might not be susceptible to PArgAM.
The higher this value, the better a polymer selects bacterial cells over RBCs. The values as displayed in Figure 5C show clearly that the homopolymer PArgAM has a high selectivity for MRSA (32), whereas for all other combinations of polymer and pathogen, only moderate values are achieved. As such, the polymer has potential for the treatment of MRSA-based infections. It should be noted that this strain already possesses resistances against various antibiotics and while the herein produced polymers are not expected to be susceptible to any of these mechanisms, it is still reassuring that PArgAM performs well against a resistant strain.
However, while RBCs are the first type of cells a drug faces when being administered via injection, the polymer has to be tolerated by other types of cells as well. In this study, we tested the cell viability of L929 mouse fibroblasts in the presence of APs as recommended by the ISO-10993-5 guideline (Biological evaluation of medical devices. Part 5: Tests for cytotoxicity: In vitro methods).
The viability of cells was determined via an MTT assay as a function of the concentration of the respective polymer (Figure 6A). Also here, no severe toxicity was detected at lower concentrations. The CC50 values (Table 1) define the concentration necessary to reduce cell viability below 50% relative to the negative control. PArgAM-co-NiPAM(80) reduced the cell viability most drastically with a CC50 value of about 600 μg mL−1. Overall, the cytocompatibility was very high, which is not granted for polymers with a high charge density, as evident by the inherent toxicity of poly(ethylene imine).41 This finding could be a result of the formation of a protein corona in the growth medium of the cells. However, it should be pointed out that tests were also performed in medium that contains proteins (Mueller–Hinton broth [MHB]), and still a notable activity was recorded.
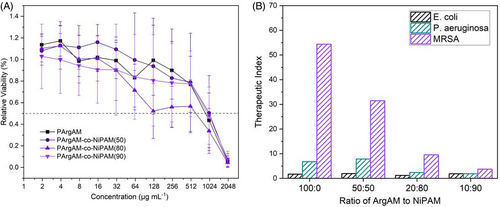
The respective values can be found in Figure 6B and again underline the suitability of PArgAM for MRSA growth inhibition. Again, PArgAM is the best-performing polymer with a TI of 54 for MRSA. The combination of both, a high selectivity and a high TI make PArgAM an interesting candidate for further studies.
As a first step in this direction, wanted to elucidate whether PArgAM is actually membrane active. As some guanidine-containing polymers have been described to act intracellularly, this is a relevant question in this context. For this purpose, large unilamellar vesicles (LUVs) were used as membrane models featuring a lipid composition that resembles that of the cellular membrane of SA. The membrane integrity was probed by the incorporation of calcein as a fluorescence dye into the LUVs in a self-quenching concentration. Membrane disruption leads to a release of the dye, which results in a decrease in concentration and hence an increase in fluorescence.26 A complete rupture was induced at the end of each measurement using the surfactant triton X. This fluorescence value was set to 100% to normalize the measurements (Figure 7A). As evident by the increase in fluorescence upon polymer addition at a concentration of 50 μg mL−1 and above, PArgAM is indeed membrane active, leading to a rapid release of the LUV content. The concentration fits well with the MIC50, and the rapid increase in fluorescence points toward an uncontrolled rupture of LUVs instead of distinct pore formation. Dynamic light scattering (DLS) measurements were performed (Figure 7B) to receive more insight into the process. Initially, LUVs were in the range of 200 nm in size, which fits well with the expectations based on the production process (extrusion through a membrane with 400 nm pores). After the addition of triton X, the size decreased to very low values indicating a complete destruction of the LUVs with micellar residuals. In contrast, upon polymer addition, larger aggregates formed, which shows that membranes were not degraded entirely but were agglomerated after rupture.
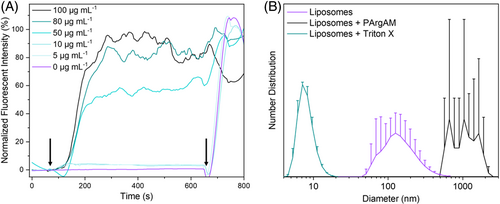
These results indicate that the presence of ammonium groups, which are not as kinetically labile as guanidinium groups regarding electrostatic interaction, governs the mode of membrane interaction. However, purely guanidinium functional polymers have been described to proceed via this mechanism as well.12, 15 An interesting feature of the herein presented polymers is their selectivity for MRSA, an effect that has to be elucidated in further studies. In general, APs that are only active for a specific pathogen are highly promising materials, as they would enable therapy of a specific infection without compromising the microbiome of the host, which is usually associated with side effects of the treatment.
3 EXPERIMENTAL
3.1 Materials and methods
Chemicals used for this study were purchased from Carl Roth, TCI (Tokyo Chemical Industry), Merck, Fisher Scientific, Alfa Aesar, and Sigma-Aldrich. PABTC and Xan were synthesized according to the literature.31, 42 Inhibitor was removed from NiPAM via recrystallization before polymerization.
1H-NMR spectra were recorded on a Bruker AVANCE NEO 400 MHz spectrometer in DMSO-D6 or D2O. Chemical shift values were reported in ppm, and as internal standard the residual proton signal of the solvent was used. SEC measurements performed with N-Methyl-2-pyrrolidone (NMP) as eluent were recorded with parallel UV, and RI detection and stationary phase was a 300 × 8 mm2 PSS GRAM Linear column at a flow rate of 0.5 mL min−1 and a temperature of 60°C. SEC in water containing 0.1 M NaCl and 0.3 V% formic acid, were performed at a flow rate of 1 mL min−1 at 40°C, stationary phase was 300 × 8 mm2 PSS NOVEMA Max column measurements were executed with synchronous UV, light scattering, and RI detection. Solutions containing polymer samples were filtered through 0.45 μm Polytetrafluoroethylene filters; the injected volume was 100 μL. PS and PEG standards (PSS, Mainz, Germany) were used for calibration, respectively. DLS measurements of polymers in milliQ water were performed on a Zetasizer Ultra from Malvern Panalytical (UK) at a measurement angle of 173° and temperature of 25°C.
For photo-polymerizations a PhotoCube from ThalesNano was used. Reactions were cooled via cooling water maintaining a temperature of 20°C. In this work, only 365 nm light was used at an intensity of 100% (setting high). The LED intensity was measured at the sample position with a commercial S170C power meter (Thorlabs). It should be noted that the flat sensor only fits into the sample chamber vertically while the reaction chamber is illuminated from all four sides. Hence, one fourth of the LEDs are behind the sensor. This was adjusted by setting the measured intensity as three fourths of the total intensity which is 75 mW cm−2.
3.2 Synthesis of ArgAM(P)
To a stirred solution of 12.5 mmol Boc-Arg(Pbf)-OH in 125 mL DCM, 0.6 mmol DMAP and 17.5 mmol N-Hydroxyethyl acrylamide (1.4 Eq.) were added. Afterward, 15.1 mmol DCC was added to the mixture, which was then stirred for 7 days at room temperature under nitrogen atmosphere. The formed precipitate (DCC urea) was filtered off through silica gel. The silica was flushed with an additional 100 mL DCM. The organic phase was then washed with water (2 × 50 mL) and brine (1 × 50 mL). The DCM phase was dried over anhydrous Na2SO4 and the drying agent was separated by filtration. The solvent was evaporated under reduced pressure. The product was obtained as a white solid with a yield of 88%.
1H-NMR (400 MHz, DMSO-d6): δ = 8.19 (1H, t), 7.21 (1H, d), 7.05–6.28 (3H, m), 6.20 (1H, dd), 6.08 (1H, dd), 5.58 (1H, dd), 4.06 (2H, m), 3.93 (1H, q), 3.37 (2H, q), 3.00 (2H, q), 2.96 (2H, s), 2.48 (3H, s), 2.43 (3H, s), 2.01 (3H, s), 1.88–1.46 (4H, m), 1.41 (6H, s), and 1.37 (9H, s) ppm.
13C-NMR (100 MHz, DMSO-d6): δ = 172.8, 165.3, 157.9, 156.6, 156.0, 137.7, 134.7, 131.9, 131.9, 125.9, 124.8, 116.7, 86.8, 78.7, 63.3, 55.2, 53.8, 43.0, 38.1, 28.8, 28.6, 25.4, 24.5, 19.4, 18.1, and 12.7 ppm.
3.3 Polymerization of ArgAM(P)
For the polymerizations, the monomers and CTAs were added into a 50-mL round-bottom flask with magnetic stirrer. The solvent (toluene/isopropanol 1:4) was added and the flask was closed with a septum. Oxygen was removed from the solution via purging with nitrogen for 10 min. After degassing, the flask was placed into the photoreactor and irradiation was performed at 365 nm (100%/high, corresponding to approximately 75 mW cm−2) under slow stirring for 60 min. After the reaction was complete, the polymer was precipitated in diethyl ether and separated via centrifugation. The precipitate was washed with diethyl ether (3 × 50 mL) and dried under reduced pressure. The polymer was obtained as a pale-yellow powder. An experimental DP cannot be determined by NMR spectroscopy as the end group signals are overlaid by polymer signals. PArgAM-co-NiPAM(90) could not be separated via precipitation. For this sample, precipitation was skipped and subsequent deprotecting was performed from the polymerization solution after removal of the majority of the solvent. The used compositions can be found in Table 2.
| Sample | m (ArgAM(P) | M (NiPAM) | M (PABTC) | m (Xan) | V (solvent) | Conversion |
|---|---|---|---|---|---|---|
| (mg) | (mg) | (mg) | (mg) | (mL) | (%) | |
| PArgAM | 1248 | 0 | 8.6 | 0.8 | 18.8 | 71.5 |
| PArgAM-co-NiPAM(50) | 936 | 191 | 12.9 | 1.2 | 28.9 | n. d. |
| PArgAM-co-NiPAM(80) | 437 | 356 | 15.0 | 1.4 | 34.2 | 75.3 |
| PArgAM-co-NiPAM(90) | 218 | 400 | 15.0 | 1.4 | 34.4 | 81.2 |
- Note: The DP was set to 50 and the ratio of PABTC to Xanthate was 9:1 for all polymers. Conversion was determined by 1H-NMR spectroscopy via comparison of t0- and t60-samples from the reaction solution.
For kinetic studies, irradiation was stopped at distinct time points and samples were taken via a syringe through the septum. The conversion was determined via 1H-NMR spectroscopy and the reaction mixture was placed back into the reactor and irradiation was resumed.
3.4 Deprotection and anion exchange of PArgAM(P) and copolymers
For deprotection of the polymers, 1 mL of TFA was used per 50 mg of polymer. Here, the solution was stirred at room temperature for 3 h and a color change from pale-yellow to orange was observed. The solution was then slowly added to cold diethyl ether. The precipitated polymer was centrifuged at −10°C, washed two times with diethyl ether and centrifuged again. The solid was dried at high vacuum for several hours. The polymers were obtained as a white solid.
For anion exchange, each polymer was dissolved in water (15 mg mL−1), and filtered through AmberLite HPR4800 Cl. Finally, 10 drops of 0.1 M HCl are added to the collected solution to ensure complete protonation of all alkaline groups. The polymers were isolated via freeze drying, and obtained as a white solid.
3.5 MIC tests
To determine the MIC50 two Gram-negative bacteria: E. coli (ATCC 25922) and P. aeruginosa (ATCC 10145) and one strain of Gram-positive bacteria: MRSA (ATCC 43300) were used. A single colony of bacteria was inoculated in 5 mL of MHB medium and incubated overnight at 37°C. Afterward, the concentration of cells was determined by measuring the optical density at 600 nm (OD600) and diluted with MHB to achieve an OD600 of 0.1 for each bacterial suspension. MHB was added to this solution in a ratio of 1:5000 to prepare the final polymer suspensions. Next, polymers were dissolved in MHB and a serial dilution was performed with three determinations for each concentration. About 100 μL of each polymer was added to a micro-well plate, followed by 100 μL of bacterial suspension. Negative control wells only containing MHB were used and wells containing only bacterial solution without polymer samples serve as positive control. The micro-well plates were incubated for 1 day at 37°C. Growth of bacteria was evaluated by measuring the OD600 and normalized using positive and negative control. The MIC50 was defined as the lowest concentration with less than 50% relative absorption, which correlates with the growth inhibition of the bacterial colony of 50%.
3.6 Hemolysis tests
To test hemotoxicity, defibrinated sheep blood was used. RBCs were isolated by centrifugation at 4500 rpm for 1 min and washed with phosphate-buffered saline (PBS) via centrifugation. The RBCs were diluted in a ratio of 1:25 with PBS. This suspension was used for further measurements. Polymers were dissolved separately in PBS and a serial dilution was performed with three determinations for each concentration. Hemolysis tests were performed by adding 600 μL of polymer solution to an Eppendorf tube, followed by 600 μL of the suspension of the RBCs. As negative control, pure PBS and positive control 600 μL of RBC suspension combined with 600 μL of a Triton X solution (1% in PBS) were used. The mixture was incubated for 1 h at 37°C. Centrifugation for 45 s at 4500 rpm was used to separate RBCs from the suspension. The supernatant was transferred into a cuvette and analyzed by UV absorption at 544 nm. The normalization was set by using negative and positive control. Hemolysis was selected as the lowest concentration which was able to induce less than 10% of RBC lysis (Hc10).
3.7 Dye leakage assay
LUVs mimicking bacterial membranes of S. aureus were prepared by the thin film method using 6 mg of 2-Oleoyl-1-palmitoyl-snglycero-3-phospho-rac-(1-glycerol) sodium salt and 4 mg of 18:1 Cardiolipin. The lipids were dissolved in chloroform (0.8 mL), and a thin film was formed by evaporation of the organic solvent on the rotary evaporator under vacuum. The dried thin film was then hydrated with 1 mL of a 40 mM calcein solution. After stirring for 1 h at room temperature, five freeze–thaw cycles in liquid nitrogen for 5 min and in a water bath at 25°C for 15 min were necessary to maximize the encapsulation of calcein into the LUVs. The vesicles were then extruded 15 times using polycarbonate membranes with pore size of 400 nm. Non-encapsulated calcein dye was removed by dilution of the suspension in PBS, followed by filtration through an Amicon Ultra-15, PLTK Ultracel-PL Membran, 15 kDa for 90 min at a centrifugation speed of 4000 rpm.
A Fluoromax 4 spectrometer was used to investigate the polymer-induced dye leakage. The excitation wavelength was set at 490 nm and the fluorescence intensity was monitored over a time period of 800 s of which the CPS signals were recorded each 5 s at the emission wavelength of 525 nm. For this time-based experiment, 1.5 mL of a solution of calcein-loaded liposomes was placed in a quartz cuvette under stirring for fluorescence monitoring. A baseline of calcein fluorescence without polymer addition was normalized for each sample for 100 s. Then, 15 μL of different polymer solutions in PBS of various concentrations were added to the cuvette. After 700 s, 20 μL of Triton X (20% solution) in PBS was added to completely disrupt the liposomes. It serves as a positive control to determine the fluorescence intensity corresponding to 100% dye leakage. The measured fluorescence intensity was normalized into percentage leakage activity.
3.8 Cytotoxicity assay
3.8.1 Cell culture
L929 cells were grown in Dulbecco's modified Eagle medium (VWR) supplemented with 10% (v/v) fetal bovine serum (VWR), 100 U mL−1 penicillin (VWR), and 100 μg mL−1 streptomycin (VWR). Cells were maintained at 37°C in a fully humidified atmosphere containing 5% CO2.
3.8.2 Cell viability
4 CONCLUSION
We demonstrate the straightforward synthesis of an arginine-based acrylamide monomer (ArgAM) from commercially available components and its polymerization using the RAFT process. More specifically, xanthate supported photo-iniferter RAFT polymerization is used to produce polymers in short reaction times (60 min) at room temperature. Homopolymerization as well as copolymerization with NiPAM proceed in a controlled fashion and cationic polymers were obtained after the removal of the protecting groups of arginine.
The investigation of the antimicrobial activity of (co)polymers showed a strong preference for the inactivation of MRSA, while tested Gram-negative bacteria were not affected at relevant concentrations. Excellent hemo and cytocompatibility of the polymers resulted in a good selectivity regarding bacterial cell membranes over interaction with mammalian cells. The mechanism was proven to be based on membrane disruption using membrane models. Overall, the specific action against MRSA in combination with the good otherwise biocompatibility makes the described polymers promising candidates for the development of new treatment actions for resistant bacteria.
Future work will focus on elucidating the reason for the selectivity between pathogens and on generating more detailed information about the interaction of these polymers with membranes.
ACKNOWLEDGMENTS
CB, AL, AB, and MH gratefully acknowledge funding by the Emmy-Noether Program of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; project number 445804074). The authors also thank the NMR core facility of the Institute of Chemistry (University of Potsdam) of Prof Dr Heiko Möller, Dr Matthias Heydenreich, and Angela Krtitschka. Prof Dr Helmut Schlaad and Sascha Prentzel from the Institute of Chemistry (University of Potsdam) are gratefully acknowledged for providing the facility to perform SEC measurements. The authors furthermore would like to thank Prof Dr Ruth Freitag for the kind access to her laboratory and equipment to perform the MTT assay. Open Access funding enabled and organized by Projekt DEAL.