Recent developments in catalytic amide bond formation
Mihajlo Todorovic
Department of Chemistry, University of British Columbia, Vancouver, British Columbia, Canada
Search for more papers by this authorCorresponding Author
David M. Perrin
Department of Chemistry, University of British Columbia, Vancouver, British Columbia, Canada
Correspondence
David M. Perrin, Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC V6T 1Z1, Canada.
Email: [email protected]
Search for more papers by this authorMihajlo Todorovic
Department of Chemistry, University of British Columbia, Vancouver, British Columbia, Canada
Search for more papers by this authorCorresponding Author
David M. Perrin
Department of Chemistry, University of British Columbia, Vancouver, British Columbia, Canada
Correspondence
David M. Perrin, Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC V6T 1Z1, Canada.
Email: [email protected]
Search for more papers by this authorFunding information: Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada
Abstract
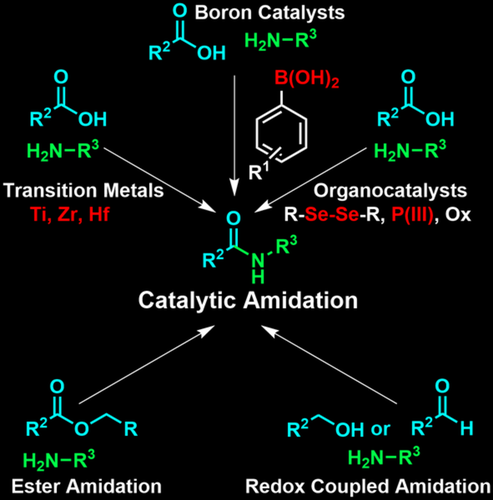
Amide bond forming reactions are critical for both polypeptide synthesis and medicinal chemistry. Most current approaches for amidation employ stoichiometric activating agents, but such methods are neither atom economical nor synthetically elegant. Catalytic approaches for amidation are potentially green and more ideal substitutes for current standard methods and thus are the subject of this review. Such methods face significant thermodynamic and kinetic barriers and have, as a result, historically conceded the use of elevated temperatures and dehydrating agents or lacked broad and relevant substrate scopes from the perspective of peptide chemistry. Recent advancements in methods for both direct amidation (the coupling of a carboxylic acid and an amine) and indirect amidation (the coupling of other partners resulting in an amide bond) based on aryl boronic acids, transition metals and organocatalysis for the former and ester amidation and redox-coupled amidation for the later, address these previous shortcomings and are examined therein.
Graphical Abstract
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as it is as review and no new data were created in this study.
REFERENCES
- 1D. G. Brown, J. Boström, J. Med. Chem. 2016, 59, 4443.
- 2N. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Lett. 1979, 20, 3437.
- 3D. Milstein, J. K. Stille, J. Am. Chem. Soc. 1978, 100, 3636.
- 4A. S. Guram, S. L. Buchwald, J. Am. Chem. Soc. 1994, 116, 7901.
- 5F. Paul, J. Patt, J. F. Hartwig, J. Am. Chem. Soc. 1994, 116, 5969.
- 6C. A. G. N. Montalbetti, V. Falque, Tetrahedron 2005, 61, 10827.
- 7H. Charville, D. Jackson, A. Whiting, Chem. Commun. 2010, 46, 1813.
- 8H. Charville, D. A. Jackson, G. Hodges, A. Whiting, M. R. Wilson, European J. Org. Chem. 2011, 2011, 5981.
- 9V. R. Pattabiraman, J. W. Bode, Nature 2011, 480, 471.
- 10Rotman, D. Chemical Week. 1993, p 12.
- 11P. T. Anastas, J. C. Warner, Green Chemistry: Theory and Practice, 1st ed., Oxford University Press, New York 1998.
- 12S. M. Dhawan, B. M. Gupta, D. K. Siddaiah, Int. J. Inf. Dissem. Technol. 2016, 6, S37.
- 13K. D. Wehrstedt, P. A. Wandrey, D. Heitkamp, J. Hazard. Mater. 2005, 126, 1.
- 14K. J. McKnelly, W. Sokol, J. S. Nowick, J. Org. Chem. 2020, 85, 1764.
- 15M. Erny, M. Lundqvist, J. H. Rasmussen, O. Ludemann-Hombourger, F. Bihel, J. Pawlas, Org. Process Res. Dev. 2020, 24, 1341.
- 16A. D. McFarland, J. Y. Buser, M. C. Embry, C. B. Held, S. P. Kolis, Org. Process Res. Dev. 2019, 23, 2099.
- 17D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks, T. Y. Zhang, Green Chem. 2007, 9, 411.
- 18A. Isidro-Llobet, M. N. Kenworthy, S. Mukherjee, M. E. Kopach, K. Wegner, F. Gallou, A. G. Smith, F. Roschangar, J. Org. Chem. 2019, 84, 4615.
- 19M. T. Sabatini, L. T. Boulton, H. F. Sneddon, T. D. Sheppard, Nat. Catal. 2019, 2, 10.
- 20K. Ishihara, S. Ohara, H. Yamamoto, J. Org. Chem. 1996, 61, 4196.
- 21L. Shteinberg, S. Kondratov, S. Shein, Zhournal Org. Khimii 1968–1972, 1988, 24.
- 22R. M. De Figueiredo, J. S. Suppo, J. M. Campagne, Chem. Rev. 2016, 116, 12029.
- 23L. Hie, N. F. Fine Nathel, X. Hong, Y. F. Yang, K. N. Houk, N. K. Garg, Angew. Chemie - Int. Ed. 2016, 55, 2810.
- 24A. Ojeda-Porras, D. Gamba-Sánchez, J. Org. Chem. 2016, 81, 11548.
- 25B. Zhang, Y. Li, W. Shi, T. Wang, F. Zhang, L. Liu, Chem. Res. Chinese Univ. 2020, 36, 733.
- 26K. E. Schwieter, J. N. Johnston, J. Am. Chem. Soc. 2016, 138, 14160.
- 27X. Wang, Nat. Catal. 2019, 2, 98.
- 28K. Hollanders, E. Renders, C. Gadais, D. Masullo, L. Van Raemdonck, C. C. D. Wybon, C. Martin, W. A. Herrebout, B. U. W. Maes, S. Ballet, ACS Catal. 2020, 10, 4280.
- 29M. B. Chaudhari, Gnanaprakasam, B. Chem. - An Asian J. 2019, 14, 76.
- 30T. Narendar Reddy, A. Beatriz, V. Jayathirtha Rao, D. P. de Lima, Chem. - An Asian J. 2019, 14, 344.
- 31A. R. Chhatwal, H. V. Lomax, A. J. Blacker, J. M. J. Williams, P. Marcé, Chem. Sci. 2020, 11, 5808.
- 32H. K. Philpott, P. J. Thomas, D. Tew, D. E. Fuerst, S. L. Lovelock, Green Chem. 2018, 20, 3426.
- 33D. Manova, F. Gallier, L. Tak-Tak, L. Yotava, N. Lubin-Germain, Tetrahedron Lett. 2018, 59, 2086.
- 34M. Winn, S. M. Richardson, D. J. Campopiano, J. Micklefield, Curr. Opin. Chem. Biol. 2020, 55, 77.
- 35M. Petchey, A. Cuetos, B. Rowlinson, S. Dannevald, A. Frese, P. W. Sutton, S. Lovelock, R. C. Lloyd, I. J. S. Fairlamb, G. Grogan, Angew. Chemie - Int. Ed. 2018, 57, 11584.
- 36M. R. Petchey, G. Grogan, Adv. Synth. Catal. 2019, 361, 3895.
- 37C. M. Lelièvre, M. Balandras, J. L. Petit, C. Vergne-Vaxelaire, A. Zaparucha, ChemCatChem 2020, 12, 1184.
- 38T. Nuijens, A. Toplak, M. Schmidt, A. Ricci, W. Cabri, Front. Chem. 2019, 7, 1.
- 39J. Pawlas, T. Nuijens, J. Persson, T. Svensson, M. Schmidt, A. Toplak, M. Nilsson, J. H. Rasmussen, Green Chem. 2019, 21, 6451.
- 40K. Yap, J. Du, F. Y. Looi, S. R. Tang, S. J. De Veer, A. R. Bony, F. B. H. Rehm, J. Xie, L. Y. Chan, C. K. Wang, D. J. Adams, L. H. L. Lua, T. Durek, D. J. Craik, Green Chem. 2020, 22, 5002.
- 41D. G. Hall, Chem. Soc. Rev. 2019, 48, 3475.
- 42A. Pelter, T. E. Levitt, P. Nelson, Tetrahedron 1970, 26, 1539.
- 43J. Tani, T. Oine, I. Inoue, Synth. 1975, 11, 714.
- 44S. Arkhipenko, M. T. Sabatini, A. S. Batsanov, V. Karaluka, T. D. Sheppard, H. S. Rzepa, A. Whiting, Chem. Sci. 2018, 9, 1058.
- 45N. Gernigon, R. M. Al-Zoubi, D. G. Hall, J. Org. Chem. 2012, 77, 8386.
- 46S. Fatemi, N. Gernigon, D. G. Hall, Green Chem. 2015, 17, 4016.
- 47R. M. Al-Zoubi, W. K. Al-Jammal, R. McDonald, New J. Chem. 2020, 44, 3612.
- 48T. Mohy El Dine, W. Erb, Y. Berhault, J. Rouden, J. Blanchet, J. Org. Chem. 2015, 80, 4532.
- 49E. K. W. Tam, Rita, L. Y. Liu, A. Chen, European J. Org. Chem. 2015, 2015, 1100.
- 50L. Gu, J. Lim, J. L. Cheong, S. S. Lee, Chem. Commun. 2014, 50, 7017.
- 51K. Ishihara, Y. Lu, Chem. Sci. 2016, 7, 1276.
- 52Y. Lu, K. Wang, K. Ishihara, Asian J. Org. Chem. 2017, 6, 1191.
- 53K. Khaldoun, A. Safer, S. Saidi-Besbes, B. Carboni, R. Le Guével, F. Carreaux, Synth. 2019, 51, 3891.
- 54K. Wang, Y. Lu, K. Ishihara, Chem. Commun. 2018, 54, 5410.
- 55P. Huy, B. Zoller, Nachrichten aus der Chemie 2019, 67, 51.
- 56D. N. Sawant, D. B. Bagal, S. Ogawa, K. Selvam, S. Saito, Org. Lett. 2018, 20, 4397.
- 57N. Shimada, M. Hirata, M. Koshizuka, N. Ohse, R. Kaito, K. Makino, Org. Lett. 2019, 21, 4303.
- 58K. Michigami, T. Sakaguchi, Y. Takemoto, ACS Catal. 2020, 10, 683.
- 59Y. Du, T. Barber, S. E. Lim, H. S. Rzepa, I. R. Baxendale, A. Whiting, Chem. Commun. 2019, 55, 2916.
- 60H. Noda, M. Furutachi, Y. Asada, M. Shibasaki, N. Kumagai, Nat. Chem. 2017, 9, 571.
- 61H. Noda, Y. Asada, M. Shibasaki, N. Kumagai, J. Am. Chem. Soc. 2019, 141, 1546.
- 62C. R. Opie, H. Noda, M. Shibasaki, N. Kumagai, Chem. - A Eur. J. 2019, 25, 4648.
- 63Z. Liu, H. Noda, M. Shibasaki, N. Kumagai, Org. Lett. 2018, 20, 612.
- 64T. M. El Dine, J. Rouden, J. Blanchet, Chem. Commun. 2015, 51, 16084.
- 65M. K. Baraniak, R. A. Lalancette, F. Jäkle, Chem. - A Eur. J. 2019, 25, 13799.
- 66R. M. Lanigan, V. Karaluka, M. T. Sabatini, P. Starkov, M. Badland, L. Boulton, T. D. Sheppard, Chem. Commun. 2016, 52, 8846.
- 67V. Karaluka, R. M. Lanigan, P. M. Murray, M. Badland, T. D. Sheppard, Org. Biomol. Chem. 2015, 13, 10888.
- 68M. T. Sabatini, V. Karaluka, R. M. Lanigan, L. T. Boulton, M. Badland, T. D. Sheppard, Chem. - A Eur. J. 2018, 24, 7033.
- 69C. E. Coomber, V. Laserna, L. T. Martin, P. D. Smith, H. C. Hailes, M. J. Porter, T. D. Sheppard, Org. Biomol. Chem. 2019, 17, 6465.
- 70M. T. Sabatini, L. T. Boulton, T. D. Sheppard, Sci. Adv. 2017, 3, 1.
- 71Y. Y. Jiang, B. Hu, Z. Y. Xu, R. X. Zhang, T. T. Liu, S. Bi, Chem. - An Asian J. 2018, 13, 2685.
- 72M. Janvier, S. Moebs-Sanchez, F. Popowycz, J. European, Org. Chem. 2016, 2016, 2308.
- 73S. A. Ghorpade, D. N. Sawant, N. Sekar, Tetrahedron 2018, 74, 6954.
- 74P. Tang, Org. Synth. 2005, 81, 262.
- 75P. Tang, B. Acid, C. Amidation, Org. Synth. 2012, 89, 432.
- 76H. Lundberg, F. Tinnis, N. Selander, H. Adolfsson, Chem. Soc. Rev. 2014, 43, 2714.
- 77L. Shteinberg, V. V. Marshalova, V. M. Dibrova, S. M. Shein, Russ. J. Gen. Chem. 2011, 81, 1839.
- 78C. L. Allen, A. R. Chhatwal, J. M. J. Williams, Chem. Commun. 2012, 48, 666.
- 79H. Lundberg, F. Tinnis, H. Adolfsson, Chem. - A Eur. J. 2012, 18, 3822.
- 80H. Lundberg, H. Adolfsson, ACS Catal. 2015, 5, 3271.
- 81H. Lundberg, F. Tinnis, J. Zhang, A. G. Algarra, F. Himo, H. Adolfsson, J. Am. Chem. Soc. 2017, 139, 2286.
- 82H. Lundberg, F. Tinnis, H. Adolfsson, Appl. Organomet. Chem. 2019, 33, 1.
- 83F. De Azambuja, T. N. Parac-Vogt, ACS Catal. 2019, 9, 10245.
- 84A. Leggio, J. Bagalà, E. L. Belsito, A. Comandè, M. Greco, A. Liguori, Chem. Cent. J. 2017, 11, 1.
- 85H. Wang, W. Dong, Z. Hou, L. Cheng, X. Li, L. Huang, Appl. Organomet. Chem. 2020, 34, 9.
- 86N. Li, L. Wang, L. Zhang, W. Zhao, J. Qiao, X. Xu, Z. Liang, ChemCatChem 2018, 10, 3532.
- 87A. Maleki, R. Taheri-Ledari, J. Rahimi, M. Soroushnejad, Z. Hajizadeh, ACS Omega 2019, 4, 10629.
- 88M. V. Zakharova, F. Kleitz, F. G. Fontaine, Dalt. Trans. 2017, 46, 3864.
- 89B. Xiong, L. Zhu, X. Feng, J. Lei, T. Chen, Y. Zhou, L. B. Han, C. T. Au, S. F. Yin, J. European, Org. Chem. 2014, 2014, 4244.
- 90Basavaprabhu, K. Muniyappa, N. R. Panguluri, P. Veladi, V. V. Sureshbabu, New J. Chem. 2015, 39, 7746.
- 91M. A. Ali, S. M. A. H. Siddiki, W. Onodera, K. Kon, K. I. Shimizu, ChemCatChem 2015, 7, 3555.
- 92A. P. Zarecki, J. L. Kolanowski, W. T. Markiewicz, Molecules 2020, 25, 1761.
- 93L. Cheng, X. Ge, L. Huang, R. Soc. Open Sci. 2018, 5, 171870.
- 94W. Muramatsu, H. Yamamoto, J. Am. Chem. Soc. 2019, 141, 18926.
- 95E. Calcio Gaudino, D. Carnaroglio, M. A. G. Nunes, L. Schmidt, E. M. M. Flores, C. Deiana, Y. Sakhno, G. Martra, G. Cravotto, Catal. Sci. Technol. 2014, 4, 1395.
- 96L. T. M. Hoang, L. H. Ngo, H. L. Nguyen, H. T. H. Nguyen, C. K. Nguyen, B. T. Nguyen, Q. T. Ton, H. K. D. Nguyen, K. E. Cordova, T. Truong, Chem. Commun. 2015, 51, 17132.
- 97P. Mäki-Arvela, J. Zhu, N. Kumar, K. Eränen, A. Aho, J. Linden, J. Salonen, M. Peurla, A. Mazur, V. Matveev, D. Y. Murzin, Appl. Catal. A Gen. 2017, 542, 350.
- 98F. Arena, C. Deiana, A. F. Lombardo, P. Ivanchenko, Y. Sakhno, G. Trunfio, G. Martra, Catal. Sci. Technol. 2015, 5, 1911.
- 99Z. Wang, X. Bao, M. Xu, Z. Deng, Y. Han, N. Wang, ChemistrySelect 2018, 3, 2599.
- 100S. M. A. H. Siddiki, M. N. Rashed, M. A. Ali, T. Toyao, P. Hirunsit, M. Ehara, K. i. Shimizu, ChemCatChem 2019, 11, 383.
- 101A. Rimola, M. Fabbiani, M. Sodupe, P. Ugliengo, G. Martra, ACS Catal. 2018, 8, 4558.
- 102T. H. M. Petchey, J. W. Comerford, T. J. Farmer, D. J. Macquarrie, J. Sherwood, J. H. Clark, ACS Sustain. Chem. Eng. 2018, 6, 1550.
- 103B. List, Chem. Rev. 2007, 107, 5413.
- 104D. C. Lenstra, F. P. J. T. Rutjes, J. Mecinović, Chem. Commun. 2014, 50, 5763.
- 105D. F. J. Hamstra, D. C. Lenstra, T. J. Koenders, F. P. J. T. Rutjes, J. Mecinović, Org. Biomol. Chem. 2017, 15, 6426.
- 106P. Gangireddy, V. Patro, L. Lam, M. Morimoto, L. S. Liebeskind, J. Org. Chem. 2017, 82, 3513.
- 107S. M. Akondi, P. Gangireddy, T. C. Pickel, L. S. Liebeskind, Org. Lett. 2018, 20, 538.
- 108Handoko, S. Satishkumar, N. R. Panigrahi, P. S. Arora, J. Am. Chem. Soc. 2019, 141, 15977.
- 109T. V. Nguyen, D. J. M. Lyons, Chem. Commun. 2015, 51, 3131.
- 110M. A. Hussein, V. T. Huynh, R. Hommelsheim, R. M. Koenigs, T. V. Nguyen, Chem. Commun. 2018, 54, 12970.
- 111P. H. Huy, C. Mbouhom, Chem. Sci. 2019, 10, 7399.
- 112S. K. Mangawa, S. K. Bagh, K. Sharma, S. K. Awasthi, Tetrahedron Lett. 2015, 56, 1960.
- 113A. A. Lamar, L. S. Liebeskind, Tetrahedron Lett. 2015, 56, 6034.
- 114H. Yue, L. Guo, H. H. Liao, Y. Cai, C. Zhu, M. Rueping, Angew. Chemie - Int. Ed. 2017, 56, 4282.
- 115T. Ben Halima, J. Masson-Makdissi, S. G. Newman, Angew. Chemie - Int. Ed. 2018, 57, 12925.
- 116Y. L. Zheng, S. G. Newman, ACS Catal. 2019, 9, 4426.
- 117C. W. Cheung, M. L. Ploeger, X. Hu, Nat. Commun. 2017, 8, 14878.
- 118T. Ben Halima, J. K. Vandavasi, M. Shkoor, S. G. Newman, ACS Catal. 2017, 7, 2176.
- 119M. A. Ali, S. M. A. H. Siddiki, K. Kon, K. I. Shimizu, ChemCatChem 2015, 7, 2705.
- 120W. Muramatsu, H. Tsuji, H. Yamamoto, ACS Catal. 2018, 8, 2181.
- 121W. Muramatsu, T. Hattori, H. Yamamoto, J. Am. Chem. Soc. 2019, 141, 12288.
- 122H. Tsuji, H. Yamamoto, J. Am. Chem. Soc. 2016, 138, 14218.
- 123G. Homerin, D. Baudelet, P. Dufrénoy, B. Rigo, E. Lipka, X. Dezitter, C. Furman, R. Millet, A. Ghinet, Tetrahedron Lett. 2016, 57, 1165.
- 124D. C. Lenstra, D. T. Nguyen, J. Mecinović, Tetrahedron 2015, 71, 5547.
- 125D. T. Nguyen, D. C. Lenstra, J. Mecinović, RSC Adv. 2015, 5, 77658.
- 126H. Morimoto, R. Fujiwara, Y. Shimizu, K. Morisaki, T. Ohshima, Org. Lett. 2014, 16, 2018.
- 127Z. Li, C. Guo, J. Chen, Y. Yao, Y. Luo, Appl. Organomet. Chem. 2020, 34, 1.
- 128Z. Li, C. Wang, Y. Wang, D. Yuan, Y. Yao, Asian J. Org. Chem. 2018, 7, 810.
- 129S. A. Miller, N. E. Leadbeater, RSC Adv. 2015, 5, 93248.
- 130N. Caldwell, C. Jamieson, I. Simpson, A. J. B. Watson, Chem. Commun. 2015, 51, 9495.
- 131C. G. McPherson, N. Caldwell, C. Jamieson, I. Simpson, A. J. B. Watson, Org. Biomol. Chem. 2017, 15, 3507.
- 132A. Tillack, I. Rudloff, M. Beller, European J. Org. Chem. 2001, 3, 523.
10.1002/1099-0690(200102)2001:3<523::AID-EJOC523>3.0.CO;2-Z Google Scholar
- 133C. Gunanathan, Y. Ben-David, D. Milstein, Science. 2007, 80, 790.
- 134P. Hu, Y. Ben-David, D. Milstein, Angew. Chemie - Int. Ed. 2016, 55, 1061.
- 135T. T. Nguyen, K. L. Hull, ACS Catal. 2016, 6, 8214.
- 136A. Mukherjee, D. Milstein, ACS Catal. 2018, 8, 11435.
- 137C. Gunanathan, D. Milstein, Appl. Org. Synth. Catal 2014, 9783527334, 1.
- 138O. P. S. Patel, D. Anand, R. K. Maurya, P. P. Yadav, Green Chem. 2015, 17, 3728.
- 139S. L. Yedage, B. M. Bhanage, Synth. 2015, 47, 526.
- 140W. Wang, Y. Cong, L. Zhang, Y. Huang, X. Wang, T. Zhang, Tetrahedron Lett. 2014, 55, 124.
- 141H. Miyamura, H. Min, J. F. Soulé, S. Kobayashi, Angew. Chemie - Int. Ed. 2015, 54, 7564.
- 142N. S. Thirukovela, R. Balaboina, R. Vadde, C. Sekhar Vasam, Tetrahedron Lett. 2018, 59, 3749.
- 143Y. Wang, D. Zhu, L. Tang, S. Wang, Z. Wang, Angew. Chemie - Int. Ed. 2011, 50, 8917.
- 144X. Guo, L. Tang, Y. Yang, Z. Zha, Z. Wang, Green Chem. 2014, 16, 2443.
- 145Y. B. Sutar, S. B. Bhagat, V. N. Telvekar, Tetrahedron Lett. 2015, 56, 6768.
- 146K. Azizi, M. Karimi, A. Heydari, RSC Adv. 2014, 4, 31817.
- 147M. Karimi, D. Saberi, K. Azizi, M. Arefi, A. Heydari, Tetrahedron Lett. 2014, 55, 5351.
- 148G. Wang, Q. Y. Yu, S. Y. Chen, X. Q. Yu, Org. Biomol. Chem. 2014, 12, 414.
- 149T. K. Achar, P. Mal, J. Org. Chem. 2015, 80, 666.
- 150R. W. M. Davidson, M. J. Fuchter, Chem. Commun. 2016, 52, 11638.