Chinese patients with p.Arg756 mutations of ATP1A3: Clinical manifestations, treatment, and follow-up
ABSTRACT
Importance
The phenotypes of ATP1A3 gene mutations are diverse. Relapsing encephalopathy with cerebellar ataxia and fever-induced paroxysmal weakness and encephalopathy (FIPWE) are considered non-classical phenotypes caused by p.Arg756 mutations of ATP1A3.
Objective
To summarize the clinical manifestations, treatment, and follow-up of Chinese patients with p.Arg756 mutations of ATP1A3.
Methods
We analyzed the clinical features, treatment, and genotypes of eight children with p.Arg756 mutations of ATP1A3 who were treated in Beijing Children's Hospital from January 2014 to December 2019.
Results
Eight patients (six boys and two girls) were included; seven had been misdiagnosed with encephalitis. The age of onset ranged from 0.8 to 4.5 years. All patients had encephalopathy and had at least one episode of FIPWE. Cerebellar ataxia was present in nine episodes. Reversible splenial lesions of the corpus callosum were found in two patients in the acute phase. Three types of heterozygous ATP1A3 mutations were found: c.2267G > T (p.R756L) (patient 3 [P3]), c.2266C > T (p.R756C) (P2 and P4), and c.2267G > A (p.R756H) (P1, P5, P6, P7, and P8). Six mutations were de novo; two mutations were inherited. Both patients with p.R756C and one patient (P7) with p.R756H had four episodes of severe ataxia as the main manifestations. However, in the other three episodes, limb weakness was more prominent than ataxia. P5 with p.R756H exhibited overlap with FIPWE and rapid-onset dystonia-parkinsonism.
Interpretation
Acute encephalopathy followed by febrile disease was characteristic of the disease in patients with p.Arg756 mutations of ATP1A3. However, the weakness and ataxia were variable. Phenotypic crossover and overlap were observed among these patients.
INTRODUCTION
Mutations in the ATP1A3 gene, which encodes the α3 subunit of sodium-potassium ATPase, have been linked to a spectrum of neurological diseases, including rapid-onset dystonia-parkinsonism (RDP), alternating hemiplegia of childhood (AHC), and cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome.1 Two recognized phenotypes are associated with mutations of ATP1A3 residue 756, including relapsing encephalopathy with cerebellar ataxia (RECA)2 and fever-induced proximal weakness and encephalopathy (FIPWE).3
Fever-induced acute neurological dysfunction is characteristic of p.Arg756 mutations of ATP1A3; thus, it must be differentiated from infectious or autoimmune encephalitis to ensure an accurate diagnosis. In this study, we investigated the clinical characteristics of patients with p.Arg756 mutations of ATP1A3 in China. We also explored the differential diagnosis of these patients. Importantly, we identified some novel manifestations and attempted ketogenic diet (KD) therapy for two patients.
METHODS
Ethical approval
This study was approved by the Ethics Committee of Beijing Children's Hospital (2021-E-125-R). All patients or their legal guardians provided written informed consent for participation.
Patient data
Eight patients with p.Arg756 mutations of ATP1A3 were included and followed up from January 2014 to December 2019. Four of them (P1, P2, P3, and P4) were previously described.4 Whole-exome sequencing was performed, and the candidate mutations were confirmed by Sanger sequencing in each proband and both parents. Variants were interpreted according to the guidelines published by the American College of Medical Genetics and Genomics Guideline (ACMG) using the HGVS (https://varnomen.hgvs.org/) nomenclature.
RESULTS
Clinical features
Eight patients (six boys and two girls) were included in this study. The age at onset ranged from 0.8 to 4.5 years; six patients were under 3 years of age. Relapse-remission was found in five patients, with intervals ranging from 3 to 34 months (Table 1). All patients developed neurological symptoms within 12–48 h after fever onset. The fever was moderate to high, lasting for 2–6 days; it was associated with respiratory tract infection (upper respiratory tract infection [n = 7] and bronchitis [n = 1]). There were a total of 14 episodes (Table 1).
| Patient number | Sex | Motor development retardation | Family History | Episodes | Age | Fever | Encephalopathy | Flaccid paralysis | Cerebellar Ataxia | Others | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | + | − | 1 | 2 years 9 months | + | Drowsiness and irritability | + | − | Athetosis, mild dystonia | c.2267G > A (R756H) |
| 2 | 5 years 9 months | + | Drowsiness | ++ | ++ | Involuntary movement of eyeball, chorea/athetosis, dystonia | |||||
| 2 | M | + | − | 1 | 2 years | + | Subcoma | ++ | ++ | c.2266C > T (R756C) | |
| 2 | 2 years 4 months | + | Drowsiness | + | ++ | Involuntary movement of eyeball, athetosis | |||||
| 3 | M | − | − | 1 | 8 months | + | Lethargy and irritability | ++ | + | Involuntary movement of eyeball, athetosis | c.2267G > T (R756L) |
| 4 | M | + | + | 1 | 2 years 5 months | + | Drowsiness | + | ++ | / | c.2266C > T (R756C) |
| 2 | 3 years 4 months | + | Drowsiness | + | ++ | / | |||||
| 3 | 3 years 7 months | + | Subcoma | ++ | + | Involuntary movement of eyeball, chorea/athetosis, mild dystonia | |||||
| 5 | M | − | − | 1 | 4 years 7 months | + | Coma | + | − | Dystonia crisis | c.2267G > A (R756H) |
| 6 | M | + | + | 1 | 1 year 6 months | + | Drowsiness | + | − | / | c.2267G > A (R756H) |
| 2 | 3 years 3 months | + | Drowsiness | + | − | / | |||||
| 7 | F | − | − | 1 | 11 months | + | Drowsiness and irritability | + | ++ | Involuntary movement of eyeball | c.2267G > A (R756H) |
| 2 | 2 years 9 months | + | Drowsiness | ++ | + | Involuntary movement of eyeball, athetosis, mild dystonia | |||||
| 8 | M | + | − | 1 | 4 years 6 months | + | Coma | + | − | Involuntary movement of eyeball, seizures | c.2267G > A (R756H) |
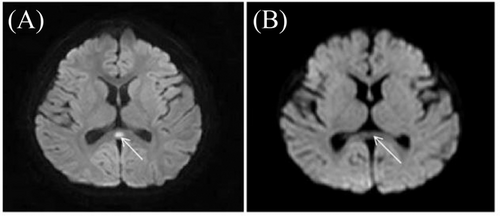
The clinical manifestations mainly included encephalopathy, flaccid paralysis, and cerebellar ataxia. All patients had encephalopathy, which manifested as coma, subcoma, drowsiness, or lethargy. P8 had frequent seizures. Hypotonia and areflexia were present in all patients, but the limb weakness ranged from mild to severe. Cerebellar ataxia was present in nine episodes. Other symptoms included involuntary eyeball movement (7/14, 50%), dystonia (5/14, 36%), and chorea/athetosis (6/14, 42%). In P5, dystonia rapidly progressed to dystonia crisis at 2 weeks after encephalopathy and limb weakness; this presentation manifested as continuous posture abnormalities and increases in both creatine kinase and carbon dioxide retention. Therefore, the phenotype was regarded as an overlap of FIPWE and RDP. No sign of pes cavus was evident. In addition to the fever-induced events, two patients (P4 and P8) had non-fever induced paroxysmal events, which lasted for several minutes to a few hours. Two patients (P1 and P8) had abnormal oval signals in the splenium of the corpus callosum during the acute phase of the disease, with limited diffusion visible in magnetic resonance images. The lesions disappeared after 2 weeks (Figure 1), and no cerebellar atrophy was evident. In three patients (P1, P4, and P7), slow waves were visible during electroencephalography assessment. Ocular fundus and audiometry findings were normal (Table 1).
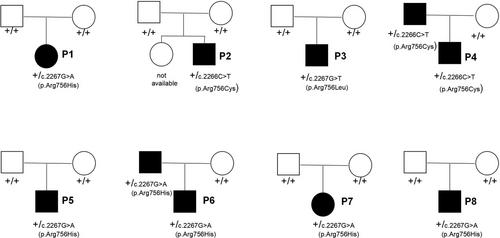
Two patients had a positive family history (Figure 2). The father of P4 had been diagnosed with Guillain–Barré syndrome attributable to fever and weakness at 6 years of age; in adulthood, the father exhibited mild dysarthria, mild limb ataxia, and focal dystonia of the right upper limb. The father of P6 had been diagnosed with viral encephalitis with symptoms of fever and dysphagia at 3 years of age; at 5 years of age, dysarthria, dysphagia, and limb weakness recurred, followed by fever. The father of P6 continued to exhibit mild dysarthria, dysphagia, involuntary facial movements, and mild limb ataxia at 27 years of age. Two patients exhibited perinatal abnormalities. P1 and P6 were born prematurely at 34 and 35 weeks, respectively; P6 was intubated after birth because of respiratory failure. In five patients (P1, P2, P4, P6, and P8), mild delays of motor development milestones were observed. All patients had almost normal language and cognition before disease onset.
Diagnosis, treatment, and follow-up
Seven of the eight patients were misdiagnosed with autoimmune or infectious encephalitis or cerebellitis at the onset of symptoms. All seven patients were administered immunotherapy, including intravenous immunoglobulins and corticosteroids (Table 2). P5 was treated with plasma exchange. Trihexyphenidyl, levodopa, or tiapride hydrochloride were used to alleviate extrapyramidal system symptoms. Four patients were treated with flunarizine after the last episodes, and no episode recurred. P4 was successively treated with three types of antiepileptic drugs to manage the paroxysmal events; these treatments had no effect. However, after flunarizine therapy (2.5 mg · day−1), the paroxysmal symptoms disappeared. We attempted KD therapy (fat/(carbohydrate+protein) = 1.5–2:1) in two patients (P5 and P8). Athetosis and posture instability improved in P8; he was able to attend a regular school and participate in physical education (modified Rankin Scales [mRS] = 0). P5 continued to exhibit severe abnormal posture with twitching, dysphagia, and dysphonia (mRS = 5). His swallowing function improved after KD. The patients attended follow-up for a duration of 4–61 months; four patients attended follow-up for more than 2 years. At the last follow-up, all patients were in remission. P3 and P5 showed obvious dyskinesia. The other children had mild ataxia and dystonia, which did not affect their daily lives. No visual or hearing impairments were observed.
| Auxiliary examination | Treatment | Last follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Misdiagnosed | Abnormal c-MRI | Abnormal EEG | EMG | Fundus | Immunotherapy | Trihexyphenidyl | Tiapride hydrochloride | Levodopa | Flunarizine | Ketogenic diet | Age | mRS |
| 1 | + | + | + | − | − | + | + | − | − | + | − |
7 years 1 month |
0 |
| 2 | + | − | − | NA | − | + | − | + | − | − | − |
6 years 3 months |
0 |
| 3 | + | − | − | NA | − | + | − | + | − | − | − | 12 months | 4 |
| 4 | + | − | + | − | − | + | − | − | − | + | − |
4 years 4 months |
0 |
| 5 | + | − | − | − | − | + | + | + | − | + | + |
5 years 3 months |
5 |
| 6 | − | − | − | − | − | − | − | − | + | − | − |
3 years 2 months |
1 |
| 7 | + | − | + | − | − | + | − | − | + | + | − |
3 years 3 months |
2 |
| 8 | + | + | − | − | − | + | − | + | − | − | + | 9 years | 0 |
- Abbreviations: c-MRI, cranial magnetic resonance imaging; EEG, electroencephalogram; EMG, electromyogram; mRS, modified Rankin Scales; NA, not available.
Relationship between genotype and phenotype
Heterozygous mutations of the ATP1A3 gene were found in all patients: c.2266C > T (p.R756C) (P2 and P4), c.2267G > T (p.R756L) (P3), and c.2267G > A (p.R756H) (P1, P5, P6, P7, and P8). Mutations were de novo in six patients; in P4 and P6, the mutations were inherited from the patient's father. All three mutations were considered pathogenic according to the ACMG. Ataxia was more prominent than weakness in three episodes among three patients; of these, two patients (P2 and P4) had the p.R756C genotype and one patient (P7) had the p.R756H genotype. P5 with c.2267G > A (p.R756H) exhibited overlap with FIPWE and RDP. The other seven episodes corresponded to FIPWE.
DISCUSSION
ATP1A3 encodes the sodium-potassium ATPase α3 subunit, which is predominantly expressed in neurons. Its dysfunction may lead to multiple impairments in the nervous system.5, 6 Mutations in ATPA13 result in diverse phenotypes (Figure S1), both classical (e.g., AHC, RDP, and CAPOS) and non-classical (e.g., early infantile epileptic encephalopathy,7 RECA,2 FIPWE,3 rapid-onset cerebellar ataxia,8 progressive cerebellar ataxia without paroxysmal or episodic symptoms,9 and childhood-onset schizophrenia/autistic spectrum disorder10). RECA and FIPWE have been identified as distinct phenotypes associated with Arg756 mutations in ATP1A3.
Three genotypes of Arg756 mutations have been reported: c.2267G > T (p.R756L), c.2266C > T (p.R756C), and c.2267G > A (p.R756H)—most of these mutations are de novo.2, 3, 6, 11-23 Kanemasa et al.6 showed that the expression level was similar between the p.R756C mutant protein and the wild-type protein in transfected HEK293T cells; this implied that the mutation modulates the activity of the Na+/K+-ATPase pump. Arg756 is located within a sequence conserved across species. In silico modeling of the AT1A3 protein indicates that the side chain of Arg756 forms two hydrogen bonds with the backbones of Arg824 and Gln825. However, only one hydrogen bond interacts with Gly358 when Arg756 is mutated to Cys or His; varying hydrogen bond lengths may cause corresponding energy penalties. Hydrogen bond interactions are lost when Arg756 is mutated to Leu (Figure S2).
In our study, most patients had their first fever-induced episode before 3 years of age. All of the patients had encephalopathy, which was significantly higher than the proportion of such patients in a previous report (50%).11 This is presumably because our hospital is a national children's medical center; thus, all referred patients are in critical condition. Other clinical features varied among our patients. For example, patients with p.R756C exhibited prominent ataxia, while patients with p.R756H or p.R756L predominantly had hypotonia and weakness (i.e., FIPWE), similar to the findings in previous reports.2, 3 Notably, each of three patients (P2, P4, and P7) showed distinct dominant symptoms in different episodes. The degrees of flaccid paralysis and ataxia varied among episodes, according to the phenotype (FIPWE or RECA). P5 had acute encephalopathy and flaccid paralysis at disease onset; severe dystonia emerged later. Thus, the phenotype was regarded as overlapping with FIPWE and RDP. To our knowledge, this is the first report of dystonia crisis in patients with p.Arg756 mutations of ATP1A3. Overall, although there were some genotype-phenotype relationships, the individual differences in our patients should be carefully considered.
Some clinical manifestations of p.Arg756 mutations of ATP1A3 are similar to viral encephalitis or autoimmune encephalitis.3, 4 In the early stage, diseases caused by such mutations are difficult to distinguish from encephalitis (Table S1). Virological and anti-neural antibody tests can assist in accurate diagnosis. Assessments of family history and past history can also provide clues. Furthermore, because of sudden consciousness disturbance or rapid progress after acute infection, there is a need to distinguish diseases caused by ATP1A3 mutations from metabolic diseases (e.g., mitochondrial disease or amino acid/organic acid metabolic disease). For young patients with encephalitis, flaccid paralysis, and ataxia—in the absence of etiological or immunological disease, ion channel diseases should be considered. Seven patients in our series were treated with immunotherapy because of delayed diagnosis. Therefore, early accurate diagnosis is critical to avoid over-treatment and alleviate economic burdens for patients and their families.
Flunarizine is reportedly effective for patients with p.Arg756 mutations.15, 16 KD has been recommended for patients with ATP1A3 mutations. However, there remains limited evidence concerning its efficacy. In 2018, Schirinzi et al.24 reported successful KD therapy in one patient with the p.D756del genotype. Possible mechanisms underlying KD efficacy may be related to the specific actions of ketones on ATP-sensitive potassium channels.24 Two patients in our series experienced good outcomes from KD therapy. However, the effect of KD remains controversial.
In summary, we have described the clinical features of Chinese patients with p.Arg756 mutations of ATP1A3. Fever-induced encephalopathy was a consistent characteristic in affected patients, while weakness and ataxia were variable. Phenotypic crossover and overlap were important findings. Dystonia crisis and reversible splenial lesions of the corpus callosum were novel manifestations in our patients. Furthermore, some patients were easily misdiagnosed at disease onset. Larger sample sizes and multicenter studies are needed to illustrate the genotype-phenotype relationships in patients with p.Arg756 mutations of ATP1A3.
CONFLICT OF INTEREST
None.