Characteristics and prognosis of embryonal rhabdomyosarcoma in children and adolescents: An analysis of 464 cases from the SEER database
ABSTRACT
Importance
As the most common subtype of pediatric rhabdomyosarcoma (RMS), the prognosis of embryonal RMS has rarely been investigated solely.
Objective
To perform a population-based study to characterize the prognosis of embryonal RMS in children and adolescents.
Methods
Demographic and clinical features were retrospectively evaluated in selected patients with embryonal RMS registered in the Surveillance, Epidemiology, and End Results (SEER) program from 1988 to 2016. Survival curves were compared using the log-rank test. A multivariate Cox proportional hazards model was developed to assess the impact of each factor on the overall survival. A nomogram was constructed based on the results of Cox regression model.
Results
A total of 464 patients were included in the analysis, among which 64.6% were male and 70.2% were white patients. About 38.6% and 26.3% of the patients were at 1–4 years and 5–9 years, respectively. Cox analysis showed that patients at age group 5–9 years had the lowest risk of mortality (hazard ratio [HR], 0.277; 95% confidential interval [CI], 0.123–0.620), compared with patients diagnosed at less than 1-year-old, and age group 1–4 years had the second-best prognosis. Patients having distant tumors had significantly higher mortality risk (HR, 4.842; 95% CI, 2.804–8.362) than the patients with localized tumor. Compared with receiving no surgery or radiotherapy, receiving any combination of surgery and radiotherapy would lower the risk of mortality significantly (for surgery without radiotherapy: HR, 0.418; for radiotherapy without surgery: HR, 0.405; and for surgery plus radiotherapy: HR, 0.410).
Interpretation
Age, stage at diagnosis, and treatment received were found to be the most important predictors of the overall survival of pediatric embryonal RMS.
1 INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common pediatric soft-tissue sarcomas, accounting for more than 50% of all soft-tissue sarcomas in children and adolescents.1 Originating from immature striated skeletal muscle, RMS can occur at any age and any site of the body. Owing to the revolutionization brought by collaborative pediatric trials to the care of this disease, about 70% of children with nonmetastatic neoplasm can be cured using multimodality treatment.2, 3
According to the 4th edition of the World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone, RMS can be divided into four groups: embryonal, alveolar, spindle cell/sclerosing, and pleomorphic rhabdomyosarcoma; and embryonal RMS is the most common subtype.4 Researches usually combined all histology of RMS together to describe the incidence, epidemiology or prognostic prediction.5-9 However, different histology tends to have different pathogenesis and prognosis patterns. Combing the histological subtypes together may obscure their unique characteristics.
Although RMS is the most common type of soft-tissue sarcomas, it only accounts for 3% of childhood cancers and 2% of adolescent cancers.10, 11 Its rarity makes information regarding its clinical and biologic characteristics very limited, and multi-institutional trials even more difficult. Under such circumstances, population-based cancer registries demonstrate the distinguished value to the knowledge of the rare tumor. The Surveillance, Epidemiology, and End Results (SEER) database is a publicly accessible database, which regularly collects demographic, diagnostic, treatment, and survival information on all diagnosed cancer patients residing within certain geographic areas in the United States, covering approximately 28% of the US population.12
The objective of this study is to better and more specifically characterize the clinical features and prognosis of embryonal RMS in children and adolescents by performing a population-based analysis of all target patients with embryonal RMS registered in the SEER database over a 29-year time interval.
2 METHODS
2.1 Data source and study population
Utilizing the November 2019 release of the SEER database and the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) morphology code for embryonal RMS (8910/3), we identified a group of children and adolescents who were diagnosed during 1988–2016 and followed up until 31 December 2016 (records of patients who were diagnosed before the year 1988 lack of information on tumor size). Patients with no confirmation of diagnosis by microscopy and incomplete follow-up information were excluded. After selection, there were 464 cases in the cohort. The flowchart for selecting the study population is shown in Figure 1. Since the dataset is within the public domain and all patient information is de-identified, it was deemed exempt from review by the Institutional Review Board, and the informed consent was waived.
2.2 Study variables
Variables including patient demographics, age at diagnosis, sex, race, year at diagnosis, primary site, size, SEER stage, radiotherapy, chemotherapy, survival status, and survival months were extracted from the database. Missing or unknown values remained blank and unaltered. Age at diagnosis was divided into <1 year, 1–4 years, 5–9 years, 10–14 years, and 15–19 years. The race was divided into white, black, and others. Year at diagnosis was divided into eras of 1988–1996, 1997–2006, and 2007–2016; Primary sites including head and neck (nonparameningeal), genitourinary (nonbladder/prostate), and bile duct regions were classified as favorable sites, and all others were classified as unfavorable. Size was classified as ≤ 5 cm, 5–10 cm, >10 cm and unknown. SEER stage had localized (an invasive neoplasm confined entirely to the organ of origin), regional (a neoplasm that has extended beyond the limits of the organ of origin directly into surrounding organs or tissues, has extended into regional lymph nodes by way of the lymphatic system, or a combination of both), and distant (a neoplasm that has spread to parts of the body remote from the primary tumor either by direct extension or by discontinuous metastasis to distant organs, tissues, or via the lymphatic system to distant lymph nodes). Blank or unstaged observations were coded as unknown. Since surgery is often accompanied by radiotherapy, we combined surgery and radiotherapy information and created a variable presentative of the treatment received by patients. The variable treatment includes four categories: no surgery or radiotherapy, surgery without radiotherapy, radiotherapy without surgery, surgery plus radiotherapy. The endpoint of this study is death due to any cause.
2.3 Statistical analysis
Baseline patient characteristics were presented as the number of patients (n) and the corresponding percentage for each category. For the outcome measure, we used the Kaplan-Meier method to evaluate overall survival and compared the survival curves using the log-rank test. Cox proportional hazards regression was performed on variables including age, sex, race, primary site, tumor size, stage, and treatment received, to investigate their impact on the risk of mortality. Besides, we utilized variables significantly associated with overall survival (OS) in the Cox regression analysis to draw a nomogram to predict the 1-, 3-, and 5-year survival probability. All data processing and statistical analysis were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA). The figures for survival curves were drawn with GraphPad 8.0 and the nomogram was drawn on the R platform (http://www.r-project.org/, version 3.2.6) using the rms package (cran.r-project.org/web/packages/rms). A P < 0.05 was considered as statistically significant.
3 RESULTS
3.1 Clinical characteristics of embryonal RMS in children and adolescents
Patient characteristics are listed in Table 1. A total of 464 children and adolescents aged 0–19 years and diagnosed between 1988 and 2016 were included in the analysis. There was 299 boys and 165 girls with a ratio of 1.81:1. Most patients were at the age intervals 1–4 years (38.6%) and 5–9 years (26.3%), and about 70.2% of patients were white people. For tumor characteristics, the number of patients having prognostically favorable primary sites (233, 50.2%) was close to the number having the unfavorable sites (231, 49.8%). More than 70% of patients had localized (36.9%) or regional (34.1%) tumors and more than half of patients had tumors less than 10 cm, most of which were less than 5 cm (38.6%). Only 34 patients (7.3%) did not receive surgery nor radiotherapy; 129 patients received surgery but did not receive radiotherapy, and the same number of patients received radiotherapy but did not receive surgery; 172 patients (37.1%) not only underwent surgery but also received radiotherapy.
| Characteristics | Number of patient, n (%) | Overall Survival rate (standard error) (%) | P | ||
|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | |||
| Sex | 0.910 | ||||
| Male | 299 (64.4) | 92.3 (1.6) | 80.5 (2.4) | 77.6 (2.6) | |
| Female | 165 (35.6) | 92.4 (2.1) | 78.5 (3.3) | 75.6 (3.5) | |
| Age (years) | <0.001 | ||||
| <1 | 28 (6.0) | 81.4 (7.5) | 65.1 (9.5) | 56.3 (10.0) | |
| 1–4 | 179 (38.6) | 94.2 (1.8) | 81.2 (3.1) | 79.1 (3.2) | |
| 5–9 | 122 (26.3) | 97.4 (1.5) | 92.8 (2.5) | 90.7 (2.8) | |
| 10–14 | 67 (14.4) | 89.2 (3.9) | 73.2 (5.8) | 69.1 (6.2) | |
| 15–19 | 68 (14.7) | 86.3 (4.3) | 65.6 (6.0) | 62.1 (6.1) | |
| Race | 0.548 | ||||
| White | 326 (70.2) | 92.0 (1.5) | 81.0 (2.3) | 78.0 (2.4) | |
| Black | 95 (20.5) | 91.3 (2.9) | 74.3 (4.6) | 71.7 (4.8) | |
| Other | 43 (9.3) | 97.4 (2.5) | 83.6 (6.1) | 80.6 (6.6) | |
| Era of diagnosis | 0.020 | ||||
| 1988–1996 | 140 (30.2) | 91.4 (2.4) | 76.4 (3.6) | 72.9 (3.8) | |
| 1997–2006 | 136 (29.3) | 92.6 (2.3) | 74.8 (3.7) | 72.6 (3.8) | |
| 2007–2016 | 188 (40.5) | 92.8 (2.0) | 87.9 (2.6) | 85.1 (3.0) | |
| Prognostic site | 0.002 | ||||
| Favorable | 233 (50.2) | 94.3 (1.5) | 83.8 (2.5) | 81.7 (2.7) | |
| Unfavorable | 231 (49.8) | 90.4 (2.0) | 75.7 (3.0) | 71.7 (3.2) | |
| Size (cm) | 0.007 | ||||
| ≤5 | 179 (38.6) | 95.4 (1.6) | 85.1 (2.8) | 83.7 (2.9) | |
| 5–10 | 126 (27.1) | 91.5 (2.6) | 81.0 (3.8) | 77.7 (4.1) | |
| >10 | 65 (14.0) | 86.8 (4.3) | 67.3 (6.2) | 60.8 (6.6) | |
| Unknown | 94 (20.3) | 91.2 (3.0) | 76.7 (4.5) | 73.3 (4.7) | |
| SEER stage | <0.001 | ||||
| Localized | 171 (36.9) | 96.3 (1.5) | 92.5 (2.1) | 91.8 (2.2) | |
| Regional | 158 (34.0) | 96.1 (1.5) | 81.8 (3.2) | 75.8 (3.6) | |
| Distant | 86 (18.5) | 78.4 (4.5) | 52.3 (5.6) | 49.6 (5.6) | |
| Unknown | 49 (10.6) | 89.7 (4.9) | 77.0 (7.2) | 77.0 (7.2) | |
| Treatment | <0.001 | ||||
| No surgery or radiotherapy | 34 (7.3) | 65.0 (8.5) | 45.5 (8.9) | 45.5 (8.9) | |
| Surgery without radiotherapy | 129 (27.8) | 92.8 (2.3) | 85.9 (3.2) | 81.0 (3.7) | |
| Radiotherapy without surgery | 129 (27.8) | 96.0 (1.8) | 76.7 (3.9) | 76.7 (3.9) | |
| Surgery plus radiotherapy | 172 (37.1) | 94.5 (1.8) | 84.1 (2.9) | 79.8 (3.3) | |
- SEER, Surveillance, Epidemiology, and End Results.
3.2 Overall survival
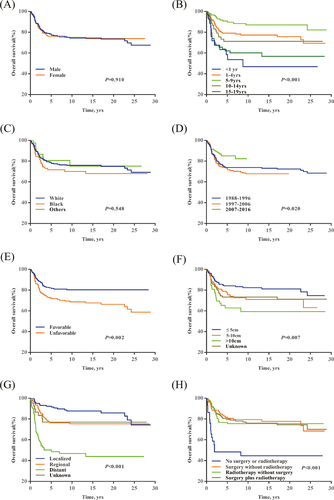
The 1-, 3-, and 5- overall survival rate for each category of all study variables and the P-values derived from the log-rank tests are listed in Table 1. The survival curves for each variable are shown in Figure 2. According to the results, male patients had similar prognosis as female patients (P = 0.910). Patients of different races did not show significantly different survival either (P = 0.548). Of the five age groups, patients aged 5–9 years tend to have the best prognosis with a 5-year survival rate of 90.7%, while patients less than 1-year-old had the poorest survival with a 5-year survival of 56.3%, and patients aged 15–19 years had the second-worst prognosis. Patients diagnosed during the period 2007–2016 had better prognosis than patients diagnosed during the previous two decades. Patients’ survival rate decreased with increasing tumor size, and patients having localized or regional tumors had a far better prognosis than patients having distant tumors. Treatment including surgery and radiotherapy showed an impact on overall survival since the patients receiving no treatment had the lowest survival rate at all time points.
3.3 Multivariable analysis
The result of Cox proportional hazard regression (Table 2) showed that age at diagnosis, SEER stage, and treatment received had a significant impact on the risk of mortality, after adjusting other potentially influencing factors. Patients at age group 5–9 still had the lowest risk of mortality (hazard ratio [HR], 0.277; 95% confidence interval (CI), 0.123–0.620; P = 0.002), compared with patients diagnosed at less than one year old, and age group 1–4 had the second-best prognosis. Sex, race, era of diagnosis, primary site, and tumor size had no significant impact on the mortality risk in the multivariable regression. Patients having distant tumors had the highest mortality risk (HR, 4.842; 95% CI, 2.804–8.362; P < 0.001), compared with the patients with localized tumor; patients having regional tumor did not show significantly higher mortality risk (HR, 1.685; 95% CI, 0.963–2.949; P = 0.068). Results showed that receiving any combination of surgery and radiotherapy would lower the risk of mortality significantly. The HR for surgery without radiotherapy is 0.418; for radiotherapy without surgery is 0.405; for surgery plus radiotherapy is 0.410.
| Variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Sex | ||
| Male | Reference | |
| Female | 0.843 (0.560–1.269) | 0.413 |
| Age (years) | ||
| <1 | Reference | |
| 1–4 | 0.509 (0.259–1.000) | 0.050 |
| 5–9 | 0.277 (0.123–0.620) | 0.002 |
| 10–14 | 0.625 (0.295–1.324) | 0.220 |
| 15–19 | 0.986 (0.481–2.018) | 0.968 |
| Race | ||
| White | Reference | |
| Black | 1.256 (0.796–1.981) | 0.328 |
| Other | 0.916 (0.434–1.935) | 0.819 |
| Era of diagnosis | ||
| 1988–1996 | Reference | |
| 1997–2006 | 1.211 (0.774–1.895) | 0.402 |
| 2007–2016 | 0.623 (0.358–1.083) | 0.093 |
| Prognostic site | ||
| Favorable | Reference | |
| Unfavorable | 1.355 (0.868–2.116) | 0.181 |
| Size (cm) | ||
| ≤5 | Reference | |
| 5–10 | 1.049 (0.619–1.779) | 0.858 |
| >10 | 1.499 (0.834–2.697) | 0.176 |
| Unknown | 0.885 (0.495–1.582) | 0.680 |
| SEER stage | ||
| Localized | Reference | |
| Regional | 1.685 (0.963–2.949) | 0.068 |
| Distant | 4.842 (2.804–8.362) | <0.001 |
| Unknown | 1.646 (0.698–3.883) | 0.255 |
| Treatment | ||
| No surgery or radiotherapy | Reference | |
| Surgery without radiotherapy | 0.418 (0.202–0.863) | 0.018 |
| Radiotherapy without surgery | 0.405 (0.203–0.809) | 0.011 |
| Surgery plus radiotherapy | 0.410 (0.210–0.799) | 0.009 |
- CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
Since the nomogram was intended to perform prediction based on patient characteristics, observations with unknown stage were excluded in this part. As tumor size is an essential part of the identification of tumor stage and the impact of tumor size estimated in the multivariable analysis part was not significant, we, therefore, dropped the variable tumor size when fitting the new Cox regression model. Based on the requirement of the nomogram, we also deleted observations with a survival time of 0. Finally, 414 patients were selected, and the sex, age group, race, prognostic site, SEER stage, and treatment were included in the analysis (Figure 3). To predict the 1-, 3-, and 5-year survival probability of a patient using the nomogram, we draw a vertical line to the Points axis to assign a point value for each category of each variable, sum the point values for all variable to obtain a total point of this patient, and then drop a vertical line from the Total points axis to the 1-, 3-, and 5-year Survival Probability axes, respectively.

4 DISCUSSION
The current study provided detailed demographics, tumor characteristics, and survival information of 464 children and adolescents diagnosed with embryonal RMS between 1988 and 2016. We demonstrated significant differences in epidemiological factors and investigated the impact of each factor on the patient’s overall survival rate. We also constructed a nomogram to predict 1-, 3-, and 5-year survival probability, which is a useful tool to evaluate the prognosis of pediatric embryonal RMS. In this study, age at diagnosis, SEER stage, and treatment received are three statistically significant factors associated with the overall survival both in the univariable and multivariable analysis.
Several studies had demonstrated that age at diagnosis is an important predictor of cancer prognosis in the pediatric population.13-16 In this study, we found that patients diagnosed at age 5–9 years had the most promising prognosis, while the ones diagnosed at less than 1-year-old had the worst, and that the association between age at diagnosis and the survival was not linear at all. The phenomenon indicates that for pediatric cancers, the cutoff value of age group should be investigated carefully, and age should not be included simply as a continuous variable. Our result is consistent with the findings of another study by Joshi et al17 who also confirmed that infants (<1 year) and adolescents (>10 years) had significantly worse outcomes. Some studies showed that infants have higher rates of therapy-related mortality and are at greater risk of serious infectious complications due to their immature immune system, so infants historically received less than the usually prescribed doses of chemotherapy and radiotherapy.17 Therefore, the challenges related to local control in infants are major causes of their poor outcomes.
According to the results of multivariable analysis and the nomogram, we concluded that the tumor stage is the most significant predictor of children’s and adolescents’ survival, and distant tumors have a far worse prognosis than the localized and regional ones. The results emphasize the important role of early diagnosis which may need a comprehensive community screening plan.
Many studies utilizing the SEER database mentioned that the database did not provide information on chemotherapy.9, 18, 19 In fact, the information on radiotherapy and chemotherapy can be obtained through custom databases on request. In this study, only 7 out of 464 patients did not undergo chemotherapy, which is why our analysis excludes chemotherapy as a risk factor. Therefore, when interpreting the result of treatment, researchers should keep in mind that almost all patients in this study received chemotherapy. We also repeated the analysis with chemotherapy included and the results remained unchanged. However, the results of Cox regression did not show significant differences among surgery without radiotherapy, radiotherapy without surgery, and surgery plus radiotherapy. The first possible reason is the rough classification of surgery and radiotherapy, the detailed information of which may vary on patient conditions. Secondly, studies had demonstrated that multimodality treatment had limited effect for metastatic RMS, so the difference among treatment regimens may appear when analyzed with stage stratification.
In the task of predicting cancer patients’ prognosis, a nomogram has advantages over a staging or scoring system, since it directly quantifies the prognosis of individual patients based on proven prognostic factors and it considers multiple factors simultaneously.20, 21 The prediction result is presented with a probability, which can be better understood by clinicians as well as patients. To the best of our knowledge, the nomogram constructed in this study is the first for the 1-, 3-, and 5-year overall survival of pediatric embryonal RMS. However, the nomogram should be used with caution because it is impossible to include all risk factors. Nomogram is a useful tool in clinical practice, but it cannot be used as the only reference for the selection of treatment.
The SEER database makes it possible to dive deep into the characteristics and prognosis for rare tumors such as embryonal RMS. However, the results are also restricted by the inherent limitations and biases shared by most registry databases. When interpreting the conclusions drawn by this analysis, researchers should be aware that there are several limitations to this study. Firstly, our study performed a retrospective cross-sectional analysis using the cases collected over the span of 25 years during which the surgical, radiotherapy and other diagnosis and treatment techniques had been improved. Secondly, the database does not include patients’ comorbidity, detailed information of surgery, radiotherapy or chemotherapy, which are all very important predictors of survival. Finally, the SEER database is confined to the United States and the patients are mostly consist of white or black people, which may influence the generalization of the conclusion to children and adolescents of other races.
In conclusion, we used a population-based dataset to investigate the clinical features and prognosis of embryonal RMS in children and adolescents. Age, stage, and treatment received were found to be predictive of overall survival. The reported differences in demographics and survival rates enable a better understanding of this most common subtype of RMS. The nomogram constructed in this study can provide clinicians information on the prognosis of the target patients for reference.
5 Funding source
Beihang University & Capital Medical University Advanced Innovation Center for Big Data-Based Precision Medicine Plan [BHME-201801]
CONFLICT OF INTEREST
All authors declare that they have no competing interest.




