Quantitative Measurement of the Direct Public Health Impact of Medicines Withdrawals in Europe: Development of a Modelling Method and Proof-of-Concept Study to Estimate the Morbidity and Mortality Prevented by Regulatory Action
Funding: The authors received no specific funding for this work.
ABSTRACT
Purpose
Removing medicines from market may benefit public health by preventing adverse drug reactions (ADRs), which should be quantified. This study's aim was to identify a model to quantify the impact of medicines' marketing authorisation (MA) withdrawal and revocation in terms of preventing morbidity and mortality.
Methods
MA withdrawals and revocations for safety reasons in France, Germany and/or the United Kingdom between July 2012 and December 2016 were identified for prescription medicines. Annual exposure was estimated for each medicine, using IQVIA Medical Research Data (IMRD)-France, IMRD-Germany and IMRD-UK primary care electronic health record databases. European Medicines Agency records provided reasons for regulatory action for each medicine. Absolute risks of ADRs which led to MA withdrawal were estimated for patients exposed to each medicine by systematic review of quantitative research. Public health impact, expressed as annual number of ADRs avoided, was estimated by modelling exposure and ADR risk.
Results
Four MA withdrawals and two revocations met study inclusion criteria. Each product's usage decreased following MA withdrawal or revocation. Absolute risk for ADRs was 0.1%–41.25%. To estimate impact of each withdrawal or revocation, its average annual exposure within each IMRD population was multiplied by the absolute risk to give the crude number of ADRs prevented annually due to regulatory action.
Conclusions
This model quantifies the public health impact of MA withdrawal and revocation in terms of serious morbidity, resulting from eliminated or reduced usage of medicines. This method can be applied to products in other settings to quantify the impact of other pharmacovigilance actions.
Summary
- Monitoring the success of pharmacovigilance processes is becoming more common, with MAHs required to measure effectiveness of risk minimisation measures under the 2012 European Union (EU) pharmacovigilance legislation.
- The impact of regulatory actions on public health in terms of morbidity and mortality was not previously understood.
- This project successfully developed a method which can estimate with acceptable accuracy the impact of withdrawals and revocations, in terms of morbidity and mortality avoided.
- This model is applicable to other situations where a measurable change in exposure to the medicine of interest occurs and results can be extrapolated to the wider population.
1 Purpose
In health sciences, impact is most often measured relative to guidelines and policies rather than health outcomes, and until recently pharmacovigilance actions would normally be examined for their impacts on healthcare delivery processes [1, 2]. However, in pharmacoepidemiology, there has been a recent shift towards evidence generation about direct or indirect effects of risk minimisation activities, whether these are intended or not. Monitoring the success of pharmacovigilance processes is becoming more common, with marketing authorisation holders (MAHs) required to measure effectiveness of risk minimisation measures and compliance with pharmacovigilance obligations under the 2012 European Union (EU) pharmacovigilance legislation [3]. However, it is not currently understood how regulatory actions taken since the introduction of the 2012 legislation have impacted public health in terms of morbidity and mortality. Such regulatory actions include withdrawal or revocation of marketing authorisation (MA).
MAs may be withdrawn or revoked for many reasons, such as a safety concern, quality issues or the inability of a MAH to supply data requested by regulators. MA withdrawal can be a voluntary action made by MAHs, or recommended by regulators when such an issue arises. When withdrawal of the MA is at the request of a regulator it is known as a revocation [4].
Removing a medicine from market may significantly benefit public health by reducing or eliminating adverse drug reactions (ADRs) associated with its use, which should be measured quantitatively wherever possible. The aim of this study was to identify a model to estimate the public health impact of removing medicines from market, in terms of serious morbidity and mortality. The model was applied to medicines which were withdrawn or revoked in France, Germany or the United Kingdom between July 2012 and December 2016.
2 Methods
2.1 Product Identification
Prescription medications which had undergone MA withdrawal, revocation or suspension within Europe for a post-marketing safety reason between July 2012 and December 2016 were identified using methodology described previously [5]. Briefly, detailed searches of European Medicines Agency (EMA) and EU Member States' regulatory agencies' webpages, plus those of Norway, Lichtenstein and Iceland, were conducted, reflecting the countries within the European Union and the European Economic Area. These searches identified 18 product withdrawals, revocations and suspensions throughout Europe during the study period. Of these, the nine medicines which had undergone regulatory action in any or all of France, Germany and the United Kingdom were selected for analysis of the public health impact of their removal from market. These countries were selected due to accessibility of data sources; electronic health records databases for these countries are contractually available to the European Medicines Agency, with whom this study was conducted [6].
This analysis focuses on the six medicines which were permanently removed from market via withdrawal or revocation of MA during the time period of interest. Products for which MAs were suspended (n = 3) were handled separately.
2.2 Utilisation
Using IMRD-France, IMRD-Germany and IMRD-UK [previously The Health Improvement Network (THIN)] electronic health databases, patients exposed to each of the six medicines were counted in 3-month (quarterly) periods for 12 months before and after regulatory action, giving utilisation patterns for the 24 month period [7, 8]. Where the regulatory action affected only specific strengths or formulations, utilisation data for these specific products were obtained. Prevalence of exposure and the crude number of patients exposed to each medicine annually were estimated.
2.3 Adverse Drug Reaction and Risk
Each ADR given as the reason for withdrawal or revocation was identified using regulatory documentation.
Published quantitative research addressing the medicines and ADR of interest was identified by systematic searches of PubMed/MEDLINE, and in publicly available documents regarding the regulatory action. Studies were considered for inclusion if they reported on the association between exposure to the medicine of interest and the event leading to regulatory action. All results from study designs identified in the systematic review were included regardless of study design, given that the number of ADRs and the number of exposed patients were presented within the published paper. Both the number of ADRs, and the number of exposed patients or participants were summed, respectively. Absolute risk (%) was then calculated. Supporting Information 1 shows the search strategy and all studies identified during this process which contributed to risk estimates, including studies reported within the EMA documentation.
2.4 Impact Modelling
Public health impact, expressed as a crude annual number of cases of ADRs avoided as a result of each medicine's withdrawal or revocation, was estimated by modelling product utilisation figures and the estimated risk of the ADR in exposed patients.
The expected quarterly number of ADR cases in patients exposed to each drug was calculated by multiplying quarterly exposure to each drug by the ADR's risk in exposed patients. This figure was multiplied by four to provide a crude estimate of the annual number of cases amongst exposed patients, and thus the number of ADRs avoided per year as a result of the medicine's removal from market in each IMRD population. Figure 1 outlines the methodology used. Figure 2 gives a worked example, using the withdrawal of calcitonin due to risk of cancer to demonstrate the method. Figure 3 details the calculations used to quantify impact of regulatory actions.
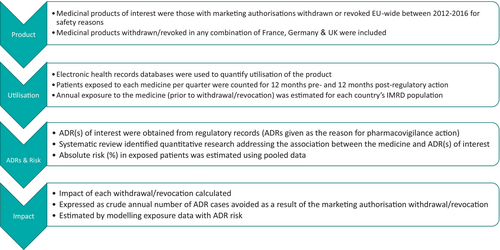
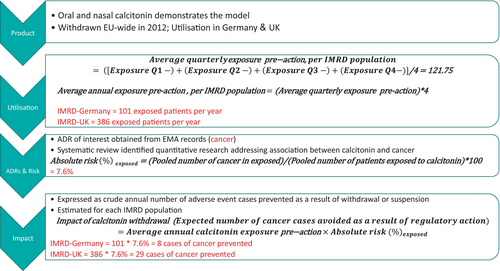

2.5 Validation of the Model
Validation of the model was conducted using only the IMRD-UK population. The number of patients exposed to each medicine was counted in 3-month periods for 12 months before and after the regulatory action. The change in the number of exposed patients was estimated by calculating the difference between exposed patients in the 12 months before and after regulatory action. Meta-analysis was used to calculate risk ratios (with 95% confidence intervals) for each ADR leading to regulatory action. The same studies which contributed to absolute risk in the primary analysis were used to calculate the risk ratio (Supporting Information 1).
Incidence rates per year were calculated for each event within the overall IMRD-UK population. Incidence rates were then applied to the calculated reductions in numbers of patients exposed, to provide an estimate of the number of incident events that would be expected in this population if not exposed to the product. These figures were then multiplied by the risk ratio to find the number of events that would be expected in the exposed IMRD-UK population. The difference between the expected number of events for exposed and unexposed patients in the database was calculated to give the expected attributable number of events in the IMRD-UK population. The expected attributable number of events was then multiplied by the actual population of the United Kingdom in that year divided by the number of patients in the IMRD-UK database.
3 Results
Throughout the study period of interest (2012–2016), four MAs were withdrawn and two revoked for safety reasons in any combination of France, Germany and the United Kingdom, meeting the criteria for inclusion in impact analyses. Date of marketing for implicated products ranged from 1955 to 2008 (Table 1). There were no apparent drug classes which had undergone MA withdrawal or revocation more than others; however, it was noted that both revoked medicines were indicated for gastrointestinal conditions (Table 1). Four regulatory actions occurred in each of France and Germany, while five occurred in the United Kingdom (Table 1). Cited reasons for regulatory action were varied (Table 1), but all were based on preventing potentially serious adverse events in patients exposed to the medicine.
| Product | Date of marketing | Therapeutic indication | Formulation(s) implicated in regulatory action | Regulatory decision date | Countries in which regulatory action applied | Reason(s) for action |
|---|---|---|---|---|---|---|
| Withdrawn products | ||||||
| Meprobamate | 1955 | Anxiety states, alcohol withdrawal, migraine attacks, digestive disorders, muscular tension and cramps, insomnia | Oral | March 2012a | France, UK | Overdose leading to profound hypotension, hypothermia, respiratory arrest, coma, cardiogenic shock |
| Nicotinic acid/laropiprant | 2008 | Dyslipidaemia | Oral | January 2013 | Germany, UK | Intracranial and gastrointestinal bleeds, myopathy, infections, new-onset diabetes, hepatotoxicity |
| Almitrine | 1982 | Respiratory stimulant (chronic respiratory diseases) | Oral | May 2013 | France | Peripheral neuropathy, severe weight loss |
| Calcitonin | 1973 | Osteoporosis, Paget's disease, conditions leading to bone loss | Nasal spray | July 2013 | Germany, UK | Cancers |
| Revoked products | ||||||
| Metoclopramide | 1966 | Nausea and vomiting, gut motility disorders, gastroesophageal disease and dyspepsia, adjuvant in surgical and radiographical procedures | Oral, rectal | October 2013 | France, Germany, UK | Neurological events including extrapyramidal symptoms and irreversible tardive dyskinesia |
| Domperidone | 1978 | Nausea and vomiting | Oral, rectal | April 2014 | France, Germany, UK | Cardiotoxicity |
- a Meprobamate withdrawal began in March 2012 and continued gradually over the duration of the study period.
All withdrawals and revocations included in the analyses occurred during the first half of the study period of interest (Table 1). No further trends were observed. The withdrawal of MA for meprobamate began prior to the start of our time period of interest; however, it was included in this analysis as meprobamate was gradually withdrawn from market throughout Europe during our study period. The reason for gradual withdrawal of meprobamate, rather than abruptly stopping access to the product, was to avoid withdrawal symptoms in patients.
Usage of each product decreased following withdrawal or revocation of MA (Figures 4 and 5). However, there was a less obvious change in usage following revocation compared with that following withdrawal. Both revocations involved specific strengths and formulations of the implicated medicines, with other strengths and formulations remaining on the market, which may in part explain this observation.
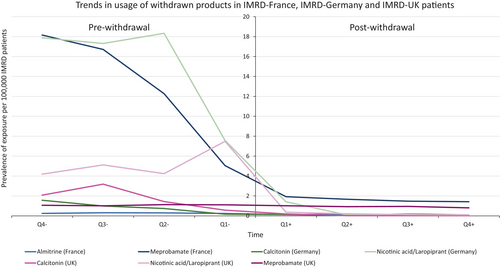
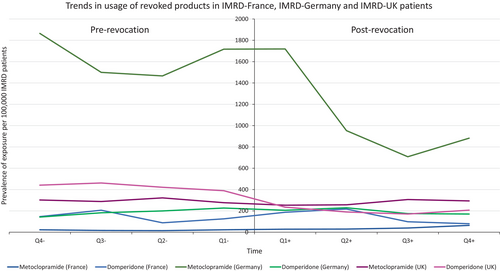
To determine the absolute risk of events leading to regulatory action, a systematic review of the literature was performed to identify published studies reporting quantitative data additional to the studies included within the EMA report. Fourteen studies containing quantitative information on exposures and events were included within EMA documentation on the withdrawals, which contributed to the estimates of absolute risk. In addition, across all products of interest, 361 additional studies were screened for relevance and to ensure they contained counts of the number of patients exposed and the number of patients experiencing the event of interest (meprobamate N = 57; calcitonin N = 41; nicotinic acid/laropiprant N = 78; almitrine N = 45; metoclopramide N = 74; domperidone N = 66). Of these, 15 studies went on to contribute to risk estimates (meprobamate n = 1; calcitonin n = 2; nicotinic acid/laropiprant n = 9; domperidone n = 3). Supporting Information 1 provides additional information on the studies included per medicine of interest.
3.1 Impact Modelling
Details of the data used for impact modelling are presented in Table 2. Average annual exposure within the database populations ranged from 81 patients (almitrine; IMRD-France patients) to 203 687 patients (metoclopramide, IMRD-Germany patients). Absolute risks of the ADRs which led to MA withdrawal or revocation, calculated using pooled study results identified by review of EMA documentation and systematic review of the literature, ranged from 0.1% to 41.25% (Table 2).
| Average number of patients exposed per year | Adverse drug reaction(s) given as reason for withdrawal or revocation | Absolute risk % | Expected number of ADRs avoided annuallya N | |
|---|---|---|---|---|
| France | ||||
| Almitrine | 81 | Peripheral nervous system disturbance | 15.5 | 13 |
| Weight loss | 7.8 | 6 | ||
| Metoclopramide | 5698 | Extrapyramidal symptoms | 0.1 | 6 |
| Domperidone | 42 150 | Cardiotoxicity | 0.7 | 295 |
| Meprobamate | 3553 | Severe hypotension | 41.2 | 1464 |
| Germany | ||||
| Calcitonin | 101 | Cancer | 7.6 | 8 |
| Nicotinic acid/laropiprant | 1818 | Cutaneous reactions | 11.4 | 207 |
| Hepatotoxicity | 1.1 | 20 | ||
| Intracranial/gastrointestinal bleeds | 2.5 | 46 | ||
| New-onset diabetes mellitus | 0.3 | 6 | ||
| Infection | 10.7 | 194 | ||
| Metoclopramide | 203 687 | Extrapyramidal symptoms | 0.1 | 204 |
| Domperidone | 23 656 | Cardiotoxicity | 0.7 | 166 |
| UK | ||||
| Calcitonin | 386 | Cancer | 7.6 | 29 |
| Nicotinic acid/laropiprant | 1110 | Cutaneous reactions | 11.4 | 37 |
| Hepatotoxicity | 1.1 | 12 | ||
| Intracranial/gastrointestinal bleeds | 2.5 | 28 | ||
| New-onset diabetes mellitus | 0.3 | 4 | ||
| Infection | 10.7 | 119 | ||
| Meprobamate | 230 | Severe hypotension | 41.2 | 95 |
| Metoclopramide | 61 088 | Extrapyramidal symptoms | 0.1 | 61 |
- a Impact estimate; rounded to nearest whole number.
Absolute risk was calculated for each reason cited for regulatory action, using pooled data from published quantitative studies obtained from systematic review of the literature (Table 2). To estimate the impact of each withdrawal and revocation, average annual exposure of the medicine within each IMRD population was multiplied by the absolute risk to give the crude number of ADRs expected to be avoided annually as a result of the regulatory action (Table 2). Estimates ranged from three cases of new-onset diabetes mellitus avoided in the IMRD-UK population following withdrawal of nicotinic acid/laropiprant, to 1464 cases of severe hypotension avoided amongst IMRD-France patients as a result of meprobamate withdrawal.
3.2 Validation of the Model
Validation of the model was conducted using the change in exposure amongst IMRD-UK patients, and risk ratio estimates calculated during meta-analyses of the same quantitative studies used to calculate absolute risk during the main impact analysis. The expected ADRs in the exposed IMRD-UK population were mostly similar when compared with results from the primary impact analysis; however, some differences were observed for cutaneous reactions, hepatotoxicity and intracranial or gastrointestinal bleeding associated with nicotinic acid/laropiprant (Table 3). As the results from the two analyses are mostly relatively consistent, the impact analysis was considered to have successfully estimated the number of ADRs avoided as a result of each withdrawal and revocation in the IMRD-UK population.
| Event cited as reason for regulatory action | Cases of the event in 2012a | Cases of the event in 2013a | Cases of the event in 2014a | Cases of the event in 2015a | Cases of the event in 2016a | Average annual cases in IMRD-UK population | Expected ADRs in exposed IMRD-UK patients (% of average annual cases) | Impact estimate (calculated in primary analysis) | |
|---|---|---|---|---|---|---|---|---|---|
| Calcitonin | Cancer | 105 789 | 100 282 | 93 328 | 80 159 | 66 078 | 89 127.2 | 30 (0.03) | 29 |
| Nicotinic acid/laropiprant | Cutaneous reactions | 460 185 | 422 780 | 367 471 | 290 874 | 230 170 | 354 296 | 10 (0.003) | 37 |
| Hepatotoxicity | 20 | 13 | 14 | 9 | 12 | 13.6 | 29 (213.24) | 12 | |
| Intracranial/gastrointestinal bleeds | 7628 | 7178 | 6739 | 5851 | 4616 | 6402.4 | 85 (1.33) | 28 | |
| New-onset diabetes mellitus | 29 456 | 28 660 | 24 292 | 22 088 | 17 838 | 24 466.8 | 4 (0.02) | 4 | |
| Bacterial infections (incident) | 45 495 | 39 988 | 34 308 | 27 873 | 22 349 | 34 002.6 | 119 (0.35) | 119 | |
| Meprobamate | Severe hypotension | 6020 | 5777 | 5203 | 4280 | 3575 | 4971 | 95 (1.91) | 95 |
| Metoclopramide | Extrapyramidal symptoms | 385 | 319 | 314 | 245 | 190 | 290.6 | 86 (0.30) | 61 |
- a Cases observed in whole IMRD-UK population during each 12-month period.
4 Discussion
This study identifies a novel technique to estimate the public health impact of pharmacovigilance regulatory actions, specifically withdrawal, revocation and suspension of MA. This is the first study to address the need for quantification of public health impact of MA withdrawals, revocations and suspensions throughout Europe.
It was observed that the products whose MAs were revoked had smaller reductions in exposure following regulatory action, when compared with those with withdrawn MAs (voluntarily by the MAH) [4]. Alternative formulations and strengths of metoclopramide and domperidone remained on the market following these revocations, therefore, it is possible that incorrect recording of medicines in GP records led to misclassification of exposure to the specific strengths and formulations affected by the regulatory action. An alternative explanation for this is that production of the medicine may have stopped but remaining stock continued to be used. It is possible that the 12-month period following revocation was too short to detect true trends in exposure. In future impact analyses, the time period of interest could be extended to include 24 months following regulatory action.
Furthermore, exposure to metoclopramide was higher in the IMRD-Germany population compared with the IMRD-France and IMRD-UK populations. This may be because the IMRD-Germany database includes records from both primary and secondary care, while IMRD-France and IMRD-UK include primary care records only; it is therefore likely that exposures to metoclopramide in primary and secondary settings have been included in the exposure figures for IMRD-Germany.
A validation analysis using an alternative method to estimate the number of ADRs expected within the IMRD-UK population produced similar estimates of expected ADRs in exposed patients overall, when compared with results using the identified model (Tables 2 and 3). A possible explanation for small differences observed is different case definitions used to identify the cases within the IMRD-UK population compared with those used in studies contributing to risk estimates in the main analysis. In future analyses, it would be useful to identify the specific case definitions used within quantitative research contributing to risk calculations, and use these specific definitions to calculate the number of cases observed within the database population.
4.1 Applicability of the Method
The six included products which underwent MA withdrawal or revocation within Europe between 2012 and 2016 successfully demonstrate application of the modelling method. Overall, these regulatory actions resulted in substantial reduction or elimination of exposure to implicated products, allowing estimation of the impact of these medicines' removal from market within the IMRD populations. However, this modelling method could be applied to any action which results in a change in usage, whether exposure is increased or decreased. Any national electronic health record or prescription database covering the country of interest may be used, providing exposure to medicines within the population can be estimated. If data allows, results of the model could be stratified by age group, sex or other measurable patient characteristic, for more detailed formal comparisons of the public health impact of an action.
Furthermore, impact analyses may be useful for regulators, pharmaceutical companies and other bodies or interested persons when making decisions about which pharmacovigilance actions to implement. Measuring the public health impact of different risk minimisation measures (using predicted changes in utilisation) before a decision is made will help regulators select the most appropriate pharmacovigilance action to protect patient safety.
Although outside the scope of this study, it would be worthwhile assessing the risks of switching to alternative treatments or leaving a condition untreated, and comparing with the risks of the product undergoing regulatory action. An additional factor to consider in future impact analyses is the cost of rectifying ADRs associated with the medicine, weighed against the cost of alternative medicines which patients would switch to and their associated ADRs, and the cost of leaving the condition untreated.
4.2 Limitations of the Method
This model is applicable to other situations where a measurable change in exposure to the medicine of interest occurs. For instance, the method can be applied to MA suspensions and restrictions placed on a medicine's license. However, some limitations were encountered which should be addressed with further refinement of the method.
The described iteration of this method is highly dependent on the accuracy of the data entered into the model. In particular, the estimation of absolute risk from the literature may introduce bias, leading to over or underestimation of the public health impact of medicines withdrawals. The external validity of clinical trials is limited; populations in trials are often different to patients who are prescribed medicines in clinical practice, and trials data are often not generalisable to alternative settings or countries [9, 10]. Results of observational studies were included in the calculation of risks for each ADR, minimising differences between the risks estimated and those for real world patients. Nonetheless, important differences may remain between the risk of each ADR in database patients and in published literature. No quality assessment was undertaken for the studies used to calculate a risk estimate. Furthermore, all study designs were included, but it may not have been appropriate to pool their data. However, due to the small number of published quantitative studies addressing the risks associated with the medicines included in this analysis, it was considered favourable to include all studies regardless of design, to maximise the sample size and to ensure unbiased inclusion of all data. The assumption that these studies are generalisable to the populations of interest may also be a limitation of this analysis. Quantitative research to calculate absolute risks should be carefully chosen in future impact analyses, however where availability of data is limited it may be advantageous to include as much data as possible. As this project progresses into its next phase where refinement and improvement of the methodology is being carried out, alternative methods to calculate the absolute risk of events associated with the medicine of interest are being considered. For example, it may be necessary to conduct epidemiological studies to estimate the risk of ADRs, to ensure accurate estimates of the risk and generalisability to the exposed patients within the database selected.
Electronic health records used in this method have inherent limitations, for example medicines used in hospitals may not be accurately recorded in primary healthcare records leading to underestimation of exposure and predicted impact. Similarly, inaccuracies in recording in patient records both within and between databases might cause inaccuracies in predictions. Examples include the medicines with increasing or static exposure following regulatory action. Some of these medicines had alternative formulations or strengths remaining on the market following their withdrawal or revocation of MA which might have increased the possibility of misclassification. Alternatively, there may have been a delay in making the medicine unavailable following the regulatory decision. Future research into impact of post-marketing removal of medications from market could include longer follow-up after the date of regulatory action to investigate trends in exposure over a longer duration. Furthermore, there are differences in coding practices between databases, so a range of possible disease codes should be used. It was these differences in disease coding between databases which meant validation analyses were undertaken only for the IMRD-UK population. In future, it would be useful to account for these differences in order to conduct validation analyses for all populations under study, to better validate the method.
The method also relies on the availability of electronic health record data. It was observed that the number of patients in the wider IMRD-UK database markedly decreased during the study period. A smaller number of patients contributing data may have led to a smaller estimate of exposed patients to the medicines which underwent regulatory action later in the study period and therefore an underestimate of the impact for these withdrawals and revocations in the IMRD-UK population, compared with actions occurring earlier in the study period. It is therefore not possible to make direct comparisons of impact numbers from across the study period within this population.
The results of this study have demonstrated that prescribing patterns differ between countries; a medicine which is widely utilised in one country may not be used as frequently in another. Different utilisation patterns of these medicines for different countries lead to differences in impact of their removal from market. Results from individual countries are therefore not comparable nor combinable, and there will be limited generalisability of the results to other populations both within and outside Europe, where utilisation varies. However, results will be generalisable to the wider population of each corresponding country, provided the patients within the database are representative of the general population.
Finally, this proof-of-concept study forms part of an ongoing project, where more recent regulatory actions, including medicines withdrawals and other actions taken for pharmacovigilance purposes, are being examined. This study has shown that the method, with further development and improvement, may be used by regulators, MAHs, public health workers and others when making pharmacovigilance decisions in future.
5 Conclusion
This project successfully developed a method which can estimate with acceptable accuracy the impact of medicines following MA withdrawals and revocations, in terms of morbidity and mortality avoided as a result of regulatory action. The model is reliant on the availability of good-quality data, including suitable usage data and quantifiable attributable risks for sufficient numbers of patients. This model is applicable to other situations where a measurable change in exposure to the medicine of interest occurs and can be adapted to use any database where exposure can be estimated. Extrapolation of results to the wider population is also possible, depending on the representativeness of the database used. Results may also be stratified by age, sex or other defining characteristics where data allows. The model can be adopted to estimate quantitatively the impact of other regulatory decisions such as restriction of use of medicinal products. This study provides proof-of-concept for the model to estimate the direct effects of MA withdrawals on morbidity and mortality, however, additional work is required to further refine the methodology for more accurate impact estimates. Studies are underway to advance the methodology and to consider the wider effects of medicines withdrawals on public health, such as risks of alternative therapeutic interventions (medicinal or others) to which patients may switch and the risks of non-treatment of the medical condition, to assess together the direct and indirect impact of pharmacovigilance actions on public health.
5.1 Plain Language Summary
The safety of medicines is thoroughly tested during clinical trials before the product is approved. However, safety concerns may arise after a medicine has received Marketing Authorisation and occasionally a Marketing Authorisation is withdrawn or revoked for safety reasons. This means the medicine is no longer available for use by patients, to protect them from potential harm. Measuring the protective effect is not straightforward. This paper describes the development of a model to measure the public health impact of withdrawal and revocation of Marketing Authorisation in terms of serious side effects. The model is tested on six medicines for which Marketing Authorisation was withdrawn or revoked between 2012 and 2016 in any of United Kingdom, France and Germany. The model uses the number of patients taking the medicine and the risk of certain side effects to estimate the number of side effects prevented following the medicine's Marketing Authorisation withdrawal or revocation. This modelling method could be applied to any action which results in a change in usage, and can be adapted to use any database where exposure to medicines can be estimated.
Acknowledgments
This study has been completed as part of a commitment to the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Special Interest Group on Measuring the Impact of Pharmacovigilance Activities. The authors would like to thank Jim Slattery for his assistance with data acquisition, interpretation and analyses, and Dr. Alison Yeomans for her review and refinement of the manuscript prior to publication.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
All authors declare no support from any organisation for the submitted work and no other relationships or activities that could appear to have influenced the submitted work. The Drug Safety Research Unit (DSRU) receives unconditional grants from multiple pharmaceutical companies. This is a methodological study funded totally by the DSRU.
Open Research
Data Availability Statement
The authors have nothing to report.




