The Effect of Sodium Glucose Cotransporter-2 Inhibitors on Hemoglobin A1c Variability and Acute Kidney Injury: A Causal Mediation Analysis
Funding: Yang Xu is supported by the Young Scientists Fund, National Natural Science Foundation of China (Grant no. 82304245). Tiansheng Wang is supported by the American Diabetes Association (Grant nos. 4-22-PDFPM-06). Til Stürmer receives investigator-initiated research funding and support as principal investigator from the National Institute on Aging (R01AG056479) and as coinvestigator from the National Institutes of Health (R01CA174453, R01HL118255, and R01MD011680).
Tiansheng Wang and Dongze Ji share first authorship.
ABSTRACT
Purpose
The role of lower hemoglobin A1c (HbA1c) variability in the effect of sodium glucose cotransporter-2 inhibitors (SGLT2i) on acute kidney injury (AKI) remains unclear. We compared AKI risk between SGLT2i and dipeptidyl peptidase 4 inhibitors (DPP4i) initiators. Additionally, we aimed to explore the extent to which SGLT2i's influence on AKI risk is mediated by reducing long-term HbA1c variability.
Methods
Using 2018–2022 year data in Yinzhou Regional Health Care Database, we included adult, type 2 diabetes patients who were new users of SGLT2i or DPP4i. The effect of SGLT2i versus DPP4i on AKI, HbA1c variability, and AKI through HbA1c variability was compared using inverse probability of treatment weighted Cox proportional hazards models, median regression models, and causal mediation analysis.
Results
With a median follow-up of 1.76 years, 19 717 adults (for SGLT2i, n = 6008; for DPP4i, n = 13 709) with type 2 diabetes were included. The adjusted hazard ratio for SGLT2i versus DPP4i was 0.79 (95% confidence interval [CI] 0.64–0.98) for AKI. The adjusted differences in median HbA1c variability score (HVS) and HbA1c reduction were −16.67% (95% CI: −27.71% to −5.62%) and −1.98% (95% CI: −14.34% to 10.38%), respectively. Furthermore, lower AKI risk associated with SGLT2i was moderately mediated (22.77%) through HVS. The results remained consistent across various subgroups and sensitivity analyses.
Conclusions
Compared to DPP4i, lower AKI risk associated with SGLT2i is moderately mediated through HbA1c variability. These findings enhance our understanding of the effect of SGLT2i on AKI and underscore the importance of considering HbA1c variability in diabetes treatment and management.
Summary
- A refined acute kidney injury (AKI) outcome identification algorithm based on changes in consecutive creatine measurements could potentially enable a more accurate estimation of AKI incidence.
- Sodium glucose cotransporter-2 inhibitors (SGLT2i) decrease AKI incidence by around 0.5 cases every 100 person-year relative to dipeptidyl peptidase 4 inhibitors (DPP4i).
- SGLT2i significantly reduce HbA1c variability, compared to DPP4i in Asian population.
- SGLT2i do not significantly reduce HbA1c levels, compared with DPP4i in Asian population.
- Around 23% of SGLT2i's effect to lower AKI is estimated to be mediated by its impact on reducing fluctuations in long-term HbA1c variability.
1 Introduction
Diabetes mellitus presents a significant global health challenge, necessitating multifaceted strategies to mitigate risks of complications and mortality beyond glycemic control [1]. Sodium glucose cotransporter-2 inhibitors (SGLT2i) have been demonstrated to benefit cardiovascular-kidney-metabolic syndrome [2-6], therefore have been recommended by current clinical guidelines for patients with established atherosclerotic cardiovascular disease, kidney disease, or heart failure [1, 7, 8]. Meta-analyses of randomized clinical trials consistently exhibit a decreased risk of acute kidney injury (AKI) associated with SGLT2i treatment compared to placebo [9-11]. Observational studies have reported divergent results about the impact of SGLT2i on AKI risk, ranging from reduction [12-14] to negligible difference [15, 16], which may be attributed to AKI identification in real-world data (RWD): (1) the International Classification of Diseases 10th Revision (ICD-10) coding to identify AKI [12, 15] has poor sensitivity [17]; (2) the infrequent serum creatinine tests according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition [13, 14, 18], leads to inaccurate identification. Consequently, a more sensitive AKI identification algorithm is needed to accurately elucidate the effect of SGLT2i on AKI.
Meanwhile, SGLT2i also distinctly reduces hemoglobin A1c (HbA1c) variability [19]. Our recent studies bring important insights for patients with type 2 diabetes (T2DM): (1) a higher HbA1c level is linked with AKI [20], and (2) greater long-term HbA1c variability is associated with an increased AKI risk [21] (Figure S1A). Yet, prior studies [12-16] have not examined whether the decreased HbA1c variability, observed with SGLT2i, influences its impact on AKI.
Mediation analysis is an epidemiologic approach to uncover the underlying mechanisms or pathways through which a treatment affects an outcome, which breaks down the total treatment effect into direct effect and indirect effects (transmitted via mediator to the outcome) [22]. However, traditional mediation analysis suffered from ambiguities when it is used to estimate the effects from mediation models with noncontinuous mediator and outcome variables [23, 24]. Thus, in the last decade, causal mediation analysis gained popularity. Based on the potential outcome framework, causal mediation analysis first defines total, direct and indirect effects explicitly, and then uses various types of mediation models to estimate them [25, 26]. By distinguishing causal effect definitions from causal effect estimation, causal mediation analysis clarifies the ambiguities that arise in traditional mediation analysis [22]. By far, this cutting-edge method has not been applied to explain SGLT2i's effect on AKI [27, 28].
Therefore, this population-based cohort study aims to: (1) evaluate the impact of SGLT2i on AKI using a refined outcome identification algorithm and (2) investigate to what extent this effect is mediated by the reduction of long-term HbA1c variability.
2 Methods
2.1 Data Source
The observational data of this study were extracted from the Yinzhou Regional Health Care Database (YRHCD) in China. Yinzhou is the largest district in Ningbo city, which lies in the middle of China's eastern coastline. In terms of gross domestic product, Yinzhou (273.48 billion Yuan) as the most economically central area of Ningbo City has ranked first among all districts in Zhejiang Province in 2022 [29]. This data repository, established by the Yinzhou District Centre for Disease Control and Prevention, encompasses records for over 2.53 million individuals, accounting for 99% of Yinzhou's permanent residents, between 2009 and 2022 [30], which is likely to represent the population in the eastern coastal areas of China. The YRHCD integrates de-identified clinical data from a range of healthcare facilities, including 5 general hospitals (including 3 tertiary hospital and 2 secondary hospitals), 24 township health centers, and 265 community health service stations, all linked with unique identifiers. The regional network of healthcare facilitates research on a broad array of datasets, including diagnosis data (e.g., disease name, ICD-10 codes, and diagnosis date), drug prescriptions (e.g., generic and brand name, Anatomical Therapeutic Chemical [ATC] codes, prescription date, dosage forms, and quantities prescribed), medical procedures, examination and laboratory tests, and death certificates. This unique setup provides a comprehensive RWD through the continuity of care across healthcare settings. The study used only de-identified data and thus was deemed not to require informed consent. It was approved by the Peking University Health Science Center Ethics Committee.
2.2 Study Design
An active-comparator new-user (ACNU) study design was implemented to mitigate the risk of confounding by indication and time-related biases [31]. Given similar indications, dipeptidyl peptidase 4 inhibitors (DPP4i) (i.e., sitagliptin, vildagliptin, linagliptin, saxagliptin, and alogliptin) were designated as an active comparator for SGLT2i (i.e., dapagliflozin, empagliflozin, and canagliflozin) (ATC codes shown in Table S1). Patients were classified as initiators of DPP4i or SGLT2i if they filled their first DPP4i or SGLT2i prescription between January 1, 2018, and December 31, 2022. This study period was selected because dapagliflozin, the first SGLT2i launched in China, was approved by the National Medical Products Administration in March 2017 and available on the market after that. Additionally, patients were required to have at least one-year history in the YRHCD. Furthermore, they should not have had any prescription for these two drugs filled in the 365 days (i.e., the baseline and washout period) before the date of the first DPP4i/SGLT2i prescription (the index date T0).
In Cohort 1, we included adult patients (aged ≥18 years old) diagnosed with T2DM (ICD-10 codes: E11–E14), who were initiators of DPP4i or SGLT2i. We excluded patients who met any of the following criteria (see Figures S2 and S3): (1) coadministered SGLT2i and DPP4i at T0; (2) had missing information on age or sex; (3) had no serum creatinine measurements during baseline period; (4) had baseline estimated glomerular filtration rate (eGFR, calculated by 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine-based equation [32]) below 30 mL/min/1.73 m2 (contradiction for SGLT2i [33]); (5) had a history of any renal replacement therapy including dialysis or kidney transplantation, or AKI diagnosis (ICD-10 codes: N17); and (6) entered the cohort later than December 31, 2021 (to increase the proportion of patients with ≥1-year follow-up).
2.3 Covariates
The covariates collected at the baseline period include demographics (age and sex), index year, body mass index (BMI, allowing for prior height measurement if only weight was recorded during the last 1 year), systolic blood pressure (SBP) and diastolic blood pressure (DBP), laboratory measurements (HbA1c, eGFR, and cholesterol levels) measured nearest to the index date, comorbidities (e.g., cardiovascular diseases, pulmonary diseases, cancer, and diabetes), co-medications including diabetes drugs (in the past 6 months), angiotensin-converting enzyme inhibitors or angiotensin receptor blocker or angiotensin receptor neprilysin inhibitors (ACEi [angiotensin-converting-enzyme inhibitors]/ARB [angiotensin receptor blocker]/ARNi [angiotensin receptor/neprilysin inhibitor]), antiplatelets, anticoagulants, calcium channel blockers, and healthcare utilization (number of outpatients and inpatients visits) (Table S1).
2.4 Outcome
The AKI outcome was ascertained by the transient creatinine elevations, using the AKI e-alert algorithm [34], which iteratively evaluates various creatine measurements during the follow-up and has been validated with a high sensitivity in different hospital settings [35-37]. By biochemistry in retrospect, it can account for patients with and without prior baseline serum creatinine, and may help clinicians recognize AKI early [37]. Further, any temporary dialysis during the follow-up would also be identified as AKI events to align with the KDIGO criteria [38] (Figure 1 and Supplementary Method S2). Patients were followed from T0 to the date of the first AKI event, death or the end of the study period (December 31, 2021), whichever came first. We implemented intention-to-treat analysis ignoring treatment switches (from SGLT2i to DPP4i or vice versa), augmentation (adding SGLT2i to DPP4i or vice versa), or discontinuation during follow-up period.
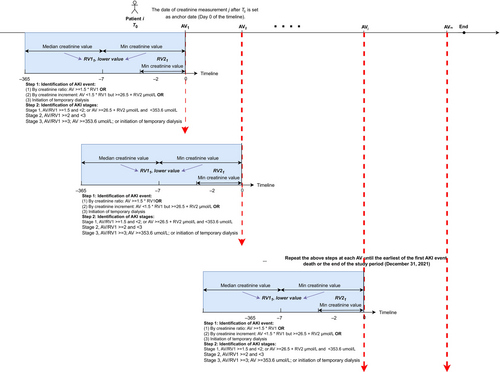
For causal median analysis, we built a restricted cohort (Cohort 2) excluding patients (1) developed AKI or died in the first year (to make sure the outcome does not precede the mediator in our mediation analysis), or (2) had fewer than three HbA1c measurements in the first year, or (3) had less than one-year follow-up. HbA1c variability was measured by calculating the HbA1c variability score (HVS) within the year after T0, employing a method previously described by Forbes et al [39]. The HVS ranges from 0% to 100%, representing the percentage of total HbA1c measures deviating by 0.5% (5.5 mmol/mol) or more compared with the preceding HbA1c measurement. For example, if a person had the following sequence of HbA1c values of 7.1%, 6.4%, 6.6%, 7.2%, 7.5%, and 6.9%, the count of differences ≥0.5% between two consecutive measurements is 3, resulting in an HVS of 60% [3/5 × 100]. Besides HbA1c variability, we also evaluated SGLT2i's effect on HbA1c reduction, defined as the difference between baseline and median HbA1c within the year after T0. For Cohort 2, follow-up also started at T0 and ended at the date of the first AKI event, death, or December 31, 2022, whichever came first.
2.5 Statistical Analysis
In Cohort 1, baseline characteristics of included patients were represented as the mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables and as count or number (percentages) for categorical variables. The proportions of missing examinations and laboratory measurements at baseline were as follows: 33.30% for BMI, 32.77% for SBP, 32.75% for DBP, 22.96% for HbA1c, and 2.64% for total cholesterol. The missing laboratory measurements of HbA1c and total cholesterol were imputed using the multiple imputation by chained equation model [40], which included treatment variable, all covariates (except BMI, SBP, and DBP), outcome indicator, and the Nelson–Aalen estimator of cumulative hazard function [41]. The probability of initiating SGLT2i versus DPP4i (i.e., propensity score [PS]) was estimated by logistic regression on covariates mentioned above (including imputed lab data) except BMI, SBP, and DBP due to high missing rate. We then used inverse probability of treatment weighting to adjust for confounding by assigning SGLT2i initiators and DPP4i initiators a weight of 1/PS and 1/(1 – PS), respectively. We used standardized absolute mean differences (SAMDs) >0.1 as the threshold for meaningful imbalance of covariates between the treatment groups. We estimated the crude incidence rates (IRs) per 100 person-years by treatment for AKI, used weighted Cox regression to estimate the adjusted hazard ratio (aHR), and plotted weighted Kaplan–Meier curves [42].
In Cohort 2, we first calculated the crude median (IQR) of HVS for SGLT2i initiators and DPP4i initiators, respectively. The difference in median HVS between SGLT2i initiators and DPP4i initiators was then computed using the weighted median regression model. Similarly, the comparison of SGLT2i's and DPP4i's effects on HbA1c reduction was also assessed by such model. For all weighted analyses, CIs were obtained by robust variance estimation to account for uncertainty around weights. To estimate the extent to which the effect of SGLT2i versus DPP4i on AKI is mediated by HbA1c reduction and HVS, we performed causal mediation analysis using the marginal structural approach with Cox regression models, as described by Lange et al [26]. We decomposed the effect of SGLT2i versus DPP4i on AKI (total effect) into the indirect effect through HVS and the remaining direct effect (Figure S1B), and computed the “proportion mediated” through HVS (PMHVS) as the ratio of the log of the indirect effect through HVS to the log of the total effect, where a PMHVS of 100% suggests complete mediation by HVS. We dichotomized HVS into high/low levels by a cutoff value of 0.5 for easy formulation and estimation of direct and indirect effects, then used different cutoff values (0.3 and 0.7) and continuous HVS to enhance the robustness. We did not proceed with further mediation analysis on HbA1c reduction as previous studies have robustly shown no significant difference in HbA1c reduction effects of SGLT2i versus DPP4i (Figure S1) [19, 43].
Subgroup analyses were conducted to investigate the potential effect modification of age (≤65 and >65 years), sex, heart failure, chronic kidney disease (CKD) stages (eGFR: 30–44, 45–59, 60–89, ≥90 mL/min/1.73 m2), and concurrent use of ACEi/ARB/ARNi. To test the robustness of our results, we conducted several sensitivity analyses in Cohort 1. First, we identified AKI by two alternative algorithms including (1) a composite of our algorithm or AKI diagnosis (to increase sensitivity) and (2) requiring to meet the severity stages two to three in our algorithm (to increase specificity). Second, we conducted complete-case analyses by restricting patients to those with no missing information on all covariates. Third, we implemented asymmetric PS trimming [44] in order to exclude patients who were treated most contrary to prediction using a PS cut points for both SGLT2i and DPP4i cohorts corresponding to the 0.5th and 99.5th percentiles of the PS distribution in patients initiated with SGLT2i versus DPP4i, respectively. Fourth, the maximum follow-up length for patients included in Cohort 1 was set at 1 year, and the main analyses were repeated to reduce the impact of different follow-up periods for SGLT2i initiators versus DPP4i initiators on study results. Last, to mitigate channeling bias caused by changes in SGLT2i initiation over time [45], we stratified Cohort 1 into two subcohorts by calendar years (2018–2020 and 2021), reperformed our main analyses in each subcohort, and then results were pooled by use of inverse variance-weighted averages of logarithmic HRs in random-effects analysis. All analyses were performed using R Statistical Software (version 4.1, Vienna, Austria).
3 Results
3.1 Baseline Characteristics
Cohort 1 included 6008 SGLT2i initiators (5742 [95.60%] on dapagliflozin, 140 [2.33%] on empagliflozin, 126 [2.10%] on canagliflozin) and 13 709 DPP4i initiators (11 516 [84.00%] on sitagliptin, 1305 [9.52%] on saxagliptin, 591 [4.31%] on linagliptin, 235 [1.71%] on vildagliptin, 58 [0.42%] alogliptin, 4 [0.03%] on unspecified DPP4i). SGLT2i initiators were older and more likely to be male, had a higher prevalence of hypertension, renal and arterial disease, coadministered medications of metformin, sulfonylureas, and ACEi/ARB/ARNi, a slightly lower median of HbA1c (7.60% vs. 7.66%) and eGFR (95.89 vs. 96.56 mL/min/1.73 m2), but higher SBP. Cohort 2 included 612 SGLT2i initiators and 1320 DPP4i initiators, and its covariates distribution was similar to Cohort 1 (Table 1). After weighting, covariates between SGLT2i and DPP4i groups were well-balanced (Figure S4).
| Cohort 1 | Cohort 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unweighted | Weighted | Unweighted | Weighted | |||||||||
| SGLT2i | DPP4i | SAMD | SGLT2i | DPP4i | SAMD | SGLT2i | DPP4i | SAMD | SGLT2i | DPP4i | SAMD | |
| (n = 6008) | (n = 13 709) | (n = 19 704) | (n = 19 796) | (n = 612) | (n = 1320) | (n = 1920) | (n = 1925) | |||||
| Demographics, n (%) | ||||||||||||
| Male | 3351 (55.8) | 7394 (53.9) | 0.037 | 10 758 (54.6) | 10 899 (55.1) | 0.009 | 359 (58.7) | 697 (52.8) | 0.118 | 1083 (56.4) | 1053 (54.7) | 0.034 |
| Age, mean (SD), years | 63 (13) | 62 (13) | 0.035 | 62 (13) | 62 (12) | 0.009 | 63 (13) | 61 (13) | 0.163 | 61 (13) | 61 (12) | 0.011 |
| Index year, n (%) | 0.832 | 0.006 | 0.738 | 0.009 | ||||||||
| 2018 | 38 (0.6) | 2805 (20.5) | 217 (1.1) | 3387 (17.2) | 7 (1.1) | 320 (24.2) | 40 (2.1) | 403 (20.9) | ||||
| 2019 | 49 (0.8) | 3213 (23.4) | 286 (1.4) | 3867 (19.6) | 2 (0.3) | 314 (23.8) | 11 (0.6) | 401 (20.8) | ||||
| 2020 | 1903 (31.7) | 3783 (27.6) | 11 406 (57.6) | 4551 (23.1) | 261 (42.6) | 394 (29.8) | 1241 (64.6) | 500 (26.0) | ||||
| 2021 | 4018 (66.9) | 3908 (28.5) | 7879 (39.8) | 7899 (40.1) | 342 (55.9) | 292 (22.1) | 628 (32.7) | 621 (32.3) | ||||
| Comorbidities, n (%) | ||||||||||||
| Acute coronary syndrome | 53 (0.9) | 80 (0.6) | 0.035 | 130 (0.7) | 125 (0.6) | 0.006 | 7 (1.1) | 10 (0.8) | 0.040 | 17 (0.9) | 17 (0.9) | 0.004 |
| Other ischemic heart diseases | 1414 (23.5) | 3161 (23.1) | 0.011 | 4608 (23.4) | 4794 (24.2) | 0.004 | 184 (30.1) | 341 (25.8) | 0.094 | 520 (27.1) | 520 (27.0) | 0.001 |
| Heart failure | 264 (4.4) | 331 (2.4) | 0.109 | 579 (2.9) | 564 (2.9) | 0.019 | 29 (4.7) | 35 (2.7) | 0.111 | 55 (2.9) | 56 (2.9) | 0.001 |
| Valvular disorders | 9 (0.1) | 11 (0.1) | 0.021 | 19 (0.1) | 17 (0.1) | 0.005 | 0 (0) | 0 (0) | <0.001 | 0 (0) | 0 (0) | <0.001 |
| Stroke | 224 (3.7) | 734 (5.4) | 0.078 | 959 (4.9) | 954 (4.8) | 0.002 | 22 (3.6) | 60 (4.5) | 0.048 | 83 (4.3) | 78 (4.0) | 0.013 |
| Other cerebrovascular diseases | 1078 (17.9) | 2576 (18.8) | 0.022 | 3664 (18.6) | 3653 (18.5) | 0.002 | 129 (21.1) | 219 (16.6) | 0.115 | 363 (18.9) | 343 (17.8) | 0.028 |
| Atrial fibrillation | 161 (2.7) | 296 (2.2) | 0.034 | 459 (2.3) | 476 (2.4) | 0.004 | 16 (2.6) | 17 (1.3) | 0.096 | 28 (1.5) | 26 (1.4) | 0.009 |
| Other arrhythmia | 411 (6.8) | 934 (6.8) | 0.001 | 1352 (6.9) | 1404 (7.1) | 0.005 | 49 (8) | 114 (8.6) | 0.023 | 170 (8.8) | 157 (8.2) | 0.024 |
| Peripheral vascular disease | 1140 (19.0) | 2349 (17.1) | 0.048 | 3527 (17.9) | 3647 (18.4) | 0.009 | 130 (21.2) | 246 (18.6) | 0.065 | 394 (20.5) | 378 (19.6) | 0.022 |
| Hypertension | 4239 (70.6) | 8479 (61.8) | 0.185 | 12 723 (64.6) | 12 934 (65.3) | 0.014 | 444 (72.5) | 786 (59.5) | 0.277 | 1241 (64.6) | 1224 (63.6) | 0.021 |
| COPD | 1623 (27.0) | 3701 (27.0) | <0.001 | 5325 (27.0) | 5326 (26.9) | 0.016 | 169 (27.6) | 387 (29.3) | 0.038 | 562 (29.3) | 550 (28.5) | 0.016 |
| Cancer | 255 (4.2) | 662 (4.8) | 0.028 | 917 (4.7) | 919 (4.6) | 0.003 | 25 (4.1) | 52 (3.9) | 0.007 | 73 (3.8) | 76 (3.9) | 0.007 |
| Arterial disease | 1203 (20) | 2505 (18.3) | 0.045 | 3740 (19.0) | 3872 (19.6) | <0.001 | 144 (23.5) | 256 (19.4) | 0.101 | 417 (21.7) | 397 (20.6) | 0.026 |
| Renal diseases | 1205 (20.1) | 1877 (13.7) | 0.171 | 3091 (15.7) | 3063 (15.5) | 0.015 | 144 (23.5) | 236 (17.9) | 0.140 | 367 (19.1) | 367 (19.1) | 0.002 |
| Obesity | 168 (2.8) | 124 (0.9) | 0.141 | 305 (1.5) | 298 (1.5) | 0.006 | 19 (3.1) | 15 (1.1) | 0.137 | 35 (1.8) | 35 (1.8) | 0.002 |
| Diabetic eye complications | 311 (5.2) | 653 (4.8) | 0.019 | 966 (4.9) | 946 (4.8) | 0.003 | 45 (7.4) | 84 (6.4) | 0.039 | 129 (6.7) | 129 (6.7) | 0.001 |
| Diabetic ketoacidosis | 35 (0.6) | 86 (0.6) | 0.006 | 127 (0.6) | 157 (0.8) | 0.006 | 3 (0.5) | 13 (1) | 0.058 | 27 (1.4) | 17 (0.9) | 0.052 |
| Diabetes, other complications | 524 (8.7) | 1392 (10.2) | 0.049 | 1947 (9.9) | 2033 (10.3) | 0.017 | 77 (12.6) | 211 (16) | 0.097 | 283 (14.7) | 289 (15.0) | 0.007 |
| Comedications, n (%) | ||||||||||||
| Metformin | 2705 (45.0) | 5512 (40.2) | 0.098 | 8224 (41.7) | 8318 (42.0) | 0.013 | 314 (51.3) | 567 (43) | 0.168 | 855 (44.6) | 867 (45.0) | 0.009 |
| Sulfonylurea | 2629 (43.8) | 4980 (36.3) | 0.152 | 7640 (38.8) | 7695 (38.9) | 0.006 | 297 (48.5) | 506 (38.3) | 0.207 | 813 (42.3) | 805 (41.8) | 0.011 |
| Insulin | 782 (13.0) | 1681 (12.3) | 0.023 | 2450 (12.4) | 2418 (12.2) | 0.002 | 88 (14.4) | 191 (14.5) | 0.003 | 271 (14.1) | 277 (14.4) | 0.008 |
| Glinide | 447 (7.4) | 1236 (9.0) | 0.057 | 1680 (8.5) | 1653 (8.4) | 0.007 | 63 (10.3) | 178 (13.5) | 0.099 | 234 (12.2) | 236 (12.3) | 0.003 |
| Alpha-glucosidase inhibitors | 1885 (31.4) | 4172 (30.4) | 0.020 | 6071 (30.8) | 6131 (31.0) | 0.006 | 220 (35.9) | 453 (34.3) | 0.034 | 659 (34.3) | 663 (34.4) | 0.002 |
| Other antidiabetics | 2154 (35.9) | 4772 (34.8) | 0.022 | 6900 (35.0) | 6803 (34.4) | 0.003 | 247 (40.4) | 544 (41.2) | 0.017 | 759 (39.5) | 777 (40.4) | 0.017 |
| ACEi/ARB/ARNi | 2978 (49.6) | 5748 (41.9) | 0.154 | 8739 (44.4) | 8874 (44.8) | 0.014 | 337 (55.1) | 555 (42) | 0.263 | 922 (48.0) | 888 (46.1) | 0.037 |
| Antiplatelets | 1284 (21.4) | 2690 (19.6) | 0.043 | 3963 (20.1) | 3921 (19.8) | 0.010 | 152 (24.8) | 278 (21.1) | 0.090 | 430 (22.4) | 421 (21.9) | 0.013 |
| Anticoagulants | 53 (0.9) | 70 (0.5) | 0.045 | 129 (0.7) | 130 (0.7) | 0.008 | 5 (0.8) | 4 (0.3) | 0.069 | 9 (0.5) | 8 (0.4) | 0.007 |
| CCBs | 2309 (38.4) | 4625 (33.7) | 0.098 | 6916 (35.1) | 6943 (35.1) | 0.001 | 253 (41.3) | 418 (31.7) | 0.202 | 654 (34.1) | 656 (34.1) | <0.001 |
| Loop diuretic | 177 (2.9) | 228 (1.7) | 0.086 | 409 (2.1) | 408 (2.1) | 0.001 | 23 (3.8) | 18 (1.4) | 0.152 | 38 (2.0) | 34 (1.8) | 0.016 |
| Thiazide diuretic | 56 (0.9) | 116 (0.8) | 0.009 | 171 (0.9) | 150 (0.8) | 0.001 | 6 (1) | 11 (0.8) | 0.016 | 16 (0.8) | 18 (0.9) | 0.012 |
| Aldosterone antagonists | 152 (2.5) | 261 (1.9) | 0.043 | 421 (2.1) | 397 (2.0) | 0.013 | 12 (2) | 27 (2) | 0.006 | 26 (1.3) | 37 (1.9) | 0.046 |
| Beta-blockers | 1059 (17.6) | 2176 (15.9) | 0.047 | 3247 (16.5) | 3288 (16.6) | 0.009 | 123 (20.1) | 233 (17.7) | 0.063 | 368 (19.2) | 356 (18.5) | 0.017 |
| Lipid-lowering agents | 2198 (36.6) | 4524 (33.0) | 0.075 | 6712 (34.1) | 6727 (34.0) | 0.004 | 244 (39.9) | 486 (36.8) | 0.063 | 733 (38.2) | 725 (37.7) | 0.011 |
| NSAIDs | 1043 (17.4) | 2400 (17.5) | 0.004 | 3439 (17.5) | 3411 (17.2) | 0.002 | 96 (15.7) | 258 (19.5) | 0.101 | 313 (16.3) | 350 (18.2) | 0.051 |
| PPIs | 1298 (21.6) | 2901 (21.2) | 0.011 | 4199 (21.3) | 4159 (21.0) | 0.006 | 141 (23) | 302 (22.9) | 0.004 | 422 (22.0) | 438 (22.8) | 0.019 |
| Examination measurement, median [IQR] | ||||||||||||
| SBP, mmHg | 129.2 [126.5, 132.4] | 128.2 [125.3, 131.5] | 0.196 | 128.3 [125.5, 131.6] | 128.9 [126.1, 132.0] | 0.124 | 129.4 [126.4, 132.4] | 128.2 [125.3, 131.3] | 0.280 | 129.2 [126.1, 132.1] | 128.5 [125.6, 131.6] | 0.220 |
| DBP, mmHg | 77.3 [75.3, 79.4] | 77.2 [75.0, 79.3] | 0.055 | 77.2 [75.0, 79.0] | 77.4 [75.4, 79.4] | 0.066 | 77.4 [75.6, 79.5] | 77.1 [75.1, 79.1] | 0.105 | 77.4 [75.7, 79.4] | 77.1 [75.1, 79.3] | 0.133 |
| BMI, kg/m2 | 24.7 [22.9, 26.8] | 24.2 [22.6, 26.1] | 0.130 | 24.3 [22.6, 26.2] | 24.6 [22.8, 26.6] | 0.072 | 24.6 [22.8, 26.5] | 24.2 [22.6, 26.0] | 0.182 | 24.5 [22.8, 26.5] | 24.2 [22.6, 26.0] | 0.150 |
| Laboratory tests, median [IQR]a | ||||||||||||
| HbA1c, % | 7.6 [6.7, 9.1] | 7.7 [6.8, 9.2] | 0.083 | 7.8 [7.0, 9.0] | 7.8 [6.9, 9.1] | 0.007 | 7.2 [6.5, 8.2] | 7.4 [6.6, 8.6] | 0.035 | 7.3 [6.6, 8.8] | 7.4 [6.7, 8.5] | 0.018 |
| Total cholesterol, mmol/L | 4.6 [4.0, 5.3] | 4.6 [4.0, 5.3] | 0.016 | 4.7 [4.0, 5.3] | 4.7 [4.0, 5.3] | 0.006 | 4.6 [4.0, 5.2] | 4.6 [4.0, 5.2] | 0.006 | 4.57 [4.0, 5.2] | 4.6 [4.0, 5.2] | 0.016 |
| eGFR, ml/min/1.73 m2 | 95.9 [85.8, 105.1] | 96.6 [86.4, 105.8] | 0.028 | 96.3 [86.3, 105.7] | 96.2 [86.7, 105.4] | 0.012 | 96.4 [85.3, 105.6] | 97.8 [87.9, 106.3] | 0.070 | 96.9 [86.5, 107.2] | 97.2 [87.5, 106.0] | 0.020 |
| Healthcare utilization, median [IQR] | ||||||||||||
| Number of inpatient visits | 0 [0, 1] | 0 [0, 1] | 0.089 | 0 [0, 1] | 0 [0, 1] | <0.001 | 0 [0, 1] | 0 [0, 1] | 0.128 | 0 [0, 1] | 0 [0, 1] | 0.007 |
| Number of outpatient visits | 37 [20, 66] | 33 [15, 61] | 0.078 | 34 [16, 62] | 35 [17.00, 63.00] | 0.004 | 47 [26, 76.25] | 38 [18, 71] | 0.098 | 44 [22, 71] | 39 [19, 73] | 0.012 |
- Note: Missing rate: (1) in Cohort 1, BMI 33.30%, SBP 32.77%, DBP 32.75%, HbA1c 22.96%, and total cholesterol 2.64%. BMI, SBP, and DBP were not included in the propensity score models; (2) in Cohort 2, BMI 31.26%, SBP 30.02%, DBP 30.02%, HbA1c 11.02%, and total cholesterol 2.12%. BMI, SBP, and DBP were not included in the propensity score models. A SAMD of >0.1 indicates meaningful imbalance between groups.
- Abbreviations: ACEi/ARB/ARNi, ACE inhibitors or angiotensin receptor blocker or angiotensin receptor neprilysin inhibitors; BMI, body mass index; CCB, calcium channel blockers; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DPP4i, dipeptidyl peptidase 4 inhibitors; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitors; SAMD, standardized absolute mean difference; SBP, systolic blood pressure; SD, standard deviation; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
- a Baseline data of HbA1c, total cholesterol, and eGFR were extracted from the nearest measurement within 1 year prior to the index date.
3.2 Effect of SGLT2i Versus DPP4i on Acute Kidney Injury
In Cohort 1, over a median follow-up time of 1.76 (IQR: 1.08, 2.77) years, the AKI IRs for initiators of SGLT2i and DPP4i were 2.99 (95% CI: 2.64–3.39) and 3.49 (3.29, 3.70) per 100 person-years, respectively. The aHR for AKI was 0.79 (0.64, 0.98) (Table 2). The 2-year weighted AKI risk difference of −1.86% (−2.82%, −0.89%), and the weighted cumulative incidence curves showed an early, stable separation throughout the follow-up (Figure 2).
| Group | No. of patients | Follow-upa (median, IQR) | No. of AKI event | Incidence rate per 100 person years, 95% CI | Crude HR, 95% CI | Adjusted HRb, 95% CI |
|---|---|---|---|---|---|---|
| Cohort 1 | ||||||
| SGLT2i | 6008 | 1.20 (0.92, 1.72) | 240 | 2.99 (2.64–3.39) | 0.77 (0.66–0.88) | 0.79 (0.64–0.98) |
| DPP4i | 13 709 | 2.16 (1.30, 3.24) | 1095 | 3.49 (3.29–3.70) | Reference | Reference |
| Cohort 2 | ||||||
| SGLT2i | 612 | 1.50 (1.20, 1.93) | 11 | 1.13 (0.64–1.89) | 0.87 (0.45–1.69) | 0.86 (0.40–1.88) |
| DPP4i | 1320 | 2.32 (1.61, 3.46) | 60 | 1.79 (1.39–2.27) | Reference | Reference |
- Abbreviations: AKI, acute kidney injury; CI, confidence interval; DPP4i, dipeptidyl peptidase 4 inhibitors; HR, hazard ratio; IQR, interquartile range; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
- a Follow-up in years is presented as median with IQR.
- b Adjusted for demographics, index year of prescription for SGLT2i or DPP4i, laboratory measurements, comorbidities, comedications, and healthcare utilization.
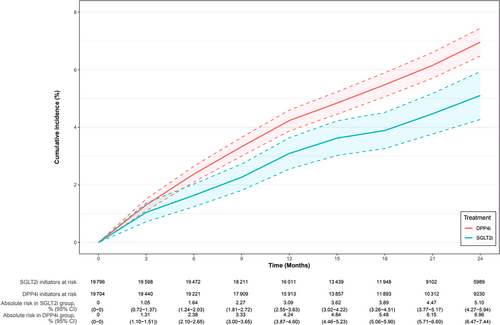
3.3 Effect of SGLT2i Versus DPP4i on Acute Kidney Injury Through Hemoglobin A1c Variability and Reduction
Similar to Cohort 1, lower risks for SGLT2i on AKI (HR 0.86, 0.40, 1.88) of patients were also observed in Cohort 2 (Table 2). The crude median HVS was 25% (IQR: 0%, 50%) for SGLT2i initiators and 50% (IQR: 0%, 66.67%) for DPP4i initiators during one-year follow-up, with an adjusted difference in median HVS of −16.67% (−27.71%, −5.62%). Weighted median regression analysis demonstrated no clinically relevant difference in HbA1c reduction effects of −1.98% (−14.34%, 10.38%) between SGLT2i and DPP4i. Causal mediation analysis revealed that, compared with DPP4i, the lower AKI risk of SGLT2i is partially mediated through lowering HVS (PMHVS of 22.77% for dichotomized HVS [0.5 as the cutoff value], and 20.57% for continuous HVS) (Table S2).
3.4 Subgroup and Sensitivity Analyses
Overall, in Cohort 1, lower AKI risks for SGLT2i were observed across different subgroups of age, sex, baseline heart failure, CKD stages, and concurrent use of ACEi/ARB/ARNi (Table S3). In Cohort 1, the findings by alternative AKI outcome algorithms aligned with our main analyses (Table S4) The complete-case analysis included 3328 SGLT2i initiators and 6628 DPP4i initiators and showed an aHR of 0.82 (0.66, 1.04) (Table S5). After asymmetric PS trimming, 4433 SGLT2i initiators and 10 310 DPP4i initiators were included into analyses, and an aHR of 0.79 (0.64, 0.99) was observed, which was similar to the main analyses (Figure S5, Tables S6 and S7). When the maximum follow-up length was set at 1 year, a lower AKI risk (aHR: 0.74 [0.61, 0.90]) was still observed for SGLT2i initiators than DPP4i initiators (Table S8). When Cohort 1 was stratified into two subcohorts by calendar years, both stratified and pooled results showed that SGLT2i use was associated with a lower AKI risk than DPP4i (Tables S9 and S10, Figure S6).
4 Discussion
Our study demonstrated that SGLT2i was associated with a 21% decreased IR of AKI and HVS (−16.67%) but not associated with HbA1c reduction, compared to DPP4i among patients with T2DM over a median follow-up of 1.76 years. The decrease in AKI risk linked to SGLT2i was partially mediated (~23%) by the effect of SGLT2i on HVS, which was consistently observed in sensitivity analyses.
Our finding on the SGLT2i's effect on AKI aligns not only with the finding of a recent meta-analysis of 13 randomized trials (median follow-up ranged from 0.8 to 4 years, a 23% reduction in the AKI risk associated with SGLT2i) [11], but also with recent four ACNU cohort studies that assessed the effect of SGLT2i versus DPP4i on AKI (aHR ranged from 0.71 to 0.89, median follow-up ranged from 130 days to 2.5 years, as shown in Table S11) [14-16, 46]. Our adapted AKI algorithm employing iterative creatinine measurements is likely to be more sensitive than previously utilized AKI identification methods, which accounts for the elevated IR observed in our study.
Our finding that SGLT2i reduced ~23% HbA1c variability relative to DPP4i is consistent with previous evidence [47] and the observed nonsignificant HbA1c reduction by SGLT2i relative to DPP4i is consistent with the negligible reduction (≤0.1%) reported in a recent meta-analysis of randomized trials [19] and an observational study [43], which reinforces the credibility of our causal mediation analysis. Our previous research indicates that both elevated HbA1c and its long-term variability are linked to a heightened AKI risk [20, 21]. Given the marginal reduction of HbA1c with SGLT2i compared to DPP4i, the decreased HbA1c variability associated with SGLT2i may play a role in its observed reduced AKI risk relative to DPP4i. Our causal mediation analysis further substantiates this observation by indicating that reduced HbA1c variability plays a ~23% role in the diminished AKI risk linked to SGLT2i. Further research is imperative to explore the underlying biomechanisms and validate such a mediation effect.
Together with previous studies [11-16], our findings bolster confidence in the positive impact of SGLT2i on AKI in diabetic patients. We further highlighted the advantage of SGLT2i in mitigating long-term HbA1c variability using RWD. Building on our earlier studies [20, 21, 48], our findings advocate for the use of SGLT2i, particularly in patients with heightened HbA1c variability, such as men and those with obesity [49]. This recommendation stems from the understanding that decreased HbA1c variability reduces risks associated with AKI, other micro- and macro-vascular outcomes [50], and mortality [39].
Our study has important clinical implications. Concerns for AKI should not inappropriately discourage clinicians from prescribing SGLT2i, especially considering the long-term benefits of preventing kidney and cardiovascular complications associated with SGLT2i treatment [3-6]. SGLT2i usage appears to be a favorable choice of glucose-lowering regimen compared with DPP4i, as it can help reduce glycemic variability that may mediate around 23% of the observed reduction in AKI.
Our study has several strengths. First, to our knowledge, our study is the first one to assess the mediation effect of glycemic variability on decreased AKI risk using RWD. Our analysis utilized visit-to-visit fluctuation of HbA1c over months calculated as HVS, which aligns with the standard monitoring procedures in routine clinical care [51] and demonstrated a more variability leads to a higher HR for AKI of 1.23 (1.16, 1.3) [21]. Second, our refined algorithm based on changes in consecutive creatinine measurements theoretically enabled a more accurate estimation of AKI incidence, irrespective of CKD status. Third, we conducted rigorous pharmacoepidemiologic analyses on a large regional healthcare dataset with laboratory tests of kidney function and HbA1c, which enabled us to sufficiently control for confounding and assess causal effects.
Our study also has several limitations. First, we did not assess the potential mediation effects of other variables, such as blood pressure, urinary albumin: creatinine ratio, hematocrit, hemoglobin, and so forth. Second, due to the nature of real-world data, some clinical variables and laboratory tests were missing, and residual confounding is likely. Third, neither the Wang's algorithm [35, 36] nor our modified version has been validated. Our ongoing validation study using YRHCD and Stockholm CREAtinine Measurements (SCREAM) databases [52] will test its performance. Fourth, measurement errors of HbA1c variability may move the indirect effect away from the null. Fifth, we were unable to choose glucagon-like peptide-1 receptor agonists (GLP1 RA, with more similar indications for patients with cardiovascular risk and CKD according to clinical guidelines [1, 2]) as active-comparator due to the small sample (currently <300) in YRHCD. Future studies using GLP1 RA as the active comparator are needed to confirm our results. Sixth, the inclusion criteria for Cohort 2, such as requiring at least three HbA1c measurements, facilitate causal median analysis but also pose a strong potential for selection bias. Generally, conditioning on future events should be avoided. Finally, as our research was based on the regional healthcare database, our findings may not be generalizable to other populations or regions.
In conclusion, our population-based cohort study found that compared to DPP4i, SGLT2i use is associated with a decreased risk for AKI, and HbA1c variability may serve as a mediator to explain the observed reduced AKI risk associated with SGLT2i.
4.1 Plain Language Summary
We conducted a study to understand the effect of sodium glucose cotransporter-2 inhibitors' (SGLT2i) on the risk of AKI and whether this effect is related to changes in HbA1c and their variability over time. Our findings, based on a comparison with dipeptidyl peptidase 4 inhibitors (DPP4i), show that: (1) SGLT2i decreased AKI incidence by ~0.5 cases every 100 person each year; (2) ~23% of SGLT2i's ability to lower AKI seems to be due to its impact on reducing fluctuations in long-term HbA1c variability; (3) SGLT2i did not significantly reduce HbA1c levels. These results suggest that the way SGLT2i helps to stabilize HbA1c levels over time may be an important factor in its protective effects against AKI.
Acknowledgments
Y.X. is supported by the Young Scientists Fund, National Natural Science Foundation of China (Grant no. 82304245). T.W. is supported by the American Diabetes Association (Grant nos. 4-22-PDFPM-06).
Ethics Statement
The study was approved by the Peking University Health Science Center Ethics Committee.
Consent
The study used only de-identified data and thus was deemed not to require informed consent.
Conflicts of Interest
Til Stürmer receives investigator-initiated research funding and support as principal investigator from the National Institute on Aging (R01AG056479) and as coinvestigator from the National Institutes of Health (R01CA174453, R01HL118255, and R01MD011680). He also receives salary support as Director of Comparative Effectiveness Research, North Carolina Translational and Clinical Sciences Institute, University of North Carolina, the Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim), from the pharmaceutical companies (Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, University of North Carolina at Chapel Hill. Til Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, and Novo Nordisk.
Open Research
Data Availability Statement
The YRCHD that supports the findings of this study restricts further dissemination of data to third parties.




