Gastrointestinal bleeding risk with rivaroxaban vs aspirin in atrial fibrillation: A multinational study
Funding information: Bayer HealthCare; Department of Education, Australian Government; Hong Kong University and University College London Strategic Partnership Fund.
Abstract
Purpose
Comparative gastrointestinal bleeding (GIB) risk between rivaroxaban and low-dose aspirin is unknown in patients with atrial fibrillation (AF). This study investigated GIB risk with rivaroxaban vs aspirin among two separate AF cohorts in Hong Kong and the United Kingdom, using a common protocol approach.
Methods
This was a population-based cohort study using separate data from the Clinical Data Analysis and Reporting System (CDARS) of the Hong Kong Hospital Authority (2010-2018) and The Health Improvement Network (THIN) database in the United Kingdom (2011-2017). Patients with AF newly prescribed aspirin or rivaroxaban were included. Cox proportional hazards regression was used to compare GIB risks for rivaroxaban vs aspirin, accounting for confounders using propensity score fine stratification approach.
Results
In CDARS, 29 213 patients were included; n = 1052 (rivaroxaban), n = 28 161 (aspirin). Crude GIB event rates per 100 patient-years in CDARS were 3.0 (aspirin) and 2.6 (rivaroxaban). No difference in GIB risk was observed between rivaroxaban and aspirin overall (HR = 1.04, 95%CI = 0.76-1.42), and in dose-stratified analyses (HR = 1.21, 95%CI = 0.84-1.74 [20 mg/day]; HR = 0.80, 95%CI = 0.44-1.45 [≤15 mg/day]). In THIN, 11 549 patients were included, n = 3496 (rivaroxaban) and n = 8053 (aspirin). Crude GIB event rates were 1.3 (aspirin) and 2.4 (rivaroxaban) per 100 patient-years. No difference in GIB risk was observed between rivaroxaban and aspirin overall (HR = 1.40, 95%CI = 1.00-1.98) and low-dose rivaroxaban (≤15 mg/day) (HR = 1.00, 95%CI = 0.56-1.30), but increased GIB risk was observed for rivaroxaban 20 mg/day vs aspirin (HR = 1.57, 95%CI = 1.08-2.29).
Conclusion
In patients with AF, GIB risk was comparable between aspirin and rivaroxaban ≤15 mg/day. GIB risk for rivaroxaban 20 mg/day vs aspirin remains uncertain and warrants further investigation.
KEY POINTS
- Aspirin monotherapy is still widely used in patients with atrial fibrillation
- Gastrointestinal bleeding (GIB) risk with rivaroxaban vs aspirin is dose-dependent
- In patients with higher baseline risk, GIB was similar for rivaroxaban and aspirin
- GIB occurred sooner after new aspirin initiation compared to rivaroxaban
1 INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a leading cause of stroke affecting 33.5 million people worldwide.1 Direct oral anticoagulants (DOACs) are the first-line treatment for stroke prevention in AF,2 but data indicate ongoing underutilization of anticoagulation.3, 4 Although aspirin is significantly less effective than anticoagulation and is not guideline-recommended for stroke prophylaxis,2 the use of aspirin monotherapy is still widely observed in patients with AF. Up to 61% of Chinese patients with AF with moderate-to-high stroke risk (CHA2DS2VASC ≥ 2) received antiplatelet drugs4 and almost one in five patients received antiplatelet therapy in place of an oral anticoagulant in the United Kingdom.3
One of the possible reasons for the continued use of aspirin is that aspirin is commonly perceived as a safer option over anticoagulation with respect to bleeding (mainly intracranial and gastrointestinal).4, 5 While previous studies have focussed on intracranial bleeding,5 little is known about the comparative risk of gastrointestinal bleeding (GIB) between aspirin and DOACs. Aspirin may cause erosion in the gastrointestinal tract and ulceration, which could predispose to GIB.6 GIB causes serious morbidity, can be fatal, and its treatment imposes substantial costs on healthcare systems.6
Therefore, generating comparative risk information for DOACs vs aspirin in relation to GIB risk in patients with AF is important for informing clinician and patient decision making. Both apixaban and dabigatran demonstrated similar GIB rates compared to aspirin in the AVERROES trial7 and in network meta-analyses,8 respectively. Currently, there are no published data comparing GIB risk for rivaroxaban vs aspirin in patients with AF in real-world clinical practice. The objective of this study was to examine GIB risks for rivaroxaban vs aspirin separately among two large patient cohorts with AF in Hong Kong and the United Kingdom, using a common protocol approach.
2 MATERIALS AND METHODS
2.1 Data sources
This study used electronic medical records of the Clinical Data Analysis and Reporting System (CDARS) of the Hong Kong Hospital Authority and The Health Improvement Network (THIN) database in the United Kingdom. The Hong Kong Hospital Authority manages Hong Kong's public hospitals and is the only public-funder of healthcare in Hong Kong.9 It serves a population of >7 million individuals, covers 80% of hospital admissions, and provides medical services through 122 outpatient (specialist and general) clinics.9 Records from the Hong Kong Hospital Authority are anonymized and centralized in CDARS and include information on demographics, hospitalization, consultation and death dates, medication dispensing, test results, and diagnoses and procedures coded using the International Classification of Disease, ninth revision, Clinical Modification (ICD-9-CM). CDARS has been used extensively for population-based research10, 11 and has demonstrated high coding accuracy in validation studies.4, 10
THIN is a longitudinal primary care database of 15 million individuals from 770 practices. Primary care physicians (PCPs) enter data, which describe patient characteristics, diagnoses, prescriptions, consultations, referrals and laboratory investigations. Diagnoses and procedures are categorized using the Read classification system. THIN data is periodically subject to internal quality checks, has been used extensively for population-based research, including bleeding studies.12
Study protocols were approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW13-468) and by THIN Scientific Review Committee (19THIN021), respectively. Informed patient consent was not required as data were anonymized.
2.2 Study design and participants
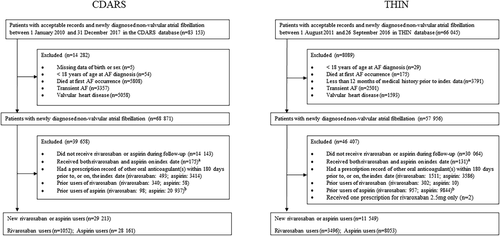
This was a new-user cohort study. Patients aged ≥18 years with a new AF diagnosis between January 1, 2010, and December 31, 2017, were identified in CDARS using ICD-9-CM code for AF (427.3), and in THIN between August 1, 2011, and September 26, 2016, using Read codes for AF (G573.00, G573000, G573200, G573300, G573400, G573500, G573700, G573z00). We excluded patients with AF due to a reversible cause, who had valvular heart disease (Table S1) or who died during their first AF episode. To ensure accuracy of patients' records; in CDARS, patients with missing birth date or sex were excluded. Similarly, in THIN, patients with ≤12 months of medical history prior to AF diagnosis were excluded to ensure the capture of incident AF diagnosis.
The index date was defined as the date of the first prescription for rivaroxaban or aspirin following AF diagnosis. To capture GIB events after treatment initiation, and to reduce possible confounding, we excluded patients who received aspirin or any anticoagulant (warfarin, dabigatran, rivaroxaban, apixaban or edoxaban) within 180 days prior to, or on, the index date. Patients who were exposed to both drugs of interest (rivaroxaban and aspirin) on the index date were also excluded (Figure 1). Patients who used non-aspirin antiplatelet drugs at baseline were included, but this was controlled in propensity score modelling (eg, patients prescribed rivaroxaban plus clopidogrel or aspirin plus clopidogrel were allocated to rivaroxaban or aspirin groups, respectively).
2.3 Outcome and cohort follow-up
The study outcome of interest was GIB which included peptic, duodenal, gastrojejunal ulcers with haemorrhage, bleeding gastritis or duodentitis, gastrointestinal haemorrhage and intestinal haemorrhage (Table S1). Previous data validation has shown high coding accuracy for GIB in CDARS (positive predicted value [PPV] of 100%).10 Validation of GIB in UK primary care demonstrated PPV of 99%,13 and GIB Read codes have a 84 to 86% confirmation rate in THIN, specifically.12
Patient follow-up commenced from the index date until GIB occurrence, 30 days after treatment discontinuation or treatment switch (to aspirin or any anticoagulant), death, transfer out of the practice (UK patients), or study end (December 31, 2018, for CDARS analysis and September 26, 2017, for THIN analysis), whichever came first (Figure S1). Treatment discontinuation was determined by the time when patients failed to refill prescriptions within the allowable refill gap. The allowable refill gap was defined as the 95% percentile of the refill gaps observed in each treatment group (CDARS: 31 days for rivaroxaban and 29 days for aspirin; THIN: 5 days for rivaroxaban and 14 days for aspirin).14
2.4 Covariates
We included age at baseline, sex, index year and the confounders listed in Table S1 as covariates. Missing data in THIN for smoking status (0.2%), alcohol consumption status (6.8%) and body mass index (BMI) (5.9%) was addressed by multiple imputation (MI) using the fully conditional specification algorithm which created 25 imputed datasets. Twenty-five imputed datasets were selected as it is the default within the PROC MI procedure in SAS, it is also more than the suggested number (between 3 and 1015), is at least equal to the percentage of incomplete cases,16 and therefore is more likely to enhance precision in effect estimates.17 To minimize bias and enhance MI precision estimates, all potential confounders outlined were included in the MI model, as well as GIB outcomes and survival time.18
2.5 Statistical analysis
Statistical analyses were separately conducted in CDARS and THIN, using a common protocol approach. We used the propensity-score (PS) fine stratification weighting19 to control for confounding. The PS stratification with fine strata results in better covariate balance and increased precision of estimated treatment effects with low exposure prevalence and similar results at relatively higher exposure prevalence compared to other PS methods.19 Given the low rivaroxaban prevalence in CDARS (3.7%) and relatively higher rivaroxaban prevalence in THIN (43%), this was an optimal method for use in both cohorts. PS was estimated as the predicted probability of receiving rivaroxaban, conditional on covariates outlined, using logistic regression. For each study sample, the fine stratification weight was calculated by creating 50 PS strata and ranked rivaroxaban patients based on their PS. Aspirin users were then assigned to these strata based on their PS. Non-overlapping areas of PS distributions among treatment groups were trimmed. After stratification, weighted regression models were used, in which each rivaroxaban-treated patient received a weight of 1, and aspirin users were weighted in proportion to the distribution of the exposed stratum in which they were assigned.19 GIB risk between rivaroxaban and aspirin users was compared using Cox proportional hazards regression based on the weighted populations and presented using Hazard Ratios (HR) with 95% confidence intervals (CI). In THIN, analyses were undertaken separately for each imputed dataset (n = 25) and results were combined using Rubin's rule with PROC MIANALYZE in SAS (v9.4).18
Standardized differences were used to assess covariate balance. A threshold of 0.1 was considered negligible.20 Baseline characteristics were presented as frequencies, percentages or medians (interquartile ranges [IQR]). A two-sided P-value of <.05 was considered statistically significant. Cohort construction was conducted separately in CDARS and in THIN. Statistical analyses were independently conducted on both datasets by two study authors for quality assurance. All data preparation and analyses were undertaken using SAS 9.4 (SAS Institute Inc, Cary, NC).
2.6 Additional analyses
Several additional analyses were conducted to test robustness of results: we accounted for the dosage effect of rivaroxaban by stratifying analyses by the standard daily dose of rivaroxaban (20 mg/day) and reduced daily dose of rivaroxaban (≤15 mg/day). As anticoagulants or aspirin can exacerbate bleeding in underlying GI lesions,21 patients with a prior history of peptic ulcer or GIB, and patients receiving gastric acid suppression therapy (proton pump inhibitor [PPI] or histamine type-2 Receptor Antagonist [H2RA]) were separately analysed. Previous data indicate women have increased DOAC-related GIB risk compared to men,22 therefore sex-specific analyses were conducted. Increasing age can also increase GIB risk,23 therefore, analyses in patients aged ≥75 years were undertaken. Finally, the maximum allowable refill gap was extended to 99th percentile of gaps in both cohorts.14
3 RESULTS
3.1 Patient characteristics
Following patient exclusions, the CDARS cohort comprised 28 161 aspirin initiators and 1052 rivaroxaban initiators; and THIN comprised 8053 aspirin initiators and 3496 rivaroxaban initiators (Figure 1). The median (IQR) follow-up time was 209 (49-864) days in CDARS and 174 (86-422) days in THIN.
Median age of the CDARS cohort was 78 years and 14 721 (50%) were women. In THIN, the median age was 74 years and 5377 (47%) were women. Before propensity score modelling, there were differences in the presence of heart failure, vascular and renal disease, and use of ACEI/ARB, beta-blockers, H2RAs and statins between new users of rivaroxaban and aspirin in CDARS; and differences for hypertension, diabetes, prior ischaemic stroke/transient ischaemic attack/systemic embolism, renal disease, and concurrent use of antiplatelet drugs, calcium channel blockers and statins in THIN. After propensity score modelling, both cohorts were well balanced across covariates (standardized differences <0.1). Low-dose rivaroxaban (≤15 mg/day) was used in 27% of patients in CDARS, compared to 17% in THIN (Table 1). For all patients, low-dose aspirin in Hong Kong comprised 80 mg/day and 75 mg/day was used in the United Kingdom.
| CDARS | THINb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Propensity-score-adjusteda | Unadjusted | Propensity-score-adjusteda | |||||||||
| Rivaroxaban | Aspirin | STDc | Rivaroxaban | Aspirin | STDc | Rivaroxaban | Aspirin | STDc | Rivaroxaban | Aspirin | STDc | |
| N = 1052 | N = 28 161 | N = 1051 | N = 22 692 | N = 3496 | N = 8053 | N = 3492 | N = 6619 | |||||
| Age (years) Median (IQR) | 76 (67-82) | 78 (67-85) | 0.16 | 76 (67–82) | 77 (66-83) | 0.02 | 75 (66-82) | 74 (65-83) | 0.04 | 75 (67-82) | 74 (66-82) | <0.01 |
| Female, n (%) | 522 (50) | 13 970 (50) | <0.01 | 522 (50) | 11 281 (50) | <0.01 | 1660 (48) | 3716 (46) | 0.03 | 1657 (48) | 3053 (46) | 0.03 |
| Comorbidities at baseline, n (%) | ||||||||||||
| Congestive cardiac failure | 158 (15) | 6075 (22) | 0.17 | 157 (15) | 3335 (15) | 0.01 | 337 (10) | 565 (7) | 0.01 | 336 (10) | 742 (11) | 0.05 |
| Hypertension | 456 (43) | 12 199 (43) | <0.01 | 456 (43) | 9959 (44) | 0.01 | 2067 (59) | 4242 (53) | 0.13 | 2063 (59) | 3994 (60) | 0.03 |
| Diabetes | 161 (15) | 4800 (17) | 0.05 | 161 (15) | 3457 (15) | <0.01 | 534 (15) | 911 (11.3) | 0.12 | 533 (15) | 1065 (16) | 0.02 |
| Prior ischemic stroke/TIA/SE | 119 (11) | 4186 (15) | 0.01 | 119 (11) | 2739 (12.1) | 0.02 | 557 (16) | 394 (4.9) | 0.37 | 553 (16) | 1123 (17) | 0.03 |
| Vascular disease | 87 (8) | 4065 (14) | 0.20 | 87 (8) | 1873 (8.3) | <0.01 | 217 (6) | 641 (8) | 0.07 | 217 (6) | 467 (7) | 0.03 |
| History of peptic ulcer/gastrointestinal bleeding | 121 (12) | 4593 (16) | 0.14 | 121 (12) | 2641 (12) | <0.01 | 574 (16) | 1169 (15) | 0.05 | 574 (16) | 1043 (16) | 0.02 |
| Renal disease | 36 (3) | 2721 (10) | 0.25 | 36 (3) | 783 (3) | <0.01 | 277 (8) | 897 (11) | 0.11 | 274 (8) | 565 (9) | 0.03 |
| Liver disease | 35 (3) | 1516 (5) | 0.10 | 35 (3) | 792 (3) | 0.01 | 31 (0.9) | 56 (0.7) | 0.02 | 31 (0.9) | 51 (0.8) | 0.01 |
| Smokingb | ||||||||||||
| Never smoked | - | - | - | - | - | - | 1896 (54) | 4447 (55.2) | 0.02 | 1894 (54) | 3525 (53) | 0.02 |
| Current smoker | - | - | - | - | - | - | 314 (9) | 803 (10) | 0.03 | 314 (9) | 664 (10) | 0.04 |
| Ex-smoker | - | - | - | - | - | - | 1286 (37) | 2803 (35) | 0.04 | 1284 (37) | 2430 (37) | <0.01 |
| Alcohol use (drinking status)b | ||||||||||||
| Never drinker | - | - | - | - | - | - | 722 (21) | 1601 (20) | 0.02 | 720 (21) | 1364 (21) | <0.01 |
| Current drinker | - | - | - | - | - | - | 2639 (76) | 6166 (77) | 0.03 | 2637 (76) | 4992 (76) | <0.01 |
| Ex-drinker | - | - | - | - | - | - | 135 (4) | 286 (4) | 0.02 | 135 (4) | 263 (4) | <0.01 |
| Body Mass Index, kg/mb | ||||||||||||
| <20 | - | - | - | - | - | - | 148 (4) | 414 (5) | 0.04 | 148 (4) | 294 (4) | 0.01 |
| 20-24 | - | - | - | - | - | - | 853 (24) | 2061 (26) | 0.03 | 851 (24) | 1539 (23) | 0.03 |
| 25-30 | - | - | - | - | - | - | 1464 (42) | 3514 (44) | 0.04 | 1464 (42) | 2855 (43) | 0.02 |
| >30 | - | - | - | - | - | - | 1031 (30) | 2064 (26) | 0.09 | 1029 (30) | 1931 (29) | <0.01 |
| Medications at baseline, n (%) | ||||||||||||
| Antiplatelet drugs | 66 (6) | 1663 (6) | 0.02 | 66 (6) | 1490 (7) | 0.01 | 504 (14) | 554 (7) | 0.25 | 501 (14) | 956 (14) | <0.01 |
| ACEI or ARB | 444 (42) | 8285 (29) | 0.27 | 443 (42) | 9455 (42) | 0.01 | 533 (15) | 938 (12) | 0.11 | 532 (15) | 955 (14) | 0.02 |
| Beta blocker | 582 (55) | 11 678 (42) | 0.28 | 581 (55) | 12 593 (56) | <0.01 | 2228 (64) | 4754 (59) | 0.01 | 2224 (64) | 4241 (64) | <0.01 |
| Calcium channel blocker | 607 (58) | 13 930 (50) | 0.17 | 607 (58) | 13 161 (58) | 0.01 | 1199 (34) | 2358 (29) | 0.11 | 1196 (34) | 2263 (34) | <0.01 |
| Loop diuretics | 225 (21) | 7037 (25) | 0.09 | 224 (21) | 4773 (21) | 0.01 | 783 (22) | 1581 (20) | 0.068 | 783 (22) | 1520 (23) | 0.01 |
| Amiodarone or dronedarone | 154 (15) | 3720 (13) | 0.04 | 154 (15) | 3342 (15) | <0.01 | 84 (2) | 154 (2) | 0.03 | 83 (2) | 177 (3) | 0.02 |
| NSAIDs | 59 (6) | 1712 (6) | 0.02 | 58 (6) | 1347 (6) | 0.02 | 438 (13) | 1133 (14) | 0.05 | 436 (13) | 827 (13) | <0.01 |
| Histamine type-2 receptor antagonists | 214 (20) | 17 548 (62) | 0.94 | 214 (20) | 4508 (20) | 0.01 | 138 (4) | 263 (3) | 0.04 | 138 (4) | 261 (4) | <0.01 |
| Proton pump inhibitors | 219 (21) | 6660 (24) | 0.07 | 219 (21) | 4540 (20) | 0.02 | 1301 (37) | 3056 (40) | 0.02 | 1299 (37) | 2614 (39) | 0.05 |
| SSRIs | 29 (3) | 579 (2) | 0.05 | 29 (3) | 598 (3) | 0.01 | 308 (9) | 612 (8) | 0.04 | 308 (9) | 639 (10) | 0.03 |
| Statins | 421 (40) | 6354 (23) | 0.38 | 420 (40) | 9255 (40.8) | 0.02 | 1465 (42) | 2665 (33) | 0.18 | 1461 (42) | 2926 (44) | 0.05 |
| CHA2DS2-VAScd | ||||||||||||
| Median (IQR) | 3 (2–4) | 3 (2–4) | 0.01 | 3 (2–4) | 3 (2–4) | <0.01 | 3 (2-4) | 3 (1-4) | 0.02 | 3 (2-4) | 3 (2-4) | <0.01 |
| 0-1 | 75 (31) | 2911 (32) | 195 (19) | 4288 (19) | 655 (19) | 2033 (25) | 655 (19) | 1224 (18) | ||||
| 2–3 | 19 (8) | 709 (8) | 512 (49) | 10 871 (48) | 1519 (43) | 3669 (46) | 1518 (44) | 2786 (42) | ||||
| ≥4 | 146 (61) | 5603 (61) | 344 (33) | 7533 (33) | 1322 (38) | 2351 (29) | 1319 (38) | 2609 (39) | ||||
| HAS-BLEDe | ||||||||||||
| Median (IQR) | 2 (1-2) | 2 (1-3) | 0.01 | 2 (1-2) | 2 (1-2) | 0.02 | 2 (1-3) | 2 (1-3) | 0.02 | 2 (1-3) | 2 (1-3) | <0.01 |
| ≥3 | 85 (35) | 3316 (36) | 243 (23) | 5426 (24) | 1194 (34) | 2171 (41) | 1190 (34) | 2396 (36) | ||||
| Low dose rivaroxaban, n (%)f | 285 (27) | - | - | 285 (27) | - | - | 598 (17) | - | - | 597 (17) | - | - |
- Abbreviations: A2RB, angiotensin-2 receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs; SE, systemic embolism; SSRIs, selective serotonin reuptake inhibitors; STD, standardized difference; TIA, transient ischemic attack.
- a Propensity score was estimated as the predicted probability of receiving rivaroxaban (exposed) vs aspirin (unexposed), conditional on the covariates described above and the index year. Patients with propensity scores that fall outside the ranges common to both the exposed and unexposed group were trimmed, and 50 strata were created based on the distribution of propensity scores among the exposed group. Weights for the unexposed observations were derived based on the number of exposed subjects in the respective stratum and were used to estimate the adjusted baseline characteristics and hazard ratios for the outcomes.
- b Propensity score analysis was performed for each of the 25 imputed datasets. The absolute standardized difference of all covariates was <0.1 for all 25 imputed datasets after propensity score fine strata adjustment. Patient characteristics of one imputed dataset are shown (of which the result yielded the least SE in the primary analysis).
- c STD = the absolute difference in mean or proportion in rivaroxaban group vs aspirin group divided by the pooled SD. A standardized difference ≤0.1 indicates a negligible difference in covariates between treatment groups.
- d CHA2DS2-VASc indicates patients with congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes mellitus, age 65 to 74 years, prior stroke or TIA or systemic embolism (doubled), vascular disease, and sex category (women). CHA2DS2-VASc score ranges from 0 to 9 (higher score indicates higher stroke risk).
- e HAS-BLED indicates patients with hypertension, renal disease, liver disease, stroke history, prior major bleeding or predisposition to bleeding, labile INR (not measured here), age > 65 years, medication use that predisposes to bleeding (clopidogrel, NSAIDs), high alcohol intake. HAS-BLED scores range from 0 to 9 (higher score indicates higher bleeding risk).
- f Low dose rivaroxaban includes patients prescribed ≤15 mg daily.
3.2 Risk of gastrointestinal bleeding
Crude GIB rates were 3.0 and 2.6 per 100 patient-years, for aspirin and rivaroxaban users, respectively, in CDARS; and 1.3 and 2.4 per 100 patient-years, respectively, in THIN. In both cohorts, the adjusted GIB event rate was higher in patients with a history of GIB or ulcer (CDARS: 5.3 [aspirin] and 5.8 [rivaroxaban] per 100 patient-years; THIN: 4.8 [aspirin] and 4.8 [rivaroxaban] per 100 patient-years), and in patients aged ≥75 years (CDARS: 4.1 [aspirin] and 3.2 [rivaroxaban] per 100 patient-years; THIN: 2.5 [aspirin] and 3.2 [rivaroxaban] per 100 patient-years) (Table 2).
| Aspirin | Rivaroxaban | Rivaroxaban vs Aspirin | ||||
|---|---|---|---|---|---|---|
| N | Crude no. of events (crude event rate per 100 patient-years) | N | Crude no. of events (crude event rate per 100 patient-years) | Crude Hazard Ratio (95%CI) | P-value | |
| GIB | ||||||
| CDARS | 28 161 | 1329 (3.0) | 1052 | 42 (2.6) | 0.83 (0.61-1.13) | .228 |
| THIN | 8053 | 85 (1.3) | 3496 | 85 (2.4) | 1.98 (1.46-2.69)* | <.001 |
| Prior use of PPI or H2RA | ||||||
| CDARS | 22 395 | 1066 (3.1) | 197 | 16 (2.8) | 0.86 (0.52-1.40) | .538 |
| THIN | 3225 | 41 (1.5) | 1387 | 45 (3.3) | 2.25 (1.47-3.44)* | <.001 |
| Prior history GIB or GI ulcer | ||||||
| CDARS | 4593 | 429 (6.9) | 121 | 9 (6.2) | 0.82 (0.42-1.59) | .558 |
| THIN | 1169 | 23 (2.3) | 574 | 24 (4.4) | 1.94 (1.09-3.44)* | .024 |
| Women | ||||||
| CDARS | 13 970 | 725 (3.3) | 522 | 16 (1.9) | 0.56 (0.34-0.92)* | .023 |
| THIN | 3716 | 40 (1.3) | 1660 | 45 (2.7) | 2.17 (1.41-3.33)* | <.001 |
| Men | ||||||
| CDARS | 14 191 | 604 (2.7) | 530 | 26 (3.3) | 1.16 (0.78-1.72) | .466 |
| THIN | 3776 | 45 (1.2) | 1832 | 40 (2.2) | 1.80 (1.17-2.77) | .007 |
| Age ≥ 75 years | ||||||
| CDARS | 16 905 | 1053 (4.1) | 565 | 27 (3.2) | 0.74 (0.51-1.09) | .129 |
| THIN | 3745 | 51 (1.6) | 877 | 54 (3.2) | 1.93 (1.31-2.83)* | .001 |
| Extended treatment gap | ||||||
| CDARS | 28 161 | 1534 (2.9) | 1052 | 45 (2.5) | 0.80 (0.59-1.07) | .138 |
| THIN | 8053 | 110 (1.3) | 3496 | 99 (2.2) | 1.75 (1.33-2.30)* | <.001 |
- Abbreviations: CDARS, Clinical Data Analysis Reporting System; CI, confidence interval; GI, gastrointestinal; GIB, gastrointestinal bleeding; H2RA, histamine-2 receptor antagonist; no., number; PPI, proton pump inhibitor; THIN, The Health Improvement Network.
- * P < .05.
In the propensity score adjusted samples, median time to GIB after first prescription for aspirin was 182 (IQR = 44-783) days in CDARS and 146 (IQR = 86-339) days in THIN; and was 408 (IQR = 108-905) days for rivaroxaban in CDARS and 254 (IQR = 122-525) days in THIN. Results for Cox proportional hazards regression analysis showed no difference in overall GIB risk between rivaroxaban or aspirin initiators in both cohorts (HR = 1.04, 95%CI = 0.76-1.42[CDARS]; 1.40, 1.00-1.98[THIN]). For additional analyses, the HR point estimates for CDARS and THIN tended to be in different directions, but overall, no statistically significant differences in GIB risks were observed for additional analyses, including prior use of PPIs or H2RAs (HR = 0.99, 95%CI = 0.60-1.62[CDARS]; 1.66, 0.92-2.98[THIN]), history of GI bleeding or ulcer (HR = 1.12, 95%CI = 0.55-2.28[CDARS]; 1.26, 0.61-2.61[THIN]), sex-specific analyses of women (HR = 0.72, 95%CI = 0.44-1.20[CDARS]; 1.50, 0.90-2.51[THIN]), increasing age (≥75 years) (HR = 0.84, 95%CI = 0.57-1.24[CDARS]; 1.28, 0.83-1.98[THIN]), or for an extended refill gap (HR = 0.96, 95%CI = 0.71-1.31[CDARS]; 1.31, 0.96-1.79[THIN]). Rivaroxaban use was significantly associated with increased GIB risk in men in CDARS (HR = 1.52, 95%CI = 1.02-2.27) (P for interaction with sex = .02), however, no significant difference in GIB risk was found for men in THIN (HR = 1.67, 95%CI = 0.99-2.82) (P for interaction with sex = .77) (Table 3).
| Aspirin | Rivaroxaban | Rivaroxaban vs aspirin | ||||
|---|---|---|---|---|---|---|
| N | Adjusted no. of events (adjusted event rate per 100 patient-years) | N | Adjusted no. of events (adjusted event rate per 100 patient-years) | Adjusted Hazard Ratio (95%CI) | P-value | |
| GIB | ||||||
| CDARS | 22 692 | 622 (2.7) | 1051 | 41 (2.5) | 1.04 (0.76-1.42) | .815 |
| THIN | 6619 | 75 (2.0) | 3492 | 84 (2.4) | 1.40 (1.00-1.98) | .052 |
| Prior use of PPI or H2RA | ||||||
| CDARS | 15 993 | 509 (3.0) | 393 | 16 (2.8) | 0.99 (0.60-1.62) | .958 |
| THIN | 2334 | 29 (2.2) | 1381 | 45 (3.3) | 1.66 (0.92-2.98) | .091 |
| Prior history GIB or GI ulcer | ||||||
| CDARS | 2602 | 139 (5.3) | 116 | 8 (5.8) | 1.12 (0.55-2.28) | .760 |
| THIN | 986 | 25 (4.8) | 523 | 24 (4.8) | 1.26 (0.61-2.61) | .528 |
| Women | ||||||
| CDARS | 9014 | 265 (2.9) | 521 | 16 (1.9) | 0.72 (0.44-1.20) | .206 |
| THIN | 2937 | 28 (1.8) | 1645 | 43 (2.6) | 1.50 (0.90-2.51) | .122 |
| Men | ||||||
| CDARS | 11 223 | 289 (2.4) | 528 | 26 (3.3) | 1.52 (1.02-2.27)* | .042 |
| THIN | 4337 | 33 (1.5) | 1836 | 40 (2.2) | 1.67 (0.99-2.82) | .053 |
| Age ≥ 75 years | ||||||
| CDARS | 10 712 | 445 (4.1) | 564 | 27 (3.2) | 0.84 (0.57-1.24) | .384 |
| THIN | 2979 | 41 (2.5) | 1736 | 53 (3.2) | 1.28 (0.83-1.98) | .268 |
| Extended treatment gap | ||||||
| CDARS | 22 692 | 731 (2.7) | 1051 | 44 (2.4) | 0.96 (0.71-1.31) | .808 |
| THIN | 6619 | 84 (1.9) | 3492 | 97 (2.2) | 1.31 (0.96-1.79) | .088 |
- Abbreviations: CDARS, Clinical Data Analysis Reporting System; CI, confidence interval; GI, gastrointestinal; GIB, gastrointestinal bleeding; H2RA, histamine-2 receptor antagonist; no., number; PPI, proton pump inhibitor; THIN, The Health Improvement Network.
- a The propensity score fine strata adjustment analysis was performed individually for all 25 imputed datasets for THIN. The adjusted hazard ratios were derived by combining the results of the imputed datasets using Rubin's rule. The event rates in one of the imputed datasets are presented for illustration.
- * P < .05.
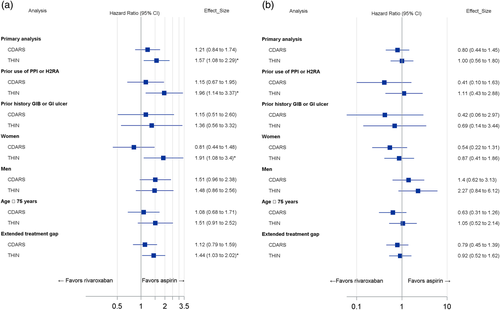
When standard dose rivaroxaban (20 mg/day) was compared to aspirin, results demonstrated significantly increased GIB risk in rivaroxaban users in THIN (HR = 1.57, 95%CI = 1.08-2.29). However, no significant difference was observed for GIB risk in CDARS for rivaroxaban 20 mg/day vs aspirin (HR = 1.21, 95%CI = 0.84-1.74). For additional analyses, standard dose rivaroxaban was also significantly associated with increased GIB risk in THIN in patients using PPIs/H2RAs (HR = 1.96, 95%CI = 1.14-3.37), in women (HR = 1.91, 95%CI = 1.08-3.40; P for interaction with sex = .53) and for an extended refill gap (HR = 1.44, 95%CI = 1.03-2.02) (Figure 2A). No differences were observed among all additional analyses for standard dose rivaroxaban vs aspirin in CDARS. Finally, no difference in GIB risk was observed for low-dose rivaroxaban (≤15 mg/day) vs aspirin in THIN or CDARS across all primary and additional analyses (Figure 2B).
4 DISCUSSION
This study compared GIB risk in patients with AF newly initiated on rivaroxaban or aspirin. CDARS and THIN data were separately analysed to explore treatment effects in different populations. We found that standard dose rivaroxaban (20 mg/day) was associated with significantly higher GIB risk compared to aspirin in the UK only. However, low-dose rivaroxaban (≤15 mg/day) had a similar GIB risk vs aspirin in both cohorts. Our results suggest that any increased GIB risk associated with rivaroxaban compared to aspirin may be dependent on total daily dose, which was exclusively observed in the UK cohort. Moreover, rivaroxaban and aspirin had more similar GIB risks when used in a population with higher baseline bleeding risk. This was demonstrated via the higher overall GIB event rate in Hong Kong compared to the UK, but no increased GIB risk was observed when comparing rivaroxaban with aspirin in CDARS. In addition, GIB rates varied between Hong Kong and UK populations which may in part be due to differences in ethnicity between these two groups of patients with AF. It was also evident that GIB presentations occurred much earlier after new treatment initiation with aspirin compared to rivaroxaban in both cohorts.
GIB plays an important role in clinical decision making.24 This present study demonstrated GIB risk may be similar for rivaroxaban ≤15 mg/day and aspirin, which is in-line with recent23 and previous work7 concerning gastrointestinal safety of antiplatelets vs other anticoagulants. Our findings suggest that aspirin should not be perceived as a safer alternative to anticoagulants with respect to GIB risks. Currently, little information exists to guide clinicians on how to assess risk-benefit trade-offs of anticoagulation. Well-managed anticoagulation provides superior efficacy in reducing stroke risk,25 which may offset similar or marginal increases in GIB risk. Previous studies have also shown that the use of gastroprotective agents may mitigate GIB risk associated with anticoagulant use.10, 26
We also observed that GIB presentations occurred much earlier for aspirin than for rivaroxaban (up to 100-200 days earlier). Earlier presentations with aspirin may be due to its direct erosive effect on the GI tract,6 in comparison to the DOACs, which presumably increase bleeding from lesions which they do not actually cause. However, the time to GIB for DOACs vs aspirin has not been investigated previously,7, 8 and more data are needed to confirm this observation.
Although the same patient selection criteria were used in both databases, the resulting cohorts were heterogenous with respect to baseline characteristics and GIB rates. First, GIB rates between Hong Kong and UK cohorts were similar for rivaroxaban (adjusted rate = 2.5 [CDARS] vs 2.4 [THIN] per 100 patient-years), however differences in GIB rates with aspirin were apparent (adjusted rate = 2.0 [CDARS] vs 2.7 [THIN] per 100 patient-years). Rate differences might be driven by a differential response to aspirin between Hong Kong and UK cohorts. It is plausible that variation in population demographics between patients with AF in Hong Kong compared to the United Kingdom could have impacted GIB rates. For example, prior to PS modelling, CDARS patients more frequently used PPIs or H2RAs compared to THIN (84% vs 41%). This may be due to greater GIB propensity in Asian populations compared to Western populations, owing to a higher prevalence of peptic ulcer disease.27 Notwithstanding the reasons, the lower GIB rate with aspirin in the UK may have ultimately driven the finding that rivaroxaban 20 mg/day appears to be associated with an increased GIB risk compared with aspirin, which was exclusively observed in the UK cohort.
The use of two databases is the strength of this study. Several reflections arose from this study that are worthy of mention and could be taken into consideration by other researchers planning to conduct multiple databases studies. In addition to the clinical heterogeneity observed, there were variable differences among CDARS and THIN databases. THIN contains additional health data such as alcohol consumption and blood pressure, whereas CDARS does not. To address this limitation, liver disease and hypertension were included as proxies to alcohol consumption and blood pressure in the CDARS population, which partially accounted for these factors. Second, the prevalence of exposure can affect the performance of PS methods in balancing covariates and precision of treatment effect estimates.19 As the prevalence of exposure varied between databases, we needed to meet a balance between efficiency and consistency of analysis methods used across databases. Given this, we opted to use the PS fine strata weighting approach.19 Compared to other PS methods, this approach has previously demonstrated better performance when the exposure is infrequent, and a comparable performance when the exposure is common.19 This was particularly important due to the lower exposure prevalence in the CDARS study population compared with THIN. Employing this method preserved the consistency of analysis methods in both study populations, which aided results interpretation.
4.1 Strengths and limitations
Our study is the first to investigate comparative GIB risk for rivaroxaban vs aspirin in patients with AF in routine care. A key strength of the current study is the utilization of longitudinal data of the largest territory-wide clinical data source in Hong Kong and representative data of the United Kingdom. Use of two different data sources enabled broad investigation of GIB risk in diverse patient groups. Study limitations include: GIB event rates in both cohorts were small, therefore results should be interpreted with caution. Consistent with most clinical databases, CDARS and THIN does not capture over-the-counter aspirin use. However, the Hospital Authority is the only public healthcare provider in Hong Kong, where medications are highly subsidized (85%-98%).28 Similarly, in the United Kingdom, most chronic aspirin use is obtained via prescription as it is free for patients aged >60 years, and there is confirmed minimal unrecorded usage.29 Ultimately, uncaptured over-the-counter aspirin use is unlikely to have impacted results. This study accounted for confounding factors using propensity score methods; however, residual confounding could remain. Finally, we focused our analysis on a new-user cohort, GIB risk may be different among prevalent users.
5 CONCLUSION
This study found a significantly increased GIB risk for standard dose rivaroxaban (20 mg/day) vs aspirin in the United Kingdom, but not in Hong Kong. Low-dose rivaroxaban (≤15 mg/day) demonstrated similar GIB risk in both cohorts. Results suggest that any increased GIB risk associated with standard dose rivaroxaban remains uncertain and more studies are needed to investigate this further. Nonetheless, the superior efficacy of rivaroxaban over aspirin for stroke prevention in AF should offset similar or marginally increased GIB risk of rivaroxaban.
ACKNOWLEDGEMENTS
Work involving Hong Kong data was supported by an education grant from Bayer Hong Kong Limited. The funder had no role in the design and conduct of the study, in the analysis, or results interpretation, or in the preparation or review of the manuscript. Laura Fanning is supported by a Research and Training PhD Scholarship from the Australian Government. The collaboration between the University of Hong Kong (HKU) and University College London (UCL) is supported by the HKU-UCL Strategic Partnership Fund.
CONFLICT OF INTEREST
ICKW has received research grants outside the submitted work from Hong Kong Research Grant Council, the Hong Kong Health and Medical Research Fund, Pfizer, BMS, Janssen, Novartis and Bayer. Dr Chan reports grants from Research Grants Council (RGC, Hong Kong), grants from Narcotics Division of the Security Bureau of the Government of the Hong Kong SAR, grants from Research Fund Secretariat of the Food and Health Bureau, grants from National Natural Science Fund of China, grants from National Health and Medical Research Council (NHMRC, Australia), grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda, personal fees (speaker fee) from Hospital Authority of Hong Kong, outside the submitted work. Kenneth K. C. Man received a personal fee from IQVIA Ltd, outside of the submitted work. All other authors have reported no conflicts relevant to this paper.




