Late Mortality Among Survivors of Childhood Cancer in Canada: A Retrospective Cohort Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Children with cancer face an increased risk of complications and death beyond the 5-year survival mark. National surveillance efforts facilitate the systematic tracking of long-term health outcomes, including treatment-related complications and late mortality, among childhood cancer survivors. We aimed to describe the population of 5-year childhood cancer survivors in Canada, quantify the risk of death among survivors relative to the general population, and identify characteristics associated with late mortality.
Methods
This retrospective cohort study used the Canadian Cancer Registry linked to the Canadian Vital Statistics-Death database (excludes Quebec). Survivors were diagnosed with cancer before 15 years old (1992–2012) and still alive five years after diagnosis. We approximated the risk of late mortality relative to the general population using standardized mortality ratios (SMRs) and absolute excess ratios (AERs). Cumulative all-cause and cause-specific mortality and time-to-event models identified characteristics associated with late mortality.
Results
Of the 10,800 5-year survivors, 405 (4%) had a late death by 2017 (median follow-up: 9.1 years). Cancer recurrence or progression caused most late deaths (64%), followed by subsequent primary neoplasms (11%) and other health-related causes (15%). Survivors had a higher risk of all-cause mortality than the general population (SMR = 9.4; 95% CI = 8.5–10.4; AER = 34.8, 95% CI = 30.8–38.8). Risk was highest in the first 5–9 years of follow-up. Cumulative mortality differed significantly by age at diagnosis, sex and cancer type.
Interpretation
Our results underline the importance of long-term surveillance of childhood cancer survivors, as mortality rates remain higher than the general population for at least two decades after diagnosis.
Abbreviations
-
- 95% CI
-
- 95% confidence interval
-
- AER
-
- Absolute excess ratio
-
- ALL
-
- Acute lymphoblastic leukaemia
-
- CCR
-
- Canadian Cancer Registry
-
- CI
-
- Confidence interval
-
- CNS
-
- Central nervous system
-
- HR
-
- Hazard ratio
-
- ICCC
-
- International Classification of Childhood Cancer
-
- ICD
-
- International Classification of Diseases
-
- PCCF+
-
- Postal Code Conversion File Plus
-
- PHAC
-
- Public Health Agency of Canada
-
- SMR
-
- Standardized mortality ratio
-
- SPN
-
- Subsequent primary neoplasm
1 Introduction
In Canada, the 5-year survival rate for children with cancer now exceeds 86% [1], largely due to improvements in treatment. Despite these encouraging gains, survivors of childhood cancer continue to experience morbidity and mortality from their diagnosis and treatment [2, 3]. Indeed, previous studies report survivors’ increased risk of second cancers [4-8], poor mental health [9-11], cardiovascular problems [12-16], respiratory problems [17-20], endocrine disorders and poor reproductive outcomes [21, 22], other chronic health challenges [3, 23-25], and late mortality [2, 4, 6, 8, 12, 14, 17, 18, 26-46].
Specifically, studies have reported that the risk of late mortality, typically defined as a death occurring more than 5 years after diagnosis, is 6-fold to 17-fold more than that expected from the general population [44]. However, as substantial advancements have been made in both the treatment of childhood cancer to improve cure rates and risk stratification and long-term supportive care to minimize late effects, the risk of late mortality in past studies may no longer reflect the true burden in contemporary survivors.
Previous studies have varied in their follow-up periods, with a median end of follow-up around 2012. While a recent study from the United States extended follow-up to 2017, it examined a historical cohort diagnosed between 1970 and 1999 [44]. In contrast, our study provides a more current perspective by extending the follow-up period to 2017 while focusing on a more contemporary cohort diagnosed from 1999 to 2012. This approach allows us to capture more recent trends in long-term outcomes.
Further, while studies have been undertaken in Europe, Australia and the United States, no pan-Canadian estimates are currently available for late mortality. In this study, late mortality among contemporary 5-year survivors of childhood cancer in Canada (excluding Quebec) was investigated.
2 Methods
2.1 Study Population
This retrospective, population-based cohort includes Canadian children with a first cancer diagnosis prior to the age of 15 years between January 1, 1992, and December 31, 2012, who survived at least 5 years, excluding cases in Ontario and the Yukon from 2016–2017 and all cases from Quebec due to data unavailability. The Canadian Cancer Registry (CCR), linked to the Canadian Vital Statistics-Death (CVSD) database for share file diagnosis years 1992–1997 provided to the Public Health Agency of Canada (PHAC), was used as the data source. The CCR and the CVSD have wide coverage and rigorous data validation processes [47, 50] and the CCR is among the highest-quality national population-based cancer registries in the world [100]. Since each Canadian province and territory has a legislated responsibility for cancer collection and control, case ascertainment is considered relatively good [47]. The CCR-CVSD PHAC share file does not include Quebec (1992–2017), Ontario (2016–2017) and Yukon (2016–2017) data as these death records are not part of the linked file. The linked file 1992–2017 holds Ontario and Yukon records in which the Cancer Registry variables are populated, while the corresponding death variables are set to missing for years 2016 and 2017.
All cases were identified using the CCR [49] and classified according to the International Classification of Childhood Cancer (ICCC), Third Edition [48, 49].
2.2 Death Ascertainment
Vital status was obtained via linkage with the CVSD Database [50], where follow-up began at the date of 5-year survival and continued until death or the exit date (December 31, 2015, for Ontario/Yukon or December 31, 2017). Deaths were classified using the International Classification of Diseases revision in use at the time of death and grouped broadly into neoplasms, external causes and other health-related diseases. Where appropriate, neoplastic deaths were further disaggregated into deaths from recurrence/progression of the primary cancer and deaths from a subsequent primary neoplasm (SPN). A diagnosis of an SPN was determined by examining the chronologic sequence number of multiple primaries for the patient, where all individuals who had an SPN and a neoplastic death were assumed to have died from their SPN [51]. Patients with only one primary cancer who experienced a neoplastic death were assumed to have died from reoccurrence/progression. External causes were disaggregated into intentional self-harm [52] and accidental injury, and other health-related causes were disaggregated into infectious, nervous, circulatory, respiratory, digestive, and all other health-related diseases [53]. Supporting Information Table S1 shows the codes used in the cause of death categorization.
2.3 Covariates of Interest
Results were stratified by sex, cancer type, age at diagnosis, diagnosis era, neighbourhood income, place of residence, SPN occurrence and attained age at study exit date. Neighbourhood income and place of residence were generated by linking the residential postal code at diagnosis with the appropriate vintage of the Postal Code Conversion File Plus (PCCF+) [54]. Residences situated in census metropolitan/agglomeration areas were classified as urban while all others were classified as ‘rural/remote’ [55].
2.4 Imputations
For persons missing dates for birth (51.8%) and diagnosis (1.3%), we imputed month and/or day using all available information such that the imputed date represented the average of all potential dates. More than 99% of the imputed dates of birth were the result of data sharing agreements prohibiting the release of the day of birth; however, the year and month of birth were available.
2.5 Statistical Analysis
Standardized mortality ratios (SMRs) and absolute excess risks (AERs) quantified the risk of mortality in this cohort relative to the age-, year- and sex-matched Canadian general population [56], using standard cohort techniques [57]. The SMR is the ratio of the observed to the expected number of deaths, whereas the AER is the difference between the number of observed and expected deaths divided by the person-years at risk since the 5-year survival mark, multiplied by 10,000. We used likelihood ratio tests comparing multivariate Poisson regression models adjusting for the simultaneous effects of sex, cancer type and age at diagnosis, to test for significant heterogeneity or a significant linear trend in the adjusted SMRs for each explanatory factor. Cox proportional hazards modelling determined the effects of several characteristics on all-cause mortality. Fine and Gray's proportional hazards modelling evaluated the effects on cause-specific late mortality, while adjusting for competing risks [58]. Causes of death other than the one of interest were treated as competing risks. Variables tested included sex, age at diagnosis, cancer type, SPN prior to the index date, SPN after the index date (time-dependent variable), diagnosis era, place of residence and neighbourhood income. The index date is the five-year anniversary of the individual's first cancer diagnosis. Variables with parameter estimates that statistically differed from zero at a significance level of α = 0.05 (Wald test) were included in the multivariate models, with sex, age and cancer type included a priori in all models. For analyses including neighbourhood income or place of residence, 3% of individuals were excluded due to missing postal codes. No independent variables violated the proportional hazards assumption. Finally, cumulative mortality was estimated by time since diagnosis, where causes of death other than the one of interest were treated as competing risks.
2.6 Disclosure Control
A disclosure control strategy is mandatory for the use of both cancer and vital statistics data in Canada. We rounded all counts of people and deaths to a multiple of 5 using unbiased random rounding and suppressed counts under 5 according to the CCR disclosure control protocol [47]. As a result, counts may not sum to total due to rounding. We calculated crude proportions with rounded counts. SMRs, AERs, regression models and cumulative mortality estimates use unrounded counts.
3 Results
Of the 13,390 children in Canada (excluding Quebec) diagnosed in the study period, 10,800 (80.6%) were 5-year survivors meeting the inclusion criteria in this study (Table 1). Fifty-four (54%) were male, leukaemia diagnoses accounted for 35% of cases, and the median age at diagnosis was 5.6 years old. Over the study follow-up (range: 0–21.0 years; median: 9.1 years; total person-years: 101,337), 405 (4%) survivors died. Supporting Information Table S2 describes the characteristics of 5-year survivors, by time since diagnosis and vital status.
| Characteristic | Vital status | |||
|---|---|---|---|---|
| Total | Died | |||
| n | % | nα | % | |
| Total | 10,800 | 100% | 405 | 100% |
| Sex | ||||
| Male | 5790 | 54% | 255 | 63% |
| Female | 5010 | 46% | 150 | 37% |
| ICCC group† | ||||
| I. Leukaemia | ||||
| ALL | 3120 | 29% | 100 | 25% |
| AML | 400 | 4% | 10 | 2% |
| Other leukemias | 200 | 2% | 10 | 2% |
| II. Lymphoma | ||||
| Hodgkin | 535 | 5% | 10 | 2% |
| Non-Hodgkin | 405 | 4% | 20 | 5% |
| Other lymphomas | 405 | 4% | 5 | 1% |
| III. CNS | ||||
| Astrocytoma | 1030 | 10% | 40 | 10% |
| Embryonal brain tumours | 390 | 4% | 50 | 12% |
| Other CNS | 480 | 4% | 30 | 7% |
| IV. Peripheral nervous cell/neuroblastoma | 675 | 6% | 40 | 10% |
| V. Retinoblastoma | 320 | 3% | <5 | n/a |
| VI. Renal | 655 | 6% | 15 | 4% |
| VII. Hepatic | 165 | 2% | 5 | 1% |
| VIII. Bone | 415 | 4% | 20 | 5% |
| IX. Soft tissue | 580 | 5% | 30 | 7% |
| X. Germ cell | 400 | 4% | 5 | 1% |
| XI. Carcinomas | 450 | 4% | 15 | 4% |
| XII. Other | 160 | 1% | <5 | n/a |
| Age at diagnosis | ||||
| <1 year old | 955 | 9% | 20 | 5% |
| 1–4 years old | 4020 | 37% | 140 | 35% |
| 5–9 years old | 2790 | 26% | 120 | 30% |
| 10–14 years old | 3040 | 28% | 130 | 32% |
| Attained age at the study exit date | ||||
| 5–9 years old | 840 | 8% | 60 | 15% |
| 10–14 years old | 1670 | 15% | 95 | 23% |
| 15–19 years old | 2355 | 22% | 110 | 27% |
| 20–24 years old | 2680 | 25% | 85 | 21% |
| 25–29 years old | 1945 | 18% | 35 | 9% |
| 30–34 years old | 990 | 9% | 10 | 2% |
| 35–40 years old | 325 | 3% | 5 | 1% |
| SPN | ||||
| No SPN | 10,555 | 98% | 355 | 88% |
| SPN(s) | 245 | 2% | 50 | 12% |
| Diagnosis era | ||||
| Early (1992–1999) | 4125 | 38% | 250 | 62% |
| Late (2000–2012) | 6670 | 62% | 150 | 37% |
| Neighbourhood income* | ||||
| Very high/high income | 4380 | 41% | 160 | 40% |
| Middle/low/very low income | 6145 | 57% | 235 | 58% |
| Place of residence* | ||||
| Urban | 8455 | 78% | 300 | 74% |
| Rural/remote | 2135 | 20% | 100 | 25% |
| Time since diagnosis | ||||
| 5–9 years | 3155 | 29% | 235 | 58% |
| 10–14 years | 2690 | 25% | 95 | 23% |
| 15–19 years | 2585 | 24% | 45 | 11% |
| 20–26 years | 2365 | 22% | 25 | 6% |
- Abbreviations: ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukemias; CNS, central nervous system; ICCC, International Classification of Childhood Cancer; SPN, subsequent primary neoplasm.
- n/a, Not applicable.
- * Missing and invalid postal codes were excluded (<3%).
- α Counts under 5 and corresponding percentages are suppressed.
- † See Supporting Information Table S3 for the unabbreviated main diagnostic groups and subtypes.
- Note. For confidentiality, we randomly rounded the number of people and deaths using an unbiased random rounding scheme with a base of five. Counts may not sum to the total due to random rounding.
Most deaths were attributed to neoplasms (n = 310; 76%), including 260 from recurrence/progression (64%). A small proportion of survivors experienced an SPN (n = 245, 2%), of which 20% died. Other health-related causes (n = 60; 15%) included circulatory (n = 10), respiratory (n = 10), nervous system (n = 10) and infectious (n = 5) diseases. The remainder of deaths were externally caused (9%), including accidents (n = 25) and intentional self-harm (n = 5).
Canadian survivors of childhood cancer had a ninefold increased risk of death (SMR = 9.4; 95% CI = 8.5–10.4) (Table 2), equating to 36 excess deaths per 10,000 person-years. Late mortality varied by cancer type, although most had significantly higher mortality than that expected.
| Variables | Person-years | Observed deaths α | SMR (95% CI) | RSMR (95% CI) | AER (95% CI) |
|---|---|---|---|---|---|
| Total | 101,337 | 405 | 9.4 (8.5–10.4) | n/a | 35.7 (31.6–39.8) |
| Sex | |||||
| Male | 54,122 | 255 | 8.4 (7.4–9.5) | 1 | 41.2 (35.1–47.3) |
| Female | 47,214 | 150 | 11.6 (9.8–13.6) | 1.3 (1.1–1.6) | 29.4 (24.1–34.8) |
| P* | <0.01 | ||||
| ICCC group† | |||||
| I. Leukaemia | 34,602 | 120 | 8.7 (7.2–10.4) | 1.4 (1.0–2.1) | 30.2 (23.7–36.7) |
| ALL | 29,302 | 100 | 8.4 (6.8–10.3) | n/a | 29.1 (22.2–36.1) |
| AML | 3673 | 10 | 8.4 (4.3–14.7) | n/a | 28.9 (9.3–48.4) |
| Other leukemias | 1650 | 10 | 13.9 (6.3–26.5) | n/a | 50.9 (14.0–87.8) |
| II. Lymphoma | 12,351 | 35 | 5.0 (3.5–7.0) | 1 | 22.7 (12.4–33.0) |
| Hodgkin | 5107 | 10 | 3.3 (1.6–6.1) | n/a | 13.5 (−0.4 to 27.4) |
| Non-Hodgkin | 3622 | 20 | 10.0 (6.1–15.5) | n/a | 49.7 (24.3–75.1) |
| Other lymphomas | 3615 | 5 | 2.6 (0.8–6.1) | n/a | 8.6 (−5.7 to 22.8) |
| III. CNS | 17,764 | 120 | 15.1 (12.5–18.0) | 2.7 (1.9–4.0) | 64.1 (51.5–76.7) |
| Astrocytoma | 10,310 | 40 | 8.7 (6.2–11.9) | n/a | 34.3 (21.6–47.0) |
| Embryonal brain tumours | 3448 | 50 | 28.8 (21.3–38.1) | n/a | 137.2 (96.7–177.7) |
| Other CNS | 4003 | 30 | 18.6 (12.8–26.1) | n/a | 77.9 (49.1–106.8) |
| IV. Peripheral nervous cell/neuroblastoma | 5890 | 40 | 24.5 (17.3–33.7) | 4.2 (2.6–6.9) | 61.8 (40.9–82.7) |
| V. Retinoblastoma | 3279 | <5 | 3.3 (0.6–9.8) | 0.5 (0.2–1.7) | 6.4 (−5.4 to 18.2) |
| VI. Renal | 6790 | 15 | 5.9 (3.1–10.1) | 0.9 (0.5–1.7) | 15.9 (4.6–27.2) |
| VII. Hepatic | 1655 | 5 | 9.5 (3.0–22.3) | 1.6 (0.6–4.2) | 27.2 (−0.7 to 55.0) |
| VIII. Bone | 4130 | 20 | 8.8 (5.4–13.6) | 1.7 (1.0–3.0) | 42.9 (20.5–65.3) |
| IX. Soft tissue | 5746 | 30 | 9.5 (6.2–13.9) | 1.8 (1.1–3.0) | 40.6 (22.3–58.8) |
| X. Germ cell | 3876 | 5 | 5.1 (2.3–9.7) | 1.0 (0.5–2.0) | 18.6 (2.0–35.2) |
| XI. Carcinomas | 3929 | 15 | 7.4 (4.1–12.2) | 1.5 (0.8–2.7) | 33.1 (12.5–53.7) |
| XII. Other | 1311 | <5 | 2.3 (0.0–13.2) | 0.4 (0.1–3.1) | 4.6 (−13.3 to 22.4) |
| P* | <0.01 | ||||
| Age at diagnosis | |||||
| <1 years | 9208 | 20 | 8.3 (4.9–13.1) | 1 | 17.2 (7.6–26.7) |
| 1–4 years | 37,805 | 140 | 12.4 (10.4–14.7) | 1.9 (1.1–3.2) | 33.5 (27.2–39.9) |
| 5–9 years | 26,385 | 120 | 9.9 (8.2–11.8) | 1.5 (0.9–2.5) | 40.5 (32.0–49.0) |
| 10–14 years | 27,939 | 130 | 7.3 (6.1–8.7) | 1.2 (0.7–2.0) | 40.2 (31.7–48.7) |
| P* | <0.01 | ||||
| Time since diagnosis | |||||
| 5–9 years | 41,815 | 235 | 18.9 (16.6–21.5) | 5.1 (3.3–7.8) | 53.9 (46.5–61.3) |
| 10–14 years | 32,683 | 95 | 7.2 (5.8–8.8) | 1.8 (1.1–2.8) | 25.8 (19.5–32.2) |
| 15–19 years | 19,537 | 45 | 4.0 (2.9–5.4) | 0.9 (0.6–1.5) | 17.3 (9.8–24.8) |
| 20+ years | 7301 | 25 | 4.3 (2.8–6.4) | 1 | 25.2 (10.6–39.8) |
| P* | <0.01 | ||||
| Neighbourhood Income** | |||||
| Very high/high income | 40,167 | 160 | 9.4 (8.0–11.0) | 1 | 36.2 (29.7–42.8) |
| Middle/low/very low income | 58,718 | 235 | 9.5 (8.3–10.8) | 1.0 (0.8–1.2) | 35.8 (30.4–41.2) |
| P* | 0.88 | ||||
| Place of residence** | |||||
| Urban | 77,726 | 300 | 9.2 (8.2–10.3) | 1 | 34.5 (29.9–39.1) |
| Rural/Remote | 21,769 | 100 | 10.4 (8.5–12.7) | 1.1 (0.9–1.4) | 41.5 (32.1–51.0) |
| P* | 0.32 |
- Note: For confidentiality, we randomly rounded the number of people and deaths using an unbiased random rounding scheme with a base of five. Counts may not sum to the total due to random rounding.
- Abbreviations: ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukemias; AER, absolute excess risk; CI, confidence interval; CNS, central nervous system; n/a, Not applicable; ICCC, International Classification of Childhood Cancer; RSMR, ratio of the standardized mortality ratios; SMR, standardized mortality ratio.
- AER is per 10,000 person-years.
- α Counts under 5 are suppressed.
- † See Supporting Information Table S3 for the unabbreviated main diagnostic groups and subtypes.
- * P values were calculated by using likelihood ratio tests within multivariable Poisson regression models that adjusted for sex, ICCC group, age at diagnosis and year of diagnosis.
- ** Missing and invalid postal codes were excluded (<3% of survivors).
When cause-specific mortality was assessed, the number of deaths due to external causes was similar to expected, whereas deaths to SPNs and health-related causes were significantly higher (Figure 1A). Although survivors of childhood cancer had a statistically significant increased risk of death for all specific types of health-related deaths, the highest SMRs were observed for deaths due to infections and respiratory disease. Supporting Information Table S4 shows cause-specific SMRs and AERs by demographic characteristics.
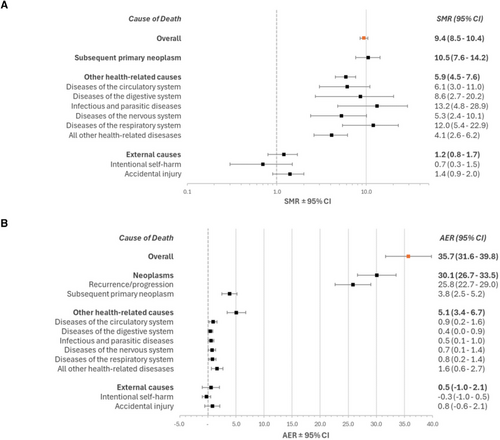
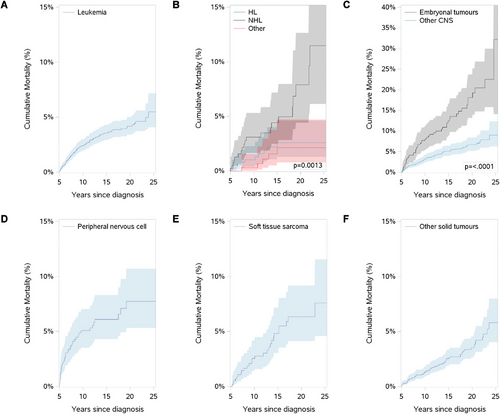
Figure 2(A–F) and Supporting Information Table S5 show cumulative mortality among 5-year survivors by cancer type. Peripheral nervous cell/neuroblastoma tumour survivors have a sharp increase in cumulative mortality in the first 5 years of follow-up, while most other cancer types show a steady increase in cumulative mortality.
Cumulative mortality differed by cause of death (Figures 3A and 3B). Mortality from recurrence/progression increased rapidly in the 5 to 10 years after diagnosis and then slowed, reaching a final cumulative mortality of 3.7%. Conversely, deaths due to other causes began to increase as neoplastic mortality slowed. Twenty-six years after diagnosis, cumulative mortality from other causes matched that of recurrence/progression of the original cancer.
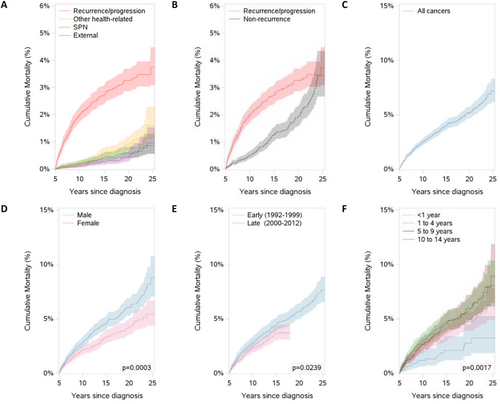
By the end of the follow-up period, the overall cumulative mortality, which can be interpreted as the probability of death, was 7.2% (95% CI = 6.1–8.3; Figure 3C). Figure 3(D–F) shows the cumulative mortality among 5-year survivors, by sex, diagnosis era and age at diagnosis. Male and female survivors had similar cumulative mortality in the first 5 years of follow-up. Past that, males’ cumulative mortality rose significantly more than females. Overall cumulative mortality was statistically significantly different between diagnosis eras. Survivors diagnosed as infants (<1 year) had significantly lower cumulative mortality compared with survivors diagnosed later in childhood.
Table 3 shows the unadjusted and adjusted associations between characteristics of survivors of childhood cancer, and the likelihood of late death during the follow-up period.
| Characteristic | Univariate | Multivariate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause | Neoplasms | Other health | All-causeδ | Neoplasmsδ | Other healthε | |||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||||||||||
| Maleα | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Female | 0.7 (0.6–0.8) | <0.01 | 0.7 (0.6–0.9) | <0.01 | 0.9 (0.5–1.5) | 0.64 | 0.6 (0.5–0.8) | <0.01 | 0.6 (0.5–0.8) | <0.01 | 0.9 (0.6–1.6) | 0.82 |
| Cancer type | ||||||||||||
| Lymphomaα | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Leukaemia | 1.2 (0.8–1.8) | 0.33 | 1.2 (0.8–1.8) | 0.33 | 1.2 (0.8–1.8) | 0.33 | 1.6 (1.1–2.3) | 0.02 | 3.5 (1.9–6.3) | <0.01 | 0.4 (0.2–0.8) | 0.01 |
| CNS tumours | 2.4 (1.7–3.5) | <0.01 | 2.4 (1.7–3.5) | <0.01 | 2.4 (1.7–3.5) | <0.01 | 2.9 (2.0–4.3) | <0.01 | 6.9 (3.8–12.3) | <0.01 | 0.4 (0.2–0.9) | 0.02 |
| Peripheral nervous cell/ neuroblastoma | 2.3 (1.4–3.6) | <0.01 | 2.3 (1.4–3.6) | <0.01 | 2.3 (1.4–3.6) | <0.01 | 4.0 (2.5–6.6) | <0.01 | 9.9 (4.9–20.1) | <0.01 | 0.4 (0.1–1.8) | 0.23 |
| Soft-tissue sarcoma | 1.6 (1.0–2.7) | 0.06 | 1.6 (1.0–2.7) | 0.06 | 1.6 (1.0–2.7) | 0.06 | 1.6 (1.0–2.7) | 0.06 | 3.4 (1.7–6.8) | <0.01 | 0.3 (0.1–1.1) | 0.08 |
| Other solid tumours* | 0.9 (0.6–1.4) | 0.78 | 0.9 (0.6–1.4) | 0.78 | 0.9 (0.6–1.4) | 0.78 | 1.3 (0.9–2.0) | 0.18 | 2.4 (1.3–4.5) | <0.01 | 0.4 (0.2–0.9) | 0.03 |
| <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.07 | |||||||
| Age at diagnosis | ||||||||||||
| <1 years oldα | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 1–4 years old | 1.9 (1.1–3.0) | 0.01 | 2.1 (1.2–3.8) | <0.01 | 1.9 (0.4–8.3) | 0.39 | 2.3 (1.4–3.8) | <0.01 | 2.7 (1.4–5.2) | <0.01 | 1.8 (0.3–9.2) | 0.50 |
| 5–9 years old | 2.3 (1.4–3.8) | <0.01 | 2.4 (1.3–4.3) | <0.01 | 3.6 (0.8–15.5) | 0.08 | 2.8 (1.7–4.7) | <0.01 | 3.1 (1.5–6.2) | <0.01 | 2.9 (0.6–15.0) | 0.20 |
| 10–14 years old | 2.4 (1.4–3.8) | <0.01 | 2.3 (1.3–4.0) | <0.01 | 3.8 (0.9–15.9) | 0.07 | 3.3 (1.9–5.6) | <0.01 | 3.6 (1.8–7.3) | <0.01 | 2.6 (0.5–13.3) | 0.25 |
| <0.01 | 0.03 | 0.06 | <0.01 | <0.01 | 0.33 | |||||||
| SPN | ||||||||||||
| No SPN | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| SPN (before 5 years) | 6.9 (3.4–13.8) | <0.01 | 7.7 (3.7–15.9) | <0.01 | 4.6 (0.6–34.1) | 0.13 | 7.4 (3.8–14.3) | <0.01 | 8.5 (4.0–18.0) | <0.01 | 6.1 (0.8–46.9) | 0.08 |
| SPN (after 5 years)** | 20.3 (13.9–29.7) | <0.01 | 28.8 (19.3–42.9) | <0.01 | 9.0 (3.5–23.0) | <0.01 | 20.0 (14.2–28.2) | <0.01 | 28.5 (18.6–43.7) | <0.01 | 8.8 (3.4–22.5) | <0.01 |
| Place of residence*** | ||||||||||||
| Urbanα | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Rural/Remote | 1.2 (1.0–1.5) | 0.09 | 1.0 (0.8–1.3) | 0.87 | 2.0 (1.2–3.4) | <0.01 | — | — | — | — | 2.0 (1.2–3.4) | <0.01 |
| Neighbourhood income*** | ||||||||||||
| Very high/highα | 1.0 | 1.0 | 1.0 | |||||||||
| Middle/low/very low | 1.0 (0.8–1.2) | 0.96 | 1.0 (0.8–1.2) | 0.88 | 1.1 (0.6–1.8) | 0.78 | — | — | — | — | — | — |
| Diagnosis era | ||||||||||||
| Early (1992–1999)α | 1.0 | 1.0 | 1.0 | |||||||||
| Late (2000–2012) | 0.8 (0.6–1.0) | 0.03 | 0.8 (0.6–1.0) | 0.05 | 0.8 (0.4–1.5) | 0.47 | — | — | — | — | — | — |
- Note: All-cause hazard ratios are calculated from Cox regression models. Cause-specific hazard ratios are calculated from Fine and Gray subdistribution models to address competing causes of death.
- Abbreviations: CI, confidence interval; CNS, central nervous system; HR, hazard ratio; SPN, subsequent primary neoplasm.
- α Reference group.
- δ Adjusted for sex, cancer type, age at diagnosis and presence of an SPN.
- ε Adjusted for sex, cancer type, age at diagnosis, presence of an SPN and place of residence.
- * Includes: retinoblastomas, renal tumours, hepatic tumours, bone tumours, germ cell tumours, carcinomas and other malignant neoplasms.
- ** Time-dependent variable.
- *** Missing and invalid postal codes were excluded from models including neighbourhood income quintile or place of residence (<3%).
After adjustment, age at diagnosis was an independent risk factor for all-cause and neoplastic deaths. As age at diagnosis increased, so did the risk of a late death. Cancer type was an independent risk factor for all-cause and neoplastic deaths. Higher hazard ratios (HRs) were observed among survivors of embryonal brain tumours and peripheral nervous cell/neuroblastoma tumours compared with lymphoma survivors. A diagnosis between 2000 and 2012 was moderately protective in the univariate model; however, after adjusting for sex, cancer type, age at diagnosis and SPNs, this association was no longer significant in any model. The occurrence of an SPN, both prior to and during follow-up, was significantly associated with all-cause and neoplastic mortality.
Independent risk factors for other health-related causes of late mortality were different than for all-cause and cancer-specific late mortality. Sex and age at diagnosis were not significant risk factors. After adjusting for the competing risk of neoplastic and external deaths, lymphoma had the highest risk of death, significantly higher than leukaemia, CNS tumours and other solid tumours. Survivors living in a rural/remote area at the time of diagnosis had an increase in risk of death, as did having an SPN during follow-up.
4 Discussion
This population-based study presents the only pan-Canadian (excluding Quebec) analysis of late mortality of childhood cancer survivors. While significant improvements in treatment mean more young patients reach the 5-year cancer survival benchmark, many face lifelong burdens [3, 59]. Results show that survivors had a ninefold increased risk of death compared with the general population.
Including health inequity data in research is an increasingly high priority [77]. This study included two area-based measures of health inequity, income and rural/remote vs. urban living. No significant results were found by income and all-cause and neoplastic mortality were the same for survivors from both rural/remote and urban areas. However, our results show a 70% increase in the risk of late mortality from other health-related causes in rural/remote survivors. Health-related deaths are often attributed to late effects associated with cancer treatment. The Children's Oncology Group recommends long-term follow-up to screen and manage late effects [78]. In Canada, aftercare clinics for survivors are typically offered in urban centres [79-82]. Survivors in rural/remote areas are often transferred to their family physician for monitoring [83, 84], who may not have experience caring for adult survivors of childhood cancer [85, 86]. The literature shows mixed results regarding the impact of Canada's urban/rural divide in this population [87-90]. One study suggested that adherence to surveillance guidelines is low in both urban and rural areas [89]. Some caution is required during interpretation, as these area-level measures are used as proxies for individual-level characteristics [55]. Additionally, a child's postal code at diagnosis may not represent access to services during adolescence and adulthood.
Our all-cause SMR is similar to the pooled SMR of a meta-analysis [44] of 11 cohort studies of late mortality (SMR = 9.3, 95% CI = 8.0–10.8) [8, 26, 28, 33, 37-39, 42, 60-62]. Similar to other studies [2, 26, 28, 38, 39, 63, 64], many late deaths (64%) were attributed to recurrence/progression. After 21 years of follow-up, cumulative mortality from non-recurrence/progression matched the cumulative mortality from recurrence/progression. Survivors still had significantly more mortality relative to the general population.
When assessed by cancer type, we noted two important findings. First, ALL had a low SMR relative to the overall SMR, unlike similar reports [30, 38]. These studies include data from the mid-20th to the 21st centuries, a period of significant improvement for the survival of children with ALL [65]. Second, our results confirmed the significantly higher risk of late mortality faced by survivors of embryonal brain tumours [66, 67]. This cancer type has one of the poorest overall 5-year survivals (less than 70% [1]), and within 5 years of reaching 5-year survivorship, over 8% of these survivors had died. Most other cancer types did not reach this level of mortality after 21 years of follow-up.
Unlike other studies [8, 12, 26-28, 30, 31, 33, 35-40, 43, 45, 68], our study only includes diagnoses in the 1990s and beyond. By then, late effects were better understood and the ‘cure at any cost’ mentality shifted to ‘cure at the least cost possible’ [69]. While comparisons within the survivor population suggest a slight reduction in risk among survivors diagnosed in the later period, this reduction was not statistically significant after adjustment. It is important to note that the treatment era serves as a surrogate marker for changes in therapy, and because therapy itself was not available for analysis, we cannot make conclusions regarding treatment changes after 2000 and whether they improved late mortality outcomes. Given this, the question of whether therapy changes beyond 2000 have an impact on late mortality remains unanswered, and future investigations are needed to explore this in greater detail.
Subsequent primary neoplasms were a strong risk factor for late mortality. Only 2% of our survivor cohort experienced an SPN; however, they represented 14% of all deaths, similar to other studies [8, 26, 28, 30, 39, 44, 68, 70]. Similarly, other studies reported that non-neoplastic deaths eventually surpass deaths due to recurrence/progression after 30 to 40 years [2, 28, 46]; reassessment with additional follow-up time is thus necessary to see how the late mortality profile changes with an ageing survivor population.
Given some concerns of poor mental health in this population [11, 71-75], we assessed the risk of suicide among survivors relative to the general population and found no significant difference. A U.S.-based study found significantly higher risk among adult survivors [75]. Almost half (45%) of our cohort were under 20 years old by the study's end, the age group with the lowest risk of suicide [76].
The data originate from population-based cancer registries from almost all Canadian provinces and territories; therefore, our results are generalizable to Canadian survivors of childhood cancer. Population-based registries and linkage to vital statistics minimize bias and maximize the completeness of cancer and death ascertainment [91]. Our cohort size was relatively large, and we were able to report some rare events. Although the death-linked CCR dataset is the most comprehensive source of cancer and mortality data in Canada, it has a few limitations. First, some cancer survivors may have left Canada during follow-up or died outside of Canada. However, in the general population under 40 years old, few (<1%) migrate out of Canada every year [56, 92], suggesting little loss-to-follow-up. Second, there are no data in CCR indicating complications or relapses, which are important contributors to mortality. Similarly, as the dataset was not designed to answer specific research questions, our study could not measure some potential confounders, such as treatment exposures [4, 6, 14, 28, 30, 39], chronic conditions [23, 93] and socioeconomic status [93, 94]. Such an analysis requires linked data, which is currently unavailable. In addition, this study used the underlying causes of death reported in death certificates, which are known to be imperfect [31, 34, 64, 95, 96]. Research has shown that physicians may sometimes misattribute deaths to a patient's cancer diagnosis even when the actual cause is unrelated [101]. Finally, while this study included data from almost all provinces and territories, it did not capture cases from Quebec, as well as Ontario and Yukon for the years 2016–2017. In 2017, Quebec had a population of 8.3 million, Ontario 14.1 million and Yukon 39,000, whose cancer status was not recorded in the CCR [102].
This study confirms that Canadian survivors of childhood cancer face an increased risk of death. However, late mortality is not inevitable [64]. Improvements in treatment have reduced late effects [97], and modifiable risk factors can reduce late mortality [62, 94, 98, 99]. While late mortality fails to capture the significant burden and morbidity experienced by many survivors, it is a key metric in population health surveillance to monitor the serious, long-term health conditions that survivors face [23]. Ensuring survivors have access to health care that follows current and forthcoming survivorship guidelines is paramount.
Acknowledgements
We acknowledge the collaboration between the provincial and territorial cancer registries and the Health Statistics Division of Statistics Canada for providing the Canadian Cancer Registry data. We thank Dr Dianne Zakaria of PHAC for discussions on the analysis method, and Dr Paul Gibson (McMaster Children's Hospital; Pediatric Oncology Group of Ontario) for discussions on risk factors. We also thank Samina Aziz and Claudia Lagace of PHAC for reviewing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.




