Treatment of relapsed/refractory severe aplastic anemia in children: Evidence-based recommendations
Abstract
Severe aplastic anemia (SAA) is a rare potentially fatal hematologic disorder. Although overall outcomes with treatment are excellent, there are variations in management approach, including differences in treatment between adult and pediatric patients. Certain aspects of treatment are under active investigation in clinical trials. Because of the rarity of the disease, some pediatric hematologists may have relatively limited experience with the complex management of SAA. The following recommendations reflect an up-to-date evidence-based approach to the treatment of children with relapsed or refractory SAA.
Abbreviations
-
- AML
-
- acute myeloid leukemia
-
- CNI
-
- calcineurin inhibitor
-
- CR
-
- complete response
-
- CSA
-
- cyclosporine
-
- Cy
-
- cyclophosphamide
-
- EBMT
-
- European Group for Blood and Marrow Transplantation
-
- EFS
-
- event-free survival
-
- FFS
-
- failure-free survival
-
- Flu
-
- Fludarabine
-
- GVHD
-
- graft versus host disease
-
- hATG
-
- horse-antithymocyte globulin
-
- HLA
-
- human leukocyte antigens
-
- HSCT
-
- hematopoietic stem cell transplant
-
- IBMFS
-
- inherited bone marrow failure syndromes
-
- IST
-
- immunosuppressive therapy
-
- MDS
-
- myelodysplastic syndrome
-
- MMF
-
- mycophenolate mofetil
-
- MSD
-
- matched sibling donor
-
- MUD
-
- matched unrelated donor
-
- NAPAAC
-
- North American Pediatric Aplastic Anemia Consortium
-
- NR
-
- nonresponder
-
- OS
-
- overall survival
-
- PR
-
- partial response
-
- rATG
-
- rabbit-antithymocyte globulin
-
- RCT
-
- randomized controlled trial
-
- RD
-
- refractory disease
-
- SAA
-
- severe aplastic anemia
-
- TBI
-
- total body irradiation
-
- TPO-RAs
-
- thrombopoietin receptor agonists
1 INTRODUCTION
Severe aplastic anemia (SAA) is an acquired bone marrow failure disorder caused by immune-mediated destruction of hematopoietic cells, with pancytopenia and bone marrow hypoplasia causing clinical features of bleeding, infections, and transfusion-dependence.1 For pediatric patients, standard first-line treatment options include hematopoietic stem cell transplant (HSCT) from a matched sibling donor (MSD) or immunosuppressive therapy (IST). Although overall survival (OS) is excellent, a significant proportion of patients will have incomplete response or relapse following IST.
Several groups have published guidelines addressing diagnosis, upfront treatment, and treatment of relapsed/refractory SAA.2-4 In 2019, Pierri and Dufour published a review of the management of aplastic anemia after failure of frontline immunosuppression.5 Development of formal pediatric SAA treatment guidelines is challenging due to the paucity of available clinical trial data, which is why patient management currently relies heavily upon expert opinion. Acknowledging these barriers, we have produced these recommendations based upon the limited evidence currently available in the literature combined with consensus-derived expert opinion, particularly when sufficient evidentiary support was lacking. This paper addresses the management of relapsed and refractory SAA. A companion publication addresses the management of newly diagnosed SAA.
2 METHODS
Details of the methods are described in the companion manuscript on recommendations for upfront treatment of newly diagnosed SAA. Summary recommendations using the GRADE process were graded as “strong” or “weak,” based on “high,” “moderate,” or “low” quality evidence (Table 1).6, 7 Table 2 lists the summary of recommendations.
| Grade | Definition | Quality of evidence | Definition |
|---|---|---|---|
| 1A | Strong recommendation, high quality evidence | High quality | Well-designed RCTs |
| 1B | Strong recommendation, moderate quality evidence | Moderate quality | RCTs with limitations; large, multiple, or well-designed observational studies |
| 1C | Strong recommendation, low quality evidence | Low quality | Few or small observational studies, case reports; expert opinion weighing risks/benefits in absence of data |
| 2A | Weak recommendation, high quality evidence | ||
| 2B | Weak recommendation, moderate quality evidence | ||
| 2C | Weak recommendation, low quality evidence |
- Abbreviation: RCT, randomized controlled trial.
| Recommendation | Grade |
|---|---|
| Nonresponder or refractory disease | |
| For patients with refractory SAA, we recommend use of HSCT from alternative donors over other currently available interventions. | 1A |
| For transplant-ineligible patients with refractory SAA, we recommend a second trial of immunosuppression with hATG/CSA with consideration of adding eltrombopag. | 2C |
| Relapsed disease | |
| We recommend use of HSCT from alternative donors over repeat immunosuppression for treatment of relapsed SAA. | 1B |
| For transplant-ineligible patients with relapsed SAA, we recommend a second course of immunosuppression with hATG/CSA, with consideration of adding eltrombopag. | 2C |
| Stem cell transplant as second-line therapy | |
| Patients should be referred early for transplant evaluation to move forward with HSCT if there is no evidence of response by 3−4 months. | 1C |
| HLA- matched unrelated donors, if available, can be considered for HSCT in patients without adequate response to immune suppression. | 1A |
| Haploidentical donors can be considered for HSCT in patients without adequate response to immune suppression. | 1A |
| There are not enough data comparing haploidentical donor and matched unrelated donor HSCT to make a recommendation for one over the other. Based upon the patient's clinical situation and available donor options, the risks and benefits should be discussed with the patient and family. | 2C |
| Due to the high rates of graft rejection using cord blood, HLA-matched unrelated donors or haploidentical donors should be prioritized over cord blood. | 1B |
- Abbreviations: CSA, cyclosporine; hATG, horse-antithymocyte globulin; HLA, human leukocyte antigens; HSCT, hematopoietic stem cell transplant; SAA, severe aplastic anemia.
3 NONRESPONDER OR REFRACTORY DISEASE
Most children with SAA will demonstrate a hematologic response within 3–6 months of IST.8-10 A patient who had not achieved an adequate partial response (PR) or complete response (CR) by 8 months following the start of IST was defined by Camitta et al.1 as a nonresponder (NR) or considered to have refractory disease (RD). In 2012, Scheinberg and Young11 reported on their experience in both children and adults and defined refractory SAA as blood counts still fulfilling criteria for SAA 6 months after initiating therapy. For pediatric SAA, we have adopted a definition of quantitative and qualitative hematologic response to IST that is defined by specific criteria for each cell line (Table 3).10
| Transfusion status | ANC | Hemoglobin | Platelet count | |
|---|---|---|---|---|
| Complete response (CR) | Independent | ≥1 × 109/L | and ≥ 10 g/dL | and ≥ 100 × 109/L |
| Partial response (PR) | Independent | ≥0.5 × 109/L | and ≥ 8 g/dL | and ≥ 20 × 109/L |
| Nonresponder (NR) or refractory disease (RD) | Dependent | or < 0.5 × 109/L | or < 8 g/dL | or < 20 × 109/L |
Considering the ongoing and cumulative risks associated with transfusion dependence and severe immunodeficiency, lack of response at 6 months should be considered RD, and providers should strongly consider a second-line treatment intervention.11-13 For a subset of patients who no longer have severe neutropenia but remain transfusion-dependent, though with decreased transfusion needs or increasing reticulocyte count, clinical judgement should be used regarding need for second-line treatment.
Care for patients with refractory SAA should be at centers familiar with this rare disease population.13, 14 Potential causes of RD include undiagnosed inherited bone marrow failure syndromes (IBMFS) and refractory autoimmune diseases. Identification of an underlying genetic disorder has implications for treatment, HSCT regimen and donor choices. Evaluation should involve a genetic counselor or medical geneticist familiar with bone marrow failure. A three-generation family pedigree should be documented, and every effort should be made to obtain genetic testing for genes associated with IBMFS, primary immunodeficiencies, and hematopoietic malignancy predisposition syndromes.
Rescue therapies for refractory SAA include HSCT for elimination of immune dysfunction and reconstitution of hematopoiesis, additional or different immunosuppression, and hematopoietic stimulatory agents. Aside from HSCT, the best choice for second line treatment has been a matter of debate. Studies specific to the pediatric population are scarce, so many of the data discussed here are extrapolated from studies performed in adults. Furthermore, described studies pre-date the expanded knowledge of genetically based IBMFS, and should be interpreted accordingly.
3.1 Hematopoietic stem cell transplant
For children refractory to up-front IST, strong evidence supports proceeding directly to HSCT from the best available donor rather than repeating IST therapy. Alternative donor is defined here as any donor other than an MSD. In 2008, Kosaka et al.15 completed a prospective study in Japan demonstrating dramatic superiority of HSCT from an alternative donor over the use of repeat IST with horse-antithymocyte globulin (hATG), with 5-year failure-free survival (FFS) of 83.9% in the HSCT group versus 9.5% in the IST group. Similarly, in a 2019 North American Pediatric Aplastic Anemia Consortium (NAPAAC) retrospective study, 90 patients received second treatment for either relapsed or RD after upfront hATG/cyclosporine (CSA). Thirty-eight patients (31 with RD) underwent HSCT and 52 patients (36 with RD) received 2nd IST. Three-year event-free survival (EFS) was 80% in patients who received HSCT compared with 55% in those who received a second course of IST (p = .0011).10
For transplant-ineligible patients (e.g., those with certain serious ongoing infections, organ dysfunction, or lacking a suitable HSCT donor), a second course of IST may be considered.
3.2 Repeat IST with hATG and CSA
In a Japanese study from 2008, out of 18 pediatric patients with RD treated with a second course of IST using hATG/CSA, two patients responded at 6 months, and four patients had later responses giving a total response rate of 33%. Three patients were unable to complete the second course of IST due to anaphylactoid reactions. The majority went on to HSCT. All patients who received the second IST survived.15
In 1998, Tichelli et al.16 reported transfusion-independent hematopoiesis in 63% of 43 patients (25 with RD) treated with a second course of IST involving hATG/CSA along with norethandrolone. There was no difference in response between those treated for RD versus those treated for relapsed disease. In the patients with RD, OS was 55%. Serum sickness occurred early in the IST course at a median of 6 days. Median age on the study was 18 years, and responses in children were not analyzed separately.16
A 2014 study by Scheinberg et al.17 evaluated the effect of hATG/CSA for RD following other immunosuppression. The study included 25 patients total, 19 of whom had received rabbit-antithymocyte globulin (rATG)/CSA and six of whom had received cyclophosphamide (Cy). Eight children were included. PR occurred in five patients (20%) and 3-year OS was 68%. Responses in children were not separately analyzed.17
3.3 Second course IST with rATG and CSA
In 1999, Di Bona et al.18 treated 30 patients with RD with rATG/CSA and granulocyte colony-stimulating factor. Response was 77% and OS was 93%. The median age of enrolled patients was 21 years, but specific analysis of response rates in children alone was not performed. As 10 out of the 30 patients received this second course of IST between 85 and 120 days after the initial IST, response could possibly be attributed to the first course of IST.18
A 2006 National Institute of Health study using rATG/CSA in 22 patients with RD showed a low response of 27%. Two out of the 19 evaluable patients were children and neither responded to treatment. Most of the non-responding patients died of complications related to severe pancytopenia or progression to leukemia.19 A slightly better response rate of 33% and OS of 60% was noted in a subsequent 2012 study.20
3.4 Cyclophosphamide
Due to its immunosuppressive effect on T cells, Cy has been used at doses of 120−200 mg/kg over 4 consecutive days, without HSCT, for upfront treatment of SAA. Studies showed conflicting response and complication rates.21-27 At Johns Hopkins in 2010, 21 patients (aged 6−63 years, median 34) with SAA refractory to hATG and CSA were treated with high dose Cy. The total response rate was 47.8%, OS was 61.8%, and FFS was low at 27%. Infectious complications were significant, with over 40% developing fungal infections, resulting in 5 patient deaths.21 Responses in children were not analyzed separately.
In a 2016 follow-up study, Gamper et al.25 analyzed responses to Cy in the pediatric population. Six out of the 28 patients had been treated with at least one course of immunosuppression, including four with ATG/CSA, and were considered to have refractory or relapsed disease. In the six with relapsed/refractory disease, there were three CR and three PR. While 10-year OS was 85% and EFS was 64%, infection rate was also high, with 62% having documented bacterial and/or fungal infection. Infectious complications were similarly high in a 2010 prospective study by Audino et al.,28 occurring in five out of five pediatric patients with RD treated with high-dose Cy, two of whom died as a result. Two patients achieved CR.28
3.5 Alemtuzumab
Alemtuzumab, a monoclonal anti-CD52 antibody causing lymphocytotoxicity, is used widely in autoimmune and inflammatory disorders as well as HSCT conditioning regimens. Its use in SAA has been limited to a few studies.20, 29, 30 A 2012 randomized controlled study evaluated patients with RD treated with either repeat IST with rATG/CSA or with alemtuzumab alone. Response rate was comparable in both groups at 33% and 37%, p = .78, respectively. OS, rate of relapse, and clonal evolution were not statistically different between the two treatment groups. Children made up 26% of the study population but their outcomes were not analyzed separately.20
3.6 Thrombopoietin receptor agonists
Eltrombopag's role in SAA treatment was first studied by Olnes et al.31 in 2012, evaluating response in 25 adult patients with RD. Eleven patients had a hematologic response in at least one lineage. An additional 18 patients, with the two youngest 17 years old, were enrolled in an extension phase and had a response rate of 40%. However, eight out of the 42 patients developed cytogenetic abnormalities, including monosomy 7. One adolescent developed clonal changes after 3 months of eltrombopag treatment and was subsequently transplanted successfully.32 A second extension study in 2019 enrolled 40 patients with RD. The study included nine children, four of whom responded, a rate comparable to that seen in the adult patients. The study noted improving responses by the 24th week of treatment, and rate of clonal evolution was similar to prior studies at 20%. Twenty-five percent of responders relapsed upon discontinuation of eltrombopag but responded to its reinitiation.33 In a 2022 prospective trial by Shimamura et al., patients with refractory/relapsed SAA after IST for SAA (Cohort A) were treated with a combination of eltrombopag with either hATG/CSA or CSA alone had an overall response (OR)of 71% at week 26 and 57% at week 52, suggesting that there may be a role for the use of thrombopoietin receptor agonists in this population.34
- For patients with refractory SAA, we recommend use of HSCT from alternative donors over other currently available interventions (grade 1A).
- For transplant-ineligible patients with refractory SAA, we recommend a second trial of immunosuppression with hATG/CSA with consideration of adding eltrombopag (grade 2C).
4 RELAPSED DISEASE
Relapse is defined as full recurrence of hematologic and bone marrow SAA diagnostic criteria after initially achieving a response (CR or PR) to primary IST or recurrence of either isolated transfusion dependence or severe neutropenia. Relapse may be seen in 10−40% of responding patients.35-38
A large retrospective study performed by NAPAAC of 264 children who were treated with hATG/CSA between 2002 and 2014 demonstrated EFS decreasing from 76% at 12 months post IST to 64% at 60 months post IST, without a plateau.10 These data indicate that durable responses in the pediatric population remain suboptimal given curative goals of treatment.
An initial response to IST supports that the SAA was indeed immune-mediated, and relapse may represent a waning effect of prior IST and/or recurrence of immune attack on hematopoiesis. Reevaluation and care of those patients should be at centers with expertise. Investigation into a primary immune dysregulation disorder through expanded immunological assessment and genetic sequencing should be considered. Similar to refractory SAA, options of treatment for relapsed disease include HSCT, repeat immunosuppression, and the use of hematopoietic stimulation agents.
Few studies have investigated and reported relapsed SAA separately from RD, and even fewer include the pediatric population. Results from these studies are summarized below.
4.1 HSCT
As described above, in a retrospective NAPAAC study, 3-year EFS was 80% in patients who received HSCT as a second therapy compared with 55% in those who received a second course of IST (p = .0011). However, the 90 patients receiving 2nd-line therapy included more patients with RD than with relapse.10
In a retrospective analysis, the Japan Childhood Aplastic Anemia Study Group reported on treatment of relapsed SAA in children from two prior prospective studies conducted from 1992 to 2007. Seventeen relapsed patients were treated with IST and eleven went directly to HSCT. EFS and OS were comparable (EFS 47% IST vs. 64% HSCT, p = .96 and OS 85% IST vs. 64% HSCT, p = .07). The authors concluded that a second course of IST was safe and effective in the relapsed setting.39 Of note, these data include a heterogenous group of patients, including some with non-SAA and some who were treated upfront with danazol. Transplants included a variety of donor sources and conditioning regimens. These factors, as well as the earlier timeframe of the studies and small patient numbers, may have contributed to the lower-than-expected survival outcomes in the transplant group.
HSCT from an alternative donor is recommended for the pediatric SAA patient who relapses post IST, as outcomes and likelihood of EFS post HSCT are very promising. However, repeat IST in the relapsed setting has a higher response rate than in RD, as detailed below.17, 40
4.2 Repeat IST with hATG and CSA
In the 2011 report from Kamio et al.39 on 42 children with relapsed SAA, 17 patients received a second course of IST with hATG/CSA, and eight of them (47%) responded within 6 months, though it was not specified whether these responses were complete or partial. Of the nine NRs, seven went on to receive alternative donor HSCT as a third line treatment, and five out of the seven survived. The FFS and OS of patients receiving a second course of IST versus HSCT in this study were not statistically different.
4.3 Second course IST with rATG and CSA
As rATG is a more potent lympholytic agent than hATG, switching between them with the goal of intensifying immunosuppression and/or minimizing the occurrence of serum sickness upon reexposure to hATG has been the reported clinical practice in some reviews.5 Data about the use of rATG for relapsed SAA in children are very limited. In one study from 2006, rATG was used as a second line in treatment of 21 patients with relapsed SAA after treatment with hATG/CSA. Of the four children in the study, two showed a PR and two had no response. Of the two children with PR, one ultimately developed monosomy 7 and underwent unrelated donor transplantation.19 In a 2015 study, Clé et al.41 used rATG as salvage therapy for 37 relapsed and refractory patients following IST with rATG/CSA. Three of the five relapsed patients showed a response. Sub-analysis of the pediatric population was not performed.41
4.4 Alemtuzumab
A 2012 single arm prospective study of 25 patients, including children, with relapsed SAA following hATG/CSA demonstrated a 56% OR and 86% 3-year survival.20 However, 23% of patients relapsed within 3 years. Outcomes specific to the children were not analyzed.
4.5 Thrombopoietin receptor agonists
As described above, the only prospective pediatric study with a refractory/relapsed cohort treated with a combination of eltrombopag with hATG/CSA or CSA demonstrated an OR of 71% at week 26 and 57% at week 52.34
- We recommend use of HSCT from alternative donors over repeat immunosuppression for treatment of relapsed SAA (grade 1B).
- For transplant-ineligible patients with relapsed SAA, we recommend a second course of immunosuppression with hATG/CSA, with consideration of adding eltrombopag (grade 2C).
5 MALIGNANT TRANSFORMATION
Pediatric studies have reported varying rates of risk of developing clonal evolution, myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML) in patients with SAA treated with IST. Fourteen percent of children treated on a 2002 prospective Japanese trial developed MDS.42 There was a 7% risk of clonal abnormalities and 1.9% risk of MDS or leukemia in a retrospective 2019 North American cohort.10 More recently, the pediatric subset analysis of a 2021 prospective trial randomizing patients to IST with or without eltrombopag had 10% risk of any clonal evolution and 6% high-risk clonal evolution or AML, without a statistically significant difference between the two treatment arms.43
Secondary MDS or AML are indications for HSCT. Management of malignant transformation is beyond the scope of this paper.
6 STEM CELL TRANSPLANT AS SECOND-LINE THERAPY
6.1 Timing/criteria
The timing and criteria to move forward with allogeneic HSCT following IST is based mostly on expert opinion. A 2008 multicenter retrospective pediatric study demonstrated 60% response (CR + PR) to IST by day 120 and 71% response by day 180.44 Another multicenter retrospective pediatric study from 2019 found a median time to response of 6 months (range 3−48 months, interquartile range 3−12 months).10 A 2015 retrospective European Group for Blood and Marrow Transplantation (EBMT) analysis of pediatric and adult patients transplanted with a MSD or matched unrelated donor (MUD) found in multivariate analysis that an interval from diagnosis to transplant of 6 months or longer was a negative predictor of survival.45
Given the potential risks of infection, bleeding, medication side effects, and possible clonal evolution, as well as the improved transplant outcomes with transplant prior to 6 months, the risks of waiting for a late response to IST must be balanced against risks associated with HSCT. Therefore, if there are no signs of early response to IST by 3−4 months, the patient should proceed to HSCT if a well-matched donor is available (Figure 1). If the patient is showing signs of response, including decreased transfusion need, rising absolute reticulocyte count, and/or rising neutrophil count, the need to move forward with HSCT can be reassessed at 6 months. As donor search, identification, workup, and insurance authorization can take 1−2 months, early referral to a HSCT center is imperative to avoid unnecessary delays in treatment while the patient is still closely monitored for response to IST.
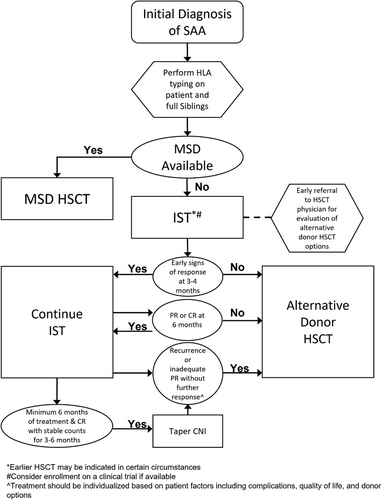
Additional considerations for moving forward with HSCT should be made on an individual patient basis. Factors to consider include severity of complications while awaiting response, need for faster neutrophil recovery in the setting of infection, life-threatening bleeding, transfusion refractoriness, degree of response, intolerance of IST, or inability to wean off immunosuppression.
- Patients should be referred early for transplant evaluation to move forward with HSCT if there is no evidence of response by 3−4 months (grade 1C).
6.2 Alternative donor sources
With improvements in HSCT approaches, alternative donor transplantation has been increasingly used (see Table S1: Studies of Alternative Donors for Aplastic Anemia). Outcomes for patients with SAA who undergo alternative donor HSCT have improved over time due to better human leukocyte antigens (HLA)-matching techniques and supportive care strategies.46 Details regarding comprehensive choice of conditioning regimens, graft versus host disease (GVHD) prophylaxis, and transplant supportive care are beyond the scope of this paper.
6.2.1 Unrelated donor transplant
OS for unrelated donor HSCT has improved over the past few decades and now approaches that of MSD transplant.47, 48 MUD HSCT performed in France from 1989 to 1998 for patients with SAA, 91% of whom received prior IST, resulted in an OS of only 29% (±7%).49 However, MUD HSCT OS improved to approximately 50% by 2004 and currently approaches 90%, both in the upfront and relapsed/refractory settings.49-51 Since 2000, multiple retrospective registry analyses have been performed comparing MUD and MSD transplantation for SAA. Studies have shown similar OS with no effect on survival by type of donor.45, 47, 52, 53 Only a single study showed superior survival outcomes with MSD compared with MUD, but that included data from the 1990s.54
However, despite similar survival outcomes, GVHD and graft rejection remain a concern with MUD.45, 52 The largest study performed by the EBMT compared transplants between 2005 and 2009 using 940 MSD (50% of patients were <20 years of age) and 508 MUD (47% of patients were <20 years of age). They reported significantly higher rates of both acute and chronic GVHD among recipients of MUD transplants. In patients receiving MUD HSCT, grades II–IV acute GVHD was reported in 25% (95% CI 21−29%) of patients and chronic GVHD in 26% (95% CI 22−31%). This was compared with 13% (95% CI 11−15%) acute GVHD and 14% (95% CI 12−18%) chronic GVHD in MSD transplants.45
In the MUD setting for SAA, preparative regimens containing fludarabine (Flu)/Cy/ATG/total body irradiation (TBI) 200 cGy are typically used.55, 56 Although both hATG and rATG are used commonly, a relatively recent 2017 Center for International Blood and Marrow Transplant Research study suggests decreased acute and chronic GVHD with improved OS for unrelated recipients associated with the use of rATG.57 Additional Flu-based approaches avoiding the use of radiation include alemtuzumab/Flu/Cy and alemtuzumab/Flu/Melphalan.58-60 These alemtuzumab containing regimens, while having similar rates of graft failure to ATG based approaches, do come with a risk of mixed chimerism.
6.2.2 Cord blood transplant
The use of cord blood transplantation for SAA has been limited by high rates of graft rejection. In a 2011 retrospective analysis from EBMT and Eurocord, engraftment rates were poor, with only 50% achieving neutrophil engraftment.61 Similar results were seen by others in both retrospective and prospective analyses.62-64 The poor engraftment translated to inferior OS of only about 40%, although many studies were performed in the early 2000s, which may not reflect more current transplantation approaches and supportive care. More recent approaches from 2013 to 2020 using cord blood have sought to decrease the time of pancytopenia following IST. In these studies, cord blood engraftment was not sustained but most patients achieved autologous reconstitution with some return of hematopoietic function.65-67 With larger cell doses, cord blood transplantation has sometimes been successful.68
6.2.3 Haploidentical related donor transplant
The use of haploidentical HSCT has increased over the past decade for all disease indications.46 For patients with SAA, the results of haploidentical HSCTs have been variable and often dependent upon the transplant approach. However, overall outcomes are improving over time. One advantage of using haploidentical donors is the rapid identification and availability of donors.
Haploidentical HSCT has been trialed for SAA in China since the early 2000s. The transplant approach described in a number of studies from China has used a reduced intensity (Busulfan/Flu/ATG) conditioning with infusion of both peripheral blood stem cells and bone marrow to reach a target total nucleated cell dose. GVHD prophylaxis was similar to standard approaches with calcineurin inhibitors (CNIs), methotrexate, and mycophenolate mofetil (MMF). In studies reported from 2012 to 2019 using this approach, survival outcomes have ranged from 70 to 89% at 3 years. Although graft failure was infrequently seen, GVHD rates remained high with approximately 30% reporting grades II–IV acute GVHD and 30% with chronic GVHD.69-74
Many centers have adopted the Johns Hopkins haploidentical transplant approach using a non-myeloablative conditioning regimen (ATG/Flu/Cy/200–400 cGy TBI) with posttransplant Cy in addition to a CNI and MMF for GVHD prophylaxis. The initial prospective studies in 2017 and 2020 using this approach had few deaths and only mild GVHD, although graft failure occurred in about 10% of patients.75, 76 Retrospective studies from the Brazilian and European transplant registries in 2020 have shown similar findings with 2-year OS of approximately 78−79% and reasonable GVHD rates (grades II–IV acute in 13 and 23%, respectively, and 10% chronic GVHD reported by both). However, graft failure remains a concern, with 20% (95% CI 12−32%) reported at 1 year in the Brazilian cohort and approximately 30% reported in the EBMT cohort.77-79 Increasing the TBI dose to 400 cGy in the treatment-naïve cohort prevented graft failure in the Johns Hopkins study.76 A 2022 multicenter prospective study using 200 cGy in relapsed/refractory patients had an 81% 1-year OS.80
- HLA-MUDs, if available, can be considered for HSCT in patients without adequate response to immune suppression (grade 1A).
- Haploidentical donors can be considered for HSCT in patients without adequate response to immune suppression (grade 1A).
- There are not enough data comparing haploidentical donor and MUD HSCT to make a recommendation for one over the other. Based upon the patient's clinical situation and available donor options, the risks and benefits should be discussed with the patient and family (grade 2C).
- Due to the high rates of graft rejection using cord blood, HLA-MUDs or haploidentical donors should be prioritized over cord blood (grade 1B).
7 CONCLUSION/FUTURE DIRECTIONS
SAA is a rare hematologic disorder in children. While OS is excellent, the management of refractory or relapsed disease remains challenging. In this paper, we have evaluated available evidence, bolstered by expert opinion where evidence is lacking, to provide recommendations for treatment options for relapsed/refractory SAA in children in North America. For patients not responding to first-line therapy with IST, early consideration of HSCT and evaluation at a center with expertise are critical. If available, enrollment on clinical trials is recommended, and data from trials will hopefully shape guidelines in the future.
CONFLICT OF INTEREST STATEMENT
Research funding—Novartis: KAS; Consulting fees—RocketPharma: NJG; Research funding—Incyte, Elixirgen Therapeutics: KM; Research funding—Amgen, Inc.: AS; Research funding—Sanofi, Roche/Genentech/Spark, Takeda. Consulting/Medical Advisory Board—Genentech, BPL, CSL, Hema Biologics, Bayer, Octapharma: CM.




